CD38 is a transmembrane molecule whose expression varies during hematopoietic cell differentiation. We used stroma-supported cultures of human myeloid cells to assess the effects of CD38 ligation on myeloid differentiation. In 8 experiments with CD34+cells purified from normal bone marrow or cord blood, flow cytometry used with antibodies to CD34 and myeloperoxidase (MPO) identified 4 cell populations after 7 days of culture. Addition of anti-CD38 (T16) to the cultures induced a profound reduction of the most mature (CD34−MPO++) cell population, which includes promyelocytes, myelocytes and metamyelocytes; mean (± SD) cell recovery was 12.8% ± 9.8% of that in parallel cultures with an isotype-matched control antibody. The suppressive effect of CD38 ligation on phenotypically more immature normal cells was inconsistent but generally less pronounced. Recovery of CD34++MPO− cells was 63.3% ± 24.4%, recovery of CD34[+/−]MPO− cells was 95.3% ± 35.1%, and recovery of CD34−MPO+cells was 42.0% ± 18.7% of that in control cultures. However, anti-CD38 suppressed recovery of cells obtained from 6 patients with CD38+ acute myeloid leukemia; after 7-day cultures, cell recovery was 25.2% ± 21.7% of that in control cultures. Cell recovery was also reduced by F(ab′)2 or Fab fragments of anti-CD38. CD38 ligation dramatically suppressed recovery of murine 32D myeloid cells transfected with human CD38 and cocultured with stroma (3.8% ± 7.3%; n = 7). CD38 ligation of CD38 + 32D cells also induced cell aggregation, tyrosine kinase activity, and Ca++ influx. We conclude that CD38 mediates signals that culminate in suppression of myeloid cell growth and survival.
CD38 is a 45-kDa transmembrane molecule that is expressed heterogeneously during lymphohematopoietic cell differentiation. Most human immature hematopoietic cells with high potential for self-renewal and multilineage differentiation express low levels of CD38 or no detectable CD38 at all.1-6 Conversely, lineage-committed myeloid and lymphoid progenitor cells express very high levels of CD38, which then decreases dramatically as maturation progresses. In the lymphoid lineage, CD38 is again expressed intensely by activated lymphocytes and plasma cells.7-10
It is not yet clear whether such remarkable changes in CD38 expression simply reflect cell cycle and activation status or whether CD38 participates in the regulation of cell growth and differentiation at certain maturation stages. The latter possibility is supported by the fact that in leukemic immature cell lines, ligation of CD38 with specific antibodies results in a rapid and transient increase in cellular tyrosine kinase activity and in phosphatidyl inositol 3-kinase activity associated with the transmembrane molecule CD19 and the adaptor molecule CBL.11-18 CD38 ligation also induces growth arrest and apoptosis in normal and leukemic immature B lymphoid cells cultured on bone marrow-derived stromal layers,10supporting the premise that CD38 plays a functional role in lymphohematopoiesis.
The cellular effects mediated by ligation of CD38 in normal immature myeloid cells, which express high levels of CD38,19 20 have not yet been thoroughly elucidated. In this study, we found that CD38 ligation transduced signals that virtually abrogated the myeloid cell differentiation of normal CD34+ cells in coculture with bone marrow stroma. The suppressive effect mediated by CD38 was also observed in experiments with patient-derived myeloid leukemic cells and with the murine cell line 32D transfected with human CD38 cDNA.
Materials and methods
Cells and antibodies
Cord blood samples were obtained after normal full-term deliveries. Bone marrow samples were taken from 6 healthy bone marrow transplant donors aged 9 to 33 years (median, 20 years) and from 7 patients aged 7 to 15 years (median, 11 years) with newly diagnosed acute myeloid leukemia (AML). The diagnosis was unequivocal by morphologic, cytochemical, and immunophenotypic criteria. These studies were approved by the Institutional Review Board, with informed consent obtained from patients or their parents or guardians. Mononucleated cells were separated on a density gradient (Lymphoprep; Nycomed, Oslo, Norway) and washed 3 times in RPMI-1640 (BioWhittaker, Walkersville, MD). Normal CD34+ cells were separated by using a MACS separation system (Miltenyi Biotec, Bergisch Gladbach, Germany), which consistently affords a purity of 90% or greater. The interleukin-3 (IL-3)-dependent murine immature myeloid cells 32D c13 (32D)21 were available in our laboratory. They were cultured in RPMI-1640 supplemented with IL-3 (25 U/mL; derived from CHO cells expressing the murine IL-3 gene), 10% fetal calf serum (FCS; BioWhittaker), l-glutamine, and antibiotics. Bone marrow-derived stromal layers were prepared in flat-bottomed 96-well plates (Costar, Cambridge, MA) and fed with RPMI-1640, 10% FCS, and 10−6 mol/L hydrocortisone (Sigma, St. Louis, MO), as previously described.10 22-26 Monoclonal anti-CD38 antibodies were T16 (IgG1; Immunotech, Westbrook, ME) and THB7 (IgG1; American Type Culture Collection [ATCC], Rockville, MD). Fab and F(ab′)2 fragments of THB7 (prepared in one of our laboratories) were used in some experiments. The purity of the latter reagents was verified by sodium dodecyl sulfate–polyacrylaminde gel electrophoresis (SDS-PAGE).
DNA constructs and electroporation conditions
The CD38 expression vector was constructed by excising the human CD38 cDNA fragment from pCDM:CD38 (a gift from Dr D. G. Jackson, Oxford, UK) with XbaI and inserting it into anXbaI-cleaved pEF-BOS mammalian expression vector (a gift from Dr S. Nagata, Osaka, Japan).27 Either the expression plasmid (32 μg) or the pEF-BOS vector without insert was electroporated into 5 × 106 32D cells with 3.2 μg of a second plasmid (pSTneoB) by using a gene pulser apparatus (Bio-Rad, Richmond, CA) set at 960 μF and 290 V. Cells were cultured for 24 hours in RPMI-1640 plus additives (see above). Transfected cells were selected after culture in the presence of 1 mg/mL G418 (Life Technologies, Gaithersburg, MD). Individual clones were obtained by single-cell sorting using a FACS Vantage flow cytometer equipped with an automatic cell deposition unit (Becton Dickinson, San Jose, CA). Clones were expanded and screened for cell surface expression of human CD38 by labeling with anti-CD38 conjugated to fluorescein isothiocyanate (FITC).
Cell culture studies
Before each experiment, we washed the adherent stromal cells with tissue culture medium. Normal CD34+ cells and leukemic myeloblasts were resuspended in AIM-V serum-free medium (Life Technologies). The 32D cells transfected with cDNA encoding the human CD38 or with the vector only were resuspended in fresh RPMI-1640 with all the additives, including IL-3 (see above). Two hundred microliters of each cell suspension was then seeded onto marrow stromal cells. In each well, we placed 0.5 to 1 × 105 normal CD34+ cells, 2 × 105 AML cells, and 0.2 to 0.5 × 105 32D cells. In some experiments, transfected 32D cells were placed into the empty wells of a 96-well flat-bottomed microtiter plate or into the wells of Transwell culture supports with 0.4-μm microporous membrane inserts (Corning Costar, Cambridge, MA). For culture experiments, anti-CD38 antibodies and nonreactive control Ig were dialyzed in phosphate-buffered saline (PBS), sterile-filtered, and used at concentrations of 2 to 10 μg/mL. All cell cultures were incubated at 37°C in 5% CO2with 90% humidity. Stromal layers remained adherent throughout the cultures.
Cell counting and assessment of ploidy and apoptosis
After culture, cells were harvested by pipetting, suspended in PBS, and passed through a 19-gauge needle to disrupt clumps. Viable cells were enumerated by flow cytometry, as previously described.10,22-26 Normal myeloid cells were stained with anti-CD34 conjugated to peridin chlorophyll protein (PerCP) or phycoerythrin (PE; both from Becton Dickinson), anti-CD14 conjugated to PE (Becton Dickinson), and anti-myeloperoxidase (MPO) conjugated to FITC (Dako, Carpinteria, CA). The latter was added to the cells after cell permeabilization with Fix & Perm (Caltag; Burlingame, CA).28 Anti-CD13 PE or FITC, anti-CD33 FITC, anti-glycophorin A FITC (all from Dako), anti-CD38 FITC and/or CD19 PE (both from Becton Dickinson) were also used to label normal and leukemic myeloid cells. Cell cycle analysis was done as previously described,29 using the ModFit software (Becton Dickinson). To detect apoptosis, we labeled phosphatidylserine residues exposed on the cell surface with FITC-conjugated Annexin-V (Trevigen, Gaithersburg, MD), following the manufacturer's instructions.30 In these experiments, cell membrane permeabilization was revealed by labeling cells with 5 μg/mL propidium iodide (Trevigen) for 15 minutes at 20°C.
SDS-PAGE and Western blotting
SDS-PAGE and Western blotting were performed essentially as previously described.11,12,16,18,27 Briefly, after exposure to anti-CD38 antibody or control Ig (5-10 μg/mL), 32D cells were lysed in 1 mL of lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% [v/v] Triton X-100, 5 μg/mL aprotinin, 1 mM phenylmethyl sulfonyl fluoride, 1 mM EDTA, and 1 mM Na3VO4). After centrifugation at 20,000g for 20 minutes, cell lysates were diluted with sample buffer (10% [v/v] glycerol, 5% 2-mercaptoethanol, 3% [w/v] SDS, 65 mM Tris-HCl [pH 6.8], and 0.002% [w/v] bromophenol blue) and separated on a 7.5% acrylamide gel.11,12,16 18 After transfer, nitrocellulose filters were incubated first in 5% albumin in TBS-T20 mM Tris (pH 7.6), 137 mM NaCl, 0.1% Tween20 for 12 hours, and then with the antiphosphotyrosine antibody 4G10 (Upstate Biotechnology, Lake Placid, NY) for 1 hour. The filters were washed in TBS-T and incubated for 1 hour with horseradish peroxidase-conjugated sheep anti-mouse Ig (Amersham Corp., Arlington Heights, IL). The filters were then washed, incubated with enhanced chemiluminescence detection reagents (Amersham), and exposed to Kodak BioMax MR film.
Measurement of Ca++ flux
The 32D cells were resuspended in RPMI-1640 at a concentration of 1 × 106/mL. The Ca++-binding fluorochrome Indo-1 (Molecular Probes, Eugene, OR) was then added at a final concentration of 10 μM and the suspension was incubated at 20°C for 30 minutes. Ca++ flux was measured by using a fluorescence-activated cell sorter (FACS) Vantage flow cytometer equipped with an ILT argon-ion laser tuned to deliver 50 mW at 488, and a Coherent 306 laser tuned to deliver multiline UV light at 80 mW, exciting Indo-1 fluorescence. After splitting with a 440 LP dichroic filter, the incident fluorescence emission was measured by 2 detectors at wavelengths of 400 ± 40 nm and 480 ± 40 nm. The ratio of the 2 linear fluorescence signals was used as the indicator of Ca++ flux.
Results
Effects of CD38 ligation in cultures of normal CD34+cells
To investigate the effects of CD38 ligation on the growth of normal myeloid progenitors, we separated CD34+ cells (> 90% of which are CD38+) from cord blood and normal bone marrow samples by using magnetic beads conjugated to anti-CD34 (Figure1). We then seeded the cells onto allogeneic bone marrow stromal layers, which in preliminary experiments supported their survival and proliferation as well as their differentiation to mature myeloid cells (unpublished observations). After the culture period, we used flow cytometry to quantify the effects of CD38 ligation on normal myeloid cell differentiation. The cells were stained simultaneously with antibodies to CD34 and to MPO, permitting identification of 4 distinct cell populations (Figure2). The most immature cells (populations 1 and 2) had very low or undetectable MPO expression; population 1 had high levels of CD34 expression, whereas population 2 was CD34dim or CD34−. The most differentiated cells (populations 3 and 4) expressed MPO; cells in population 3 had intermediate levels of MPO and were CD34dim or CD34−, whereas those in population 4 had high levels of MPO and were invariably CD34−. Cells in all 4 populations expressed CD13 and/or CD33 (myeloid markers), and lacked CD19 and glycophorin A (B cell and erythroid markers, respectively), indicating their myeloid association (not shown). Population 4 includes cells that have differentiated to the promyelocyte stage or further, whereas the remaining populations include more immature myeloid cells; monocytes constitute a proportion of population 3.28,31 32
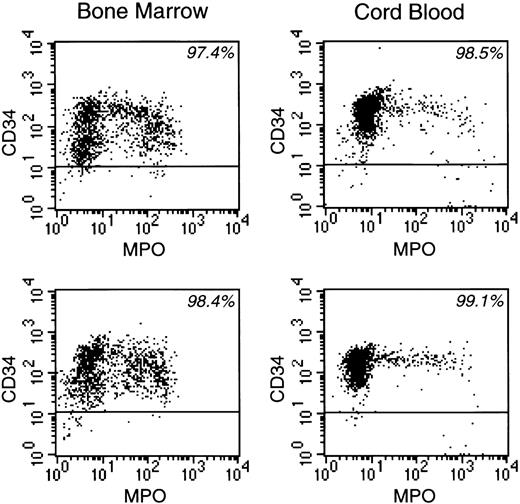
CD34 and MPO expression of enriched normal CD34+ cells from 2 bone marrow and 2 cord blood samples before culture.
The percentage of CD34+ cells in each experiment is indicated.

CD38 ligation induces a block in normal myeloid cell differentiation.
CD34+ cells were cultured for 7 days on allogeneic bone marrow stromal layers in the presence of anti-CD38 (T16) or of a control nonreactive Ig. After culture, 4 phenotypically distinct cell populations could be identified by staining with anti-CD34 PE and anti-MPO FITC (left panels). Right panel shows percent cell recovery of each of the 4 subpopulations in the presence of anti-CD38 as compared with parallel control cultures with nonreactive Ig; results are mean (± SD) of 8 experiments.

CD38 ligation induces a block in normal myeloid cell differentiation.
CD34+ cells were cultured for 7 days on allogeneic bone marrow stromal layers in the presence of anti-CD38 (T16) or of a control nonreactive Ig. After culture, 4 phenotypically distinct cell populations could be identified by staining with anti-CD34 PE and anti-MPO FITC (left panels). Right panel shows percent cell recovery of each of the 4 subpopulations in the presence of anti-CD38 as compared with parallel control cultures with nonreactive Ig; results are mean (± SD) of 8 experiments.
The number of cells recovered after 7 days of culture with a nonreactive antibody ranged from 142% to 1230% (median, 295%) of those originally seeded. Mean (± SD) cell recovery in the presence of anti-CD38 was consistently lower (49.7% ± 21.6%; n = 8) than that in control cultures with the nonreactive antibody (isotype-matched). The reduction in cell numbers, however, differed markedly between cell populations at different stages of maturation (see Figure 2). Cell recovery was particularly low among the most mature “population 4” cells (12.8% ± 9.8% of cell recovery in control wells). In line with these results, virtually no promyelocytes, myelocytes, or metamyelocytes could be identified in Wright-Giemsa-stained cytocentrifuge preparations from cultures containing anti-CD38 (not shown). By contrast, the effect of CD38 ligation on the most immature population 1 and population 2 cells was inconsistent and, overall, cell recovery was significantly less affected (63.3% ± 24.4% and 95.3% ± 35.1% of control cultures, respectively; P < 0.001 by t test for both comparisons).
Cell recovery of the intermediate population 3, which includes both granulocytic and monocytic cells,31 32 was 42.0% ± 18.7%. To determine whether CD38 ligation affected the development of both cell lineages or was selective for 1 lineage, we labeled cells at the end of the cultures with anti-CD14, a marker expressed by monocytic but not granulocytic cells. In 4 experiments (2 with cord blood and 2 with bone marrow CD34+ cells), the number of MPO+CD14+ cells at the end of 7 days of culture in the presence of anti-CD38 was 55.4%, 63.9%, 46.9%, and 15.0% of that in control cultures. In another experiment with bone marrow-derived CD34+ cells, the cultures were prolonged to 13 days; after culture in the presence of anti-CD38, the number of MPO+CD14+ cells was 18.8% of control culture values. These results indicate that both granulocytic and monocytic cells are sensitive to CD38-mediated inhibition.
Effects of CD38 ligation in cultures of patient-derived leukemic myeloid cells
The inhibitory effects of CD38 ligation were also seen in stroma-supported cultures of leukemic myeloid cells. Expression of CD38 was heterogeneous among cells derived from 6 patients with AML; cells from 1 additional patient were CD38−. Table1 summarizes the main presenting clinical and cellular features of the CD38+ cases. Percentage of cell recovery after 7 days on allogeneic bone marrow stroma ranged from 68% to 230% (median, 121%) of the viable cell input. When anti-CD38 (T16) was added to the cultures, cell recovery was decreased in all 6 CD38+ cases (mean cell recovery = 25.2% ± 21.7% of control cultures with isotype-matched nonreactive antibody; see Table1; Figure 3). A similar decrease in cell numbers, albeit less marked (46.0% ± 16.8% of recovery in control cultures), was seen in parallel cultures with the anti-CD38 antibody THB7. By contrast, in the patient whose leukemic cells did not express CD38, neither anti-CD38 antibody significantly affected cell recovery. The extent of cell recovery inhibition did not directly correlate with intensity of CD38 expression (see Table 1). On the contrary, the 2 patients with the lowest cell recovery (patients 1 and 3) expressed CD38 at the lowest level in this series, whereas the patient with highest cell recovery (patient 5) had the highest level of CD38 expression. CD38-mediated inhibition of cell recovery appeared to be caused at least in part by induction of apoptosis, as indicated by cells with nuclear fragmentation in cultures containing anti-CD38 (not shown) and by characteristic shifts in light scattering seen in some cases (eg, patient 3 in Figure 3) at the end of the cultures. After 48 hours of culture with anti-CD38, there was a marked increase in Annexin V labeling and hypodiploidy (Figure4), hallmarks of apoptosis.33
Presenting features of patients with CD38+AML and responses to culture with anti-CD38
| Pt . | Age (y) . | WBC (×109/L) . | FAB . | Karyotype . | CD38 Expression* (MFI) . | Cell Recovery After Culture With Anti-CD38† . |
|---|---|---|---|---|---|---|
| 1 | 11 | 98.4 | M2 | 46,XX,t(6;9)(p23;q34) [31] | 32.2 | 4% |
| 2 | 7 | 9.9 | M4 | 46,XX,inv(16)(p13.1q22) [10]/46,XX [12] | 82.3 | 36% |
| 47,XX,der(2)t(1;2)(q21;q35),t(5;12) | ||||||
| 3 | 14 | 26.2 | M1 | (p13;p13),del(8)(p21),+22 [19]/46,XX [1] | 17.7 | 8% |
| 4 | 7 | 88.8 | M2 | 46,XY [16] | 61.1 | 17% |
| 5 | 10 | 69.3 | M1 | 46,XY [40] | 94.6 | 63% |
| 6 | 15 | 86.2 | M5 | 48,XY,+8,+13 [4]/46,XY [19] | 57.3 | 23% |
| Pt . | Age (y) . | WBC (×109/L) . | FAB . | Karyotype . | CD38 Expression* (MFI) . | Cell Recovery After Culture With Anti-CD38† . |
|---|---|---|---|---|---|---|
| 1 | 11 | 98.4 | M2 | 46,XX,t(6;9)(p23;q34) [31] | 32.2 | 4% |
| 2 | 7 | 9.9 | M4 | 46,XX,inv(16)(p13.1q22) [10]/46,XX [12] | 82.3 | 36% |
| 47,XX,der(2)t(1;2)(q21;q35),t(5;12) | ||||||
| 3 | 14 | 26.2 | M1 | (p13;p13),del(8)(p21),+22 [19]/46,XX [1] | 17.7 | 8% |
| 4 | 7 | 88.8 | M2 | 46,XY [16] | 61.1 | 17% |
| 5 | 10 | 69.3 | M1 | 46,XY [40] | 94.6 | 63% |
| 6 | 15 | 86.2 | M5 | 48,XY,+8,+13 [4]/46,XY [19] | 57.3 | 23% |
WBC, white blood cell count; FAB, French-American-British classification; MFI, mean fluorescence intensity.
Percentage of cells with CD38 staining above the highest levels achievable with an isotype-matched control antibody was >90% in patients 2, 4, 5, and 6, 75% in patient 1 and 44% in patient 4. In the two latter patients, however, the whole cell population was shifted and no distinct CD38 negative subset was detectable.
Cell recovery after 7 days of culture on allogeneic bone marrow stroma in the presence of anti-CD38 (T16) is expressed as a percentage of cells recovered after parallel cultures with an isotype-matched nonreactive antibody. Results are the mean of 4 measurements.
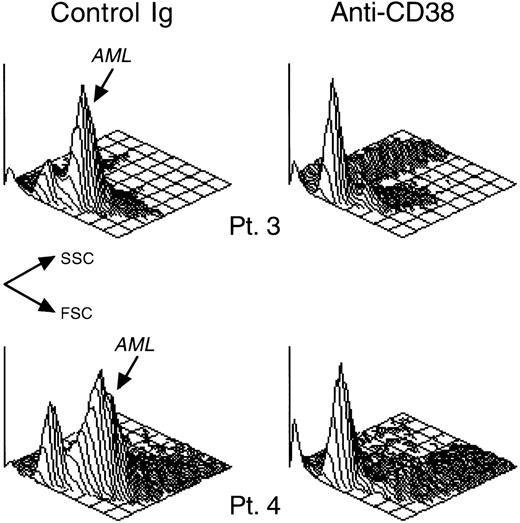
CD38 ligation inhibits in vitro growth of patient-derived leukemic myeloid cells.
AML blast cells from 2 patients (patients 3 and 4 in Table 1) were cultured for 7 days on allogeneic bone marrow stromal layers in the presence of anti-CD38 (T16) or of a control nonreactive Ig. Isometric contour plots depict the cells' light scattering (FSC, forward scatter; SSC, side scatter) after culture. In cultures with control Ig, most cells had light-scattering properties of viable myeloblasts. These cells also expressed CD13 and/or CD33 (not shown). Myeloblast cell recovery was drastically reduced in cultures with anti-CD38, where most residual viable cells had lymphoid morphology, and lacked CD13 and CD33 (not shown).

CD38 ligation inhibits in vitro growth of patient-derived leukemic myeloid cells.
AML blast cells from 2 patients (patients 3 and 4 in Table 1) were cultured for 7 days on allogeneic bone marrow stromal layers in the presence of anti-CD38 (T16) or of a control nonreactive Ig. Isometric contour plots depict the cells' light scattering (FSC, forward scatter; SSC, side scatter) after culture. In cultures with control Ig, most cells had light-scattering properties of viable myeloblasts. These cells also expressed CD13 and/or CD33 (not shown). Myeloblast cell recovery was drastically reduced in cultures with anti-CD38, where most residual viable cells had lymphoid morphology, and lacked CD13 and CD33 (not shown).
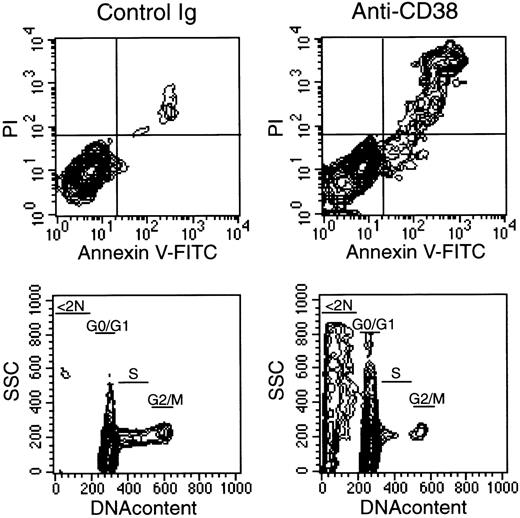
Ligation of CD38 induces apoptosis in AML cells.
Leukemic myeloblasts (patient 3 in Table 1) were cultured for 48 hours on allogeneic bone marrow stromal layers in the presence of anti-CD38 (T16) or of a control nonreactive Ig. Top panels are flow cytometric contour plots illustrating staining with Annexin-V (x-axis; a marker of apoptosis) and propidium iodide (PI, y-axis; a marker of cell membrane permeability) after culture. Bottom panels illustrate DNA content analysis; a marked increase in hypodiploid (< 2N) cells, characteristic of apoptosis, is seen in cultures containing anti-CD38. Among the viable cells, the percentage of cells in G0/G1, S, and G2/M was 90%, 8%, and 2% with control Ig, and 95%, 4%, and 1% with anti-CD38, respectively.

Ligation of CD38 induces apoptosis in AML cells.
Leukemic myeloblasts (patient 3 in Table 1) were cultured for 48 hours on allogeneic bone marrow stromal layers in the presence of anti-CD38 (T16) or of a control nonreactive Ig. Top panels are flow cytometric contour plots illustrating staining with Annexin-V (x-axis; a marker of apoptosis) and propidium iodide (PI, y-axis; a marker of cell membrane permeability) after culture. Bottom panels illustrate DNA content analysis; a marked increase in hypodiploid (< 2N) cells, characteristic of apoptosis, is seen in cultures containing anti-CD38. Among the viable cells, the percentage of cells in G0/G1, S, and G2/M was 90%, 8%, and 2% with control Ig, and 95%, 4%, and 1% with anti-CD38, respectively.
Inoue et al17 have proposed that CD38-mediated tyrosine phosphorylation in myeloid cell lines requires simultaneous engagement of CD38 and FcγRII receptors. To determine whether Fc receptor signaling was required for the cellular effects caused by CD38 ligation in leukemic myeloid cells, we performed parallel cultures to which F(ab′)2 fragments of THB7 were added. This reagent suppressed cell recovery (58.0% ± 14.6% of that in control cultures) in the 6 CD38+ AML cases. Thus, Fc receptor signaling is not required for CD38-mediated suppression of cell growth in leukemic myeloblasts. Leukemic cell recovery was also reduced (52.2% ± 27.0% of control) in cultures to which a Fab monomeric fragment of THB7 was added. Thus, conformational changes in the CD38 molecule, rather than cross-linking, appear to be important for CD38-mediated suppression of cell growth.
Effects of CD38 ligation on 32D cells transfected with human CD38
To further investigate the effects of CD38 ligation in myeloid cells, we transfected the murine cell line 32D (which does not express CD38; unpublished observation) with a human CD38 cDNA. Clonal 32D cells expressing high levels of human CD38 were obtained after transfection and single-cell sorting. In the selected clones, cell-surface CD38 expression was similar to or greater than that of human normal and leukemic immature myeloid cells. By contrast, mock-transfected 32D cells did not react with anti-human CD38 antibodies.
Ligation of human CD38 with T16 or THB7 induced aggregation of 32D cells transfected with CD38 (Figure 5). This effect was seen in all 4 CD38+ clones studied but not in cells transfected with vector only or in human myeloid cells. Cell aggregation resembled that observed with Ba/F3 murine pro-B cells transfected with human CD38.27 Aggregation became distinguishable within 2 hours of exposure to the antibody and was maximal after 24 hours. Aggregation was also induced by F(ab′)2 and Fab fragments of THB7 (see Figure 5). As we previously observed with Ba/F3 murine pro-B cells tranfected with human CD38,27 cell aggregation was not noticeably reduced by the addition of 5 mM EDTA or 5 mM EGTA to the cultures, indicating that aggregation did not require Ca++ or Mg++. Neither was aggregation inhibited by preincubating cells for 90 minutes with an antibody to leukocyte function-associated antigen 1 (LFA-1) (I21/7.7), indicating that the interaction between LFA-1/ICAM-1 was not involved.

CD38 ligation induces aggregation of murine 32D cells tranfected with human CD38.
Cultures of 32D expressing human CD38 were exposed for 24 hours to a nonreactive isotype-matched control Ig or to 3 preparations of anti-CD38 (THB7): whole Ig molecule, F(ab′)2fragments, and Fab fragments. Cell aggregation was induced by anti-CD38 irrespective of the integrity of the Ig molecule. The loose aggregation seen with control Ig was also seen in cells cultured without antibody (not shown).

CD38 ligation induces aggregation of murine 32D cells tranfected with human CD38.
Cultures of 32D expressing human CD38 were exposed for 24 hours to a nonreactive isotype-matched control Ig or to 3 preparations of anti-CD38 (THB7): whole Ig molecule, F(ab′)2fragments, and Fab fragments. Cell aggregation was induced by anti-CD38 irrespective of the integrity of the Ig molecule. The loose aggregation seen with control Ig was also seen in cells cultured without antibody (not shown).
When CD38-transfected 32D cells (3 different clones) were cultured with anti-CD38 (T16), cell recovery after 7 days varied but was slightly less overall than that in control wells that contained a control nonreactive antibody; the mean (± SD) cell recovery in 15 experiments was 77.1% ± 23.1% (Figure6). However, when bone marrow-derived stroma was present in culture wells, cell recovery in the presence of anti-CD38 antibody (T16) decreased dramatically (3.8% ± 7.3%; n = 7; see Figure 6). Similar results were seen with THB7 and with the F(ab′)2 and Fab fragments of this antibody. In a comparative experiment, mean cell recovery of duplicate cultures relative to control cultures with isotype-matched nonreactive Ig was 2.7% with T16, 3.7% with THB7 whole Ig, 2.7% with THB7 F(ab′)2, and 3.2% with THB7 Fab. No decrease in cell recovery was observed in parallel experiments with mock-transfected 32D cells. These results recall our observations that in human immature B-cell lines CD38 ligation induces growth suppression only when cells are cultured in the presence of bone marrow stroma.10

Direct contact with stroma enhances the suppressive effects of CD38 ligation in 32D cells expressing human CD38.
Cells were cultured for 7 days without or with bone marrow-derived stromal layers. In the latter cultures, 32D cells were either in direct contact with stroma (“Contact”) or separated from stroma by a 0.4-μm porous membrane (“No contact”). Results are expressed as mean (± SD) percent cell recovery in the presence of anti-CD38 (T16) as compared with parallel cultures with a control nonreactive Ig. The number of experiments for each culture condition is indicated.

Direct contact with stroma enhances the suppressive effects of CD38 ligation in 32D cells expressing human CD38.
Cells were cultured for 7 days without or with bone marrow-derived stromal layers. In the latter cultures, 32D cells were either in direct contact with stroma (“Contact”) or separated from stroma by a 0.4-μm porous membrane (“No contact”). Results are expressed as mean (± SD) percent cell recovery in the presence of anti-CD38 (T16) as compared with parallel cultures with a control nonreactive Ig. The number of experiments for each culture condition is indicated.
The 32D cells do not require contact with stroma to remain viable. To determine whether direct contact with stroma was necessary for the enhanced response of the cells to anti-CD38, we prepared cultures in which 32D-CD38 cells were separated from stroma by a 0.4-μm microporous membrane, which allowed free flow of soluble factors but blocked direct cell–cell contact. In 4 experiments under these culture conditions, CD38 ligation produced a cell recovery of 68.3% ± 23.2%. This percentage of cell recovery was similar to that seen in parallel cultures in which 32D-CD38 cells were exposed to anti-CD38 in wells without stroma (see Figure 6). Thus, direct contact with stromal layers, rather than exposure to stroma-derived soluble factors, was critical to enhance the response of cells to CD38 ligation.
CD38-mediated signaling in 32D cells
To test whether CD38 ligation in transfected 32D cells could trigger signal transduction, we engaged CD38 with an anti-CD38 antibody (T16) and performed Western blots on cell lysates. The anti-phosphotyrosine antibody 4G10 detected tyrosine phosphorylation of several proteins after 1 minute of CD38 ligation. The effect was maximal 5 minutes after CD38 ligation and decreased progressively thereafter (Figure7). The main tyrosine-phosphorylated proteins had molecular masses of approximately 68, 72, 110, and 140 kDa. By contrast, an isotype-matched nonreactive antibody and an antibody to murine CD44 (IM7), a molecule highly expressed on 32D cells (our unpublished observations), did not noticeably increase tyrosine phosphorylation. Another anti-CD38 antibody (THB7) did induce tyrosine phosphorylation, but its F(ab′)2 and Fab fragments did not.
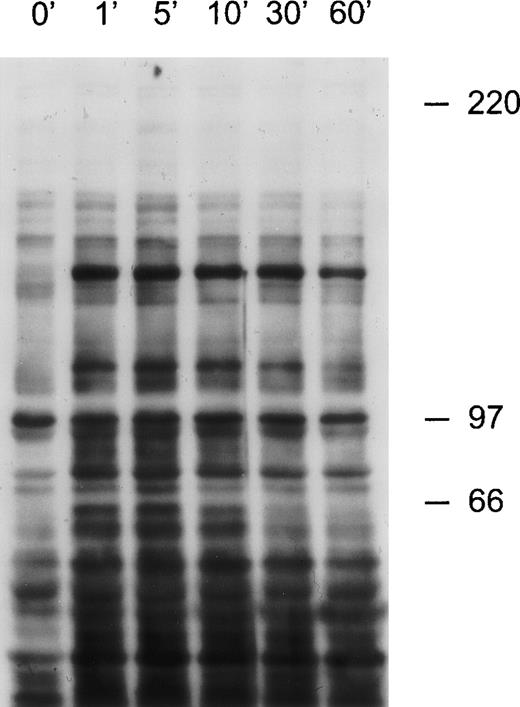
CD38 ligation in 32D cells expressing human CD38 induces tyrosine kinase activity.
32D cells were exposed to anti-CD38 (T16) for the times indicated. Cell lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane, which was probed with the anti-phosphotyrosine antibody 4G10. Molecular mass markers (in kDa) are indicated.

CD38 ligation in 32D cells expressing human CD38 induces tyrosine kinase activity.
32D cells were exposed to anti-CD38 (T16) for the times indicated. Cell lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane, which was probed with the anti-phosphotyrosine antibody 4G10. Molecular mass markers (in kDa) are indicated.
We also tested whether CD38 ligation induced Ca++ flux in the same cells. A small but consistent increase in intracellular Ca++ was detected after CD38 ligation with T16, THB7, and THB7 F(ab′)2 and Fab fragments (Figure8); this effect was not enhanced by culturing 32D cells on stroma before exposure to the antibody. Ca++ flux was abrogated by the addition of EGTA (5 mM) to the medium (see Figure 8). By contrast, an isotype-matched nonreactive antibody and the anti-CD44 (IM7) antibody did not alter the intracellular Ca++ content (see Figure 8).

CD38 ligation in 32D cells expressing human CD38 induces Ca++ and influx.
Cells were loaded with Indo-1. A shift in Indo-1 fluorescence was induced by the addition of anti-CD38 (THB7) as a whole Ig and F(ab′)2 and Fab fragments. The shift was abrogated by the presence of 5 mM EGTA and was not induced by an isotype-matched nonreactive or by an antibody to CD44, a surface molecule highly expressed in 32D cells.

CD38 ligation in 32D cells expressing human CD38 induces Ca++ and influx.
Cells were loaded with Indo-1. A shift in Indo-1 fluorescence was induced by the addition of anti-CD38 (THB7) as a whole Ig and F(ab′)2 and Fab fragments. The shift was abrogated by the presence of 5 mM EGTA and was not induced by an isotype-matched nonreactive or by an antibody to CD44, a surface molecule highly expressed in 32D cells.
Discussion
In this study, we found that CD38 ligation induces a marked inhibition of normal human myeloid cell differentiation. The suppressive effects of CD38 ligation on cell growth were particularly distinct among CD34−MPO++cells (population 4), a phenotype that includes most promyelocytes and myelocytes.28 31 Of note, both monocytic cells and granulocytic cells appeared to be affected by CD38 ligation. The human CD38 molecule could mediate suppressive signals even when ectopically expressed in murine myeloid cells, indicating that the signaling mechanism leading to this cellular effect is preserved across species. It is unclear whether the effect of CD38 was primarily caused by inhibition of cell proliferation or induction of apoptosis in normal immature myeloid cells. In cultures of leukemic myeloid cells derived from patients, however, induction of apoptosis was apparent.
Experiments by Inoue et al17 with leukemic myeloid cell lines suggested that anti-CD38 antibodies could trigger signaling in myeloid cells by serving as a bridge between the CD38 molecule and FcγRII receptors. Although anti-CD38 antibodies lacking the Fc portion failed to induce measurable tyrosine kinase activity, all other cellular effects examined could be triggered irrespective of the integrity of the Ig molecule. One interpretation for this apparent discrepancy is that CD38-mediated tyrosine phosphorylation and cellular effects are independent. Although the former may require simultaneous engagement of CD38 and Fc receptors, the latter can be triggered by CD38 ligation alone. Alternatively, changes in affinity may have been introduced by the enzymatic removal of the Fc portion, leading to reagents incapable of causing a detectable surge in tyrosine kinase activity but still able to initiate all other cellular effects. Interestingly, anti-CD38 in a monomeric Fab fragment form also reduced cell recovery and caused aggregation, an effect we had previously observed in immature lymphoid cells.27 These results are consistent with the model of CD38 extrapolated by Prasad et al34from the crystal structure of the Aplysia californicaadenosine diphosphate ribose cyclase, a CD38 homolog. This model depicts CD38 as a dimer with a hinge motion that can effect signal transduction in response to external stimuli. We speculate that anti-CD38 antibodies can exert their cellular effects by eliciting this hinge motion, rather than by dimerization or oligomerization of the receptor.
Our observations appear to contradict those of Konopleva et al,35 who found that CD38 ligation in myeloid cell lines and AML blast cells induced a variable increase in thymidine incorporation, colony formation, and cell numbers recovered after culture, and had no discernible effect on the numbers of normal granulocyte-monocyte colony-forming units. One critical difference between the studies is our addition of stromal feeder layers, which mimic the in vivo bone marrow microenvironment. Both normal hematopoietic cells and primary leukemic cells require stromal feeder layers for optimal in vitro growth.36-42 In experiments with CD38-transfected 32D cells, we found that CD38 ligation had an inconsistent effect on cell recovery after culture without stroma but radically and consistently reduced cell numbers in the presence of stroma. The accessory effect of stroma-derived signal recalls our previous observations in human lymphoid leukemic cell lines.10
The reduced cell recovery observed in stroma-dependent cultures of normal and leukemic primary cells could conceivably have been caused by interference with the adhesion of these cells to stromal elements. This possibility is suggested by the reported ability of CD38 to mediate adhesion of lymphocytes to endothelial cells,43 and to bind to hyaluronic acid,44 which is abundant in the extracellular matrix of stromal layer prepared in the presence of corticosteroids, such as those in our assay.45 However, we think this possibility unlikely because CD38 ligation also suppressed cell growth in 32D and other cell lines10 that do not require interaction with stroma for survival and proliferation. Thus, the mechanism by which CD38- and stroma-mediated signals interact and synergize remains to be clarified. It appears, however, that the relevant stroma-derived factors either are expressed on the surfaces of stromal cells or accumulate in the extracellular matrix, because separation of 32D cells and stroma by a microporous membrane markedly reduced the suppressive effects of CD38 ligation.
In summary, CD38 in conjunction with stromal elements directly mediates signals that culminate in suppression of myeloid cell development. The effects of CD38 ligation on myeloid cells described in this study have many analogies with the effects of the same stimulus in immature B lymphoid cells,10,11,27 and suggest common signaling pathways between the 2 lineages. The physiologic role of CD38 at the early stages of hematopoietic cell differentiation remains an enigma, but its powerful signaling properties, suppression of cell growth, and heterogeneous distribution indicate that this molecule could be involved in the homeostasis of hematopoiesis within the bone marrow microenvironment. Although CD38-deficient mice have apparently normal hematopoiesis,46 the distribution of CD38 differs in murine and human cells,47,48 rendering comparisons between the 2 species difficult. Natural CD38 ligands, capable of inducing events similar to those triggered by anti-CD38 monoclonal antibodies, have not yet been identified. CD31 and hyaluronic acid can bind CD38,43,44 but did not elicit any detectable biochemical or cellular response in vitro (unpublished observations).27Thus they cannot accout for the full spectrum of biologic effects mediated by CD38, suggesting the existence of other CD38 ligands. Nevertheless, our findings have important practical implications. The immunophenotype of human normal and leukemic stem cells is often defined as CD34+CD38−,6,20,49-51 because the growth potential of sorted CD34+CD38−cells is greater than that of CD38+ cells. In light of the results of this and previous studies,10 we propose that the lesser growth potential of sorted CD38+ cells may be, at least in part, caused by signals mediated by anti-CD38 antibody used in the sorting process. We suggest that, in experiments designed to compare the growth of selected cell populations, cell sorting should be done using antibodies that have no effect on cell growth.
Acknowledgments
We thank Dr P. Kelly for providing cord blood samples, Dr R. Ashmun for analysis of Ca++ flux, and S. Naron for editorial suggestions.
Supported by grants RO1-CA58297 and P30-CA21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
Reprints:D. Campana, Department of Hematology-Oncology, St. Jude Children's Research Hospital, 332 North Lauderdale, Memphis TN 38105-2794; e-mail: dario.campana@stjude.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal