Adenosine (Ado) is an important autocrine modulator of neutrophil functions. In this study, we determined the effects of endogenous Ado on fMet-Leu-Phe (fMLP)–induced phospholipase D (PLD) activity in neutrophils. The removal of extracellular Ado by Ado deaminase (ADA) or the blockade of its action by the A2a receptor antagonists 8-(3-chlorostyryl) caffeine (CSC) or CGS15943 markedly increased fMLP-induced PLD activation. The concentration-dependent stimulatory effects of CSC and CGS15943 were abolished by a pretreatment of neutrophil suspensionswith ADA. In contrast, the selective A2a receptor agonist CGS21680 suppressed fMLP-induced PLD activation. Furthermore, inhibition by CGS21680 of fMLP-induced PLD activity was reversed by CSC or CGS15943. The removal of Ado by ADA or the blockade of its action by CSC or CGS15943, markedly increased the membrane recruitment of cytosolic protein kinase C (PKC), RhoA, and ADP-ribosylation factor (ARF) in response to fMLP. As shown for PLD activity, the stimulatory effect of Ado receptor antagonists on PLD cofactors translocation was abolished by a pretreatment of the cells with ADA. Moreover, the membrane translocation of both PKC, RhoA, and ARF in response to fMLP was attenuated by CGS21680 and this effect of the A2a receptor agonist was antagonized by CSC or CGS15943. These data demonstrate that Ado released by neutrophils in the extracellular milieu inhibits PLD activation by blocking membrane association of ARF, RhoA, and PKC through Ado A2a receptor occupancy.
Adenosine (Ado)1 is an ubiquitous nucleotide autacoid that mediates a large spectrum of biologic effects by activation of at least 4 cell surface receptors designated as A1, A2a, A2b, and A3.1 Ado is well-known to modulate the functional responsiveness of inflammatory cells including neutrophils.2 Several sources have reported that Ado acts on neutrophils by binding 2 different classes of cell surface receptors, the A1 and A2 adenosine receptor subtypes. Acting through A1 receptors, Ado has been reported to increase chemotaxis,3phagocytosis of immune complexes,4 adherence to endothelium,5 and to enhance the expression of β2 integrins on neutrophil membranes.6,7 In contrast, occupancy of A2a receptors has opposite effects, decreasing phagocytosis,4 adherence to endothelial cells,5leukotriene synthesis,8 and fMet-Leu-Phe(fMLP)-stimulated respiratory burst.3,9,10 Neutrophils express the A2a receptor and the order of potency of Ado analogues provides pharmacologic evidence that the A2a subtype mediates these inhibitory effects.11,12 Thus, Ado is viewed as a potent endogenous anti-inflammatory agent. Different drugs including methotrexate13,14 and sulfasalazine15 cause Ado accumulation at inflamed sites and inhibition of neutrophil migration through occupancy of A2a receptors. A2 receptors are linked to heterotrimeric Gs proteins and regulate adenylyl cyclase activation, whereas A1 receptors are coupled to Gi proteins.2 The occupancy of A2a receptors stimulated an increase in the levels of intracellular cAMP that could only be measured after stimulation of neutrophils with fMLP.16 However, there is little experimental support for a role of cAMP as the second messenger mediating the inhibition of neutrophil functions by Ado.2
Phospholipase D (PLD) activities are present in many cell types, including human granulocytes.17 PLD catalyzes the hydrolysis of phosphatidylcholine to yield phosphatidic acid, which is further metabolized to diacylglycerol by lipid phosphate phosphohydrolases.18 PLD-derived second messengers promote the respiratory burst,19 secretion,20 the number of cell surface β2 integrins,21 and are required for phagocytosis.22 Two PLD isoforms have recently been cloned, PLD123 and PLD2.24,25 PLD1 has a low basal activity but is stimulated several times by ADP-ribosylation factors (ARFs),26,27 RhoA,28 and PKCα,29-31 in presence of phosphatidylinositol 4,5-bisphosphate (PIP2). In contrast, PLD2 shows a high basal activity but PLD cofactors other than PIP2 are not required for its activity.24,25 In granulocytes, PLD is stimulated by RhoA,32 ARF,26,33 and PKCα.34Furthermore, neutrophil activation with chemotactic peptides promotes the recruitment of RhoA, ARF, and PKCα to membranes, which in turn stimulates PLD activity. These observations together with immunoblotting analyses of PLD isoforms led to the conclusion that PLD1 is expressed and activated in neutrophils after their stimulation with chemotactic peptides.35 36
The effects of A2a receptor occupancy on fMLP-induced intracellular signals are still incompletely characterized.2 A2a receptor occupancy does not affect Ca++ mobilization from intracellular stores,37 phospholipase C-mediated inositol 1,4,5-triphosphate formation,38 and the rapid rise in the levels of diacylgylcerol stimulated by fMLP.16 However, it diminishes Ca++ influx,39 and the late and sustained accumulation of diacylglycerol.16 Because the sustained generation of diacylgylcerol induced by fMLP is secondary to dephosphorylation of PLD-derived phosphatidic acid,40 it is possible that Ado modulates neutrophil functions by interfering with the PLD signaling pathway. In this study, we examined the role of endogenous Ado removal and of Ado analogues on fMLP-stimulated PLD activity. We found that pretreatment of the cell suspensions with Ado deaminase (ADA) or A2a receptor antagonists before stimulation with fMLP markedly increased PLD activity, whereas A2a receptor agonists suppressed PLD activity. The inhibitory effects of A2a receptor agonists on fMLP-induced PLD activity were reversed by CSC, a specific A2a receptor antagonist. Furthermore, we demonstrated that the occupancy of Ado A2a receptors interferes with PLD activation by diminishing the membrane recruitment of the PLD1 activators, ARF, RhoA, and PKCα.
Materials and methods
Materials
2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxiamido adenosine hydrochloride (CGS21680), 8-(3-chlorostyryl)caffeine (CSC), 8-cyclopentyl-3,7-dihydro-1,3-dipropyl-1H-purine-2,6-dione (DPCPX), 9-chloro-2-(2-furyl)[1,2,4]triazolo[1,5-c]quinazolin-5-amine (CGS15943), 2-chloro-N-cyclopentyladenosine (CCPA), 5′-N-ethylcarboxamidoadenosine (NECA) were from RBI (Natick, MA). Dextran T-500 and Ficoll-Paque were purchased from Pharmacia Biotech (Dorval, Québec, Canada). Anti-PKCα and anti-RhoA antibodies were obtained from Santa Cruz (Santa Cruz, CA). The polyclonal anti-ARF1 antibody was described in previous studies.33ADA, di-isopropylfluorophosphate (DFP), fMLP, and all other reagents were from Sigma-Aldrich Canada (Oakville, Ontario, Canada). ADA was dialyzed against 0.9% NaCl prior use to remove ammonium salt.
Neutrophil purification
Venous blood was collected from healthy adult volunteers in isocitrate anticoagulant solution. Neutrophils were separated as described previously.35 Leukocytes were obtained after erythrocyte sedimentation in 2% Dextran T-500 and by centrifugation on Ficoll-Paque cushions. Contaminating erythrocytes in the neutrophil pellets were removed by a 20-second hypotonic lysis in water. Neutrophils were resuspended in Hanks' balanced salt solution (HBSS), pH 7.4, containing 0.8 mmol/L Ca++ but no Mg2+to reduce nonspecific aggregation of the cells.
Analysis of Ado
Neutrophils were washed in HBSS and cell suspensions (2 × 107/mL) were incubated at 37°C. After selected times, incubations were stopped by adding 100 μL of 22% TCA (2% final). NECA (10 ng/sample) was added as an internal standard and the denatured cell suspensions stored at −20°C for at least 30 minutes. Denatured samples were centrifuged at 2000g for 10 minutes and Ado was extracted from supernatants with Sep Pak Cartridges as described previously.41 The samples were analyzed by liquid chromatography-mass spectrometry with nebulizer-assisted electrospray ionization in the positive mode and by monitoring the transitions m/z 309 and m/z 268 of protonated parental ions to m/z 136 of protonated adenine, corresponding to the loss of the carbohydrate moieties from NECA and Ado.41 Ado was quantitated by extrapolating the measured Ado/NECA ratio on a calibration curve generated from standard solutions containing NECA (1 ng) and Ado (0-4 ng).
Phospholipase D measurements
Neutrophils were prelabeled with 1-O-[3H]alkyl-2-lyso-phosphatidylcholine (2 μCi/107 cells) for 90 minutes as described previously.35 The cells were then washed and resuspended at 8 × 106 cells/mL. Samples of the cell suspensions (0.5 mL) were preincubated at 37°C for 5 minutes and pretreated with 10 μmol/L cytochalasin B (CB) in the presence or the absence of ADA or of Ado analogues for 5 minutes before stimulation. Ethanol (final concentration 1.0% vol/vol) was added immediately preceding the addition of fMLP (10-7 mol/L, 10 minutes). Where indicated, neutrophils were stimulated with monosodium urate (MSU) crystals (3 mg/mL, 15 minutes) but without CB. The incubations were stopped by adding 1.8 mL cold chloroform/methanol/HCl (50:100:1, vol/vol/vol) and unlabeled phosphatidylethanol (PEt) as a standard. The lipids were extracted, dried under nitrogen, and spotted on prewashed silica gel 60 thin-layer chromatographic (TLC) plates. PEt was separated from the other lipids with the solvent mixture chloroform/methanol/acetic acid (65:15:2, vol/vol/vol). Lipids were visualized by Coomassie Brilliant Blue staining and the different lipid classes were scraped off the plates. Radioactivity in PEt was monitored by liquid scintillation counting, and the results corrected for background radioactivity and quenching.
Translocation assays
Neutrophils (4 × 107 cells/mL) were treated with 1.1 mmol/L DFP for 30 minutes at 24°C. The cell suspensions were centrifuged and resuspended in HBSS (107 cells/mL). The cells were preheated 5 minutes at 37°C, preincubated at 37°C for 5 minutes in the presence of 10 μmol/L CB with either the indicated concentrations of ADA, of Ado analogues or an equal volume of the vehicles. Cells were stimulated with 10-7 mol/L fMLP for 2 minutes, incubations were stopped by diluting the cells 1:5 with ice cold HBSS, and the samples processed as described previously.35 Briefly, cell suspensions were centrifuged as indicated and resuspended at 1.6 × 107 cells/mL in ice cold KCl-HEPES relaxation buffer (100 mmol/L KCl, 50 mmol/L HEPES, 5 mmol/L NaCl, 1 mmol/L MgCl2, 0.5 mmol/L EGTA, 2.5 μg/mL aprotinin, 2.5 μg/mL leupeptin, and 2.5 mmol/L PMSF, pH 7.2). Cell suspensions were sonicated 2 × 20 seconds and centrifuged 7 minutes at 700g. Unbroken cells and nuclei were discarded and the supernatants ultracentrifuged at 180 000g for 45 minutes. Membrane pellets were washed once and resuspended in a small volume of buffer A containing 0.25 mol/L Na2HPO4, 0.3 mol/L NaCl, 2.5% sodium dodecyl sulfate (SDS), 2.5 μg/mL aprotinin, 2.5 μg/mL leupeptin, and 2.5 mmol/L PMSF and samples were assayed for protein content. Protein samples (30-60 μg) were resolved on a 12% SDS-PAGE and transferred to Immobilon PVDF membranes (Millipore Corporation, Bedford, MA). Immunoblotting was performed with the anti-ARF (1/3500), anti-RhoA (1/1000), or anti-PKCα (1/5000) antibodies and proteins were revealed with the ECL detection system.
Statistics
Data are expressed as means ± SD. Data were analyzed with the Student paired t test (2-tailed) to determine the level of significance, *P < .05 and **P < .01, between the treated samples and the appropriate controls.
Results
Effect of ADA on fMLP-induced PLD activity and recruitment of PKC, RhoA, and ARF to membranes
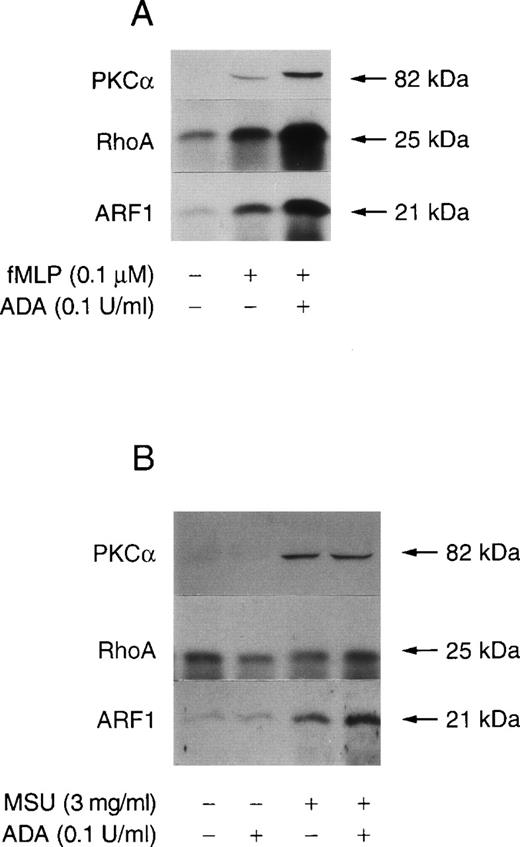
Engagement of Ado receptors has been reported to reduce the generation of the second, slow phase of diacylglycerol accumulation in fMLP-activated neutrophils.16 Although this late and sustained inhibition of diacylglycerol generation has not been fully characterized, it is now accepted that the bulk of diacylglycerol is produced by the PLD and the lipid phosphate phosphohydrolase pathways.42 Thus, we first evaluated the effects of extracellular Ado removal by ADA on fMLP-induced PLD activity. PLD activity was assayed by measuring the levels of [3H]PEt formed in presence of 1% ethanol. As shown in Figure1A, ethanol-treated but otherwise unstimulated neutrophils produced little [3H]PEt (0.031% ± 0.01% of total counts). Moreover, the addition of 0.1 U/mL ADA to neutrophil suspensions had no effect on basal PLD activity (0.042% ± 0.01% of total counts, data not shown). The amount of [3H]PEt formed reached 2.4% ± 0.3% of total counts after 10 minutes' stimulation with fMLP. The removal of extracellular Ado by ADA further increased the levels of [3H]PEt formed in response to fMLP stimulation by 2.03- ± 0.23-fold (P < .01, n = 6) compared with cells stimulated in the absence of ADA. Although both fMLP and MSU crystals stimulated PLD activity in neutrophils, we previously demonstrated that these 2 agonists act through different signal transduction pathways or PLD isoforms.35 To determine whether the effects of Ado receptor occupancy are specific to the activation of PLD by fMLP, we examined the effects of ADA on MSU crystal-induced [3H]PEt formation. As illustrated in Figure 1B, the addition of ADA to cell suspensions was without effect on MSU crystal-induced [3H]PEt formation.
Effect of ADA on fMLP- and MSU-crystals–induced PLD activity.
Neutrophils labeled with 1-O-[3H]alkyl-2-lysophosphatidylcholine were prewarmed at 37°C for 5 minutes. (A) The cell suspensions were pretreated with 10 μmol/L CB and incubated for an additional 5 minutes in the absence or presence of 0.1 U/mL ADA. Cells were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into PEt was measured as described in “Materials and Methods.” The levels of [3H]PEt formed are expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 6 separate experiments. (B) Cell suspensions were stimulated with 3 mg/mL MSU crystals in the presence of 1% ethanol for 15 minutes, without CB. The data are the means ± SEM of 4 separate experiments.
Effect of ADA on fMLP- and MSU-crystals–induced PLD activity.
Neutrophils labeled with 1-O-[3H]alkyl-2-lysophosphatidylcholine were prewarmed at 37°C for 5 minutes. (A) The cell suspensions were pretreated with 10 μmol/L CB and incubated for an additional 5 minutes in the absence or presence of 0.1 U/mL ADA. Cells were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into PEt was measured as described in “Materials and Methods.” The levels of [3H]PEt formed are expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 6 separate experiments. (B) Cell suspensions were stimulated with 3 mg/mL MSU crystals in the presence of 1% ethanol for 15 minutes, without CB. The data are the means ± SEM of 4 separate experiments.
Because previous studies have demonstrated that the PLD1 isoform expressed in human neutrophils is sensitive to activation by PKCα, RhoA, and ARF1,35 36 we conducted experiments to assess whether extracellular Ado interferes with the fMLP-induced translocation of PLD cofactors. The amounts of PKCα, RhoA, and ARF associated with membranes obtained from control and fMLP-stimulated neutrophils in the presence or absence of ADA were analyzed by immunoblotting. As shown in Figure 2, low basal levels of membrane-associated ARF and RhoA (but not of PKCα) were detected in unstimulated neutrophils. The addition of 0.1 U/mL ADA to remove Ado had no effect on the basal membrane levels of ARF, RhoA, and PKCα as estimated by immunoblotting (data not shown). In comparison, when neutrophils were stimulated with fMLP for 2 minutes, there was a detectable increase in the amount of membrane-associated ARF and RhoA. Furthermore, PKCα was also recovered in membrane fractions derived from fMLP-stimulated cells. Neutrophil pretreatment with ADA before stimulation with 0.1 μmol/L fMLP resulted in a significant enhancement of ARF, RhoA, and PKCα translocation, compared with cells stimulated in the absence of ADA. In contrast to fMLP stimulation, MSU crystal-induced translocation of ARF and PKCα but not of RhoA to membranes (Figure 2A). When neutrophils were stimulated with MSU crystals in the presence of 0.1 U/mL ADA, there was a little increase in the amount of membrane-associated ARF but not of RhoA and PKCα. Taken together the data from Figures 1 and 2 indicate that endogenous Ado accumulating in neutrophil suspensions exerts suppressive effects on fMLP but not MSU- crystal–induced PLD activity.
Effect of ADA on fMLP-stimulated translocation of PKCα, RhoA, and ARF1 to membranes.
Unlabeled neutrophils were prewarmed 5 minutes at 37°C. (A) 10 μmol/L CB was added and the cell suspension incubated for an additional 5 minutes in the presence or absence of 0.1 U/mL ADA. Neutrophils were stimulated with 0.1 μmol/L fMLP for 2 minutes. (B) Cell suspensions were stimulated with 3 mg/mL MSU crystals for 15 minutes, without CB. Incubations were stopped and neutrophil membranes prepared as described in “Materials and Methods.” The samples were analyzed for PKCα, RhoA, and ARF1 by immunoblotting. The data are from 1 experiment representative of 3 similar experiments.
Effect of ADA on fMLP-stimulated translocation of PKCα, RhoA, and ARF1 to membranes.
Unlabeled neutrophils were prewarmed 5 minutes at 37°C. (A) 10 μmol/L CB was added and the cell suspension incubated for an additional 5 minutes in the presence or absence of 0.1 U/mL ADA. Neutrophils were stimulated with 0.1 μmol/L fMLP for 2 minutes. (B) Cell suspensions were stimulated with 3 mg/mL MSU crystals for 15 minutes, without CB. Incubations were stopped and neutrophil membranes prepared as described in “Materials and Methods.” The samples were analyzed for PKCα, RhoA, and ARF1 by immunoblotting. The data are from 1 experiment representative of 3 similar experiments.
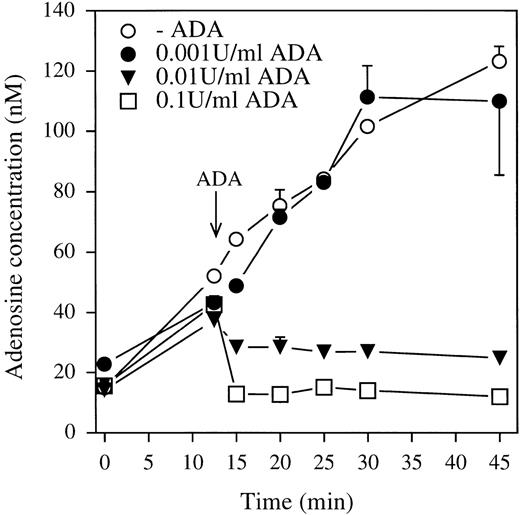
We investigated Ado accumulation in neutrophil suspensions using experimental conditions similar to those indicated for the PLD assay. The cells were resuspended in fresh HBSS and incubated at 37°C for up to 45 minutes. At selected times, samples were removed for Ado measurements. As shown in Figure 3, Ado accumulated in cell suspensions in a time-dependent manner. The concentrations of Ado increased from 20 nmol/L to up 120 nmol/L after 45 minutes incubation in HBSS. ADA (0.001 U/mL) had no effect on Ado accumulation in neutrophil suspensions. However, the addition of 0.01 and 0.1 U/mL ADA after 13 minutes incubation of the cells prevented Ado accumulation in the incubation media and reduced its extracellular concentrations below 30 and 15 nmol/L throughout the experiments, respectively. The accumulation of Ado in neutrophil suspensions was also dependent on the cell concentration, and their stimulation with fMLP did not impact on the levels of extracellular Ado (not shown and Krump et al41).
Time course of Ado accumulation in neutrophil suspensions.
Neutrophils were resuspended in HBSS at the concentration of 2 × 107 cells/mL and incubated at 37°C for 13 minutes before the addition of 0.001, 0.01, and 0.1 U/mL ADA. At selected times, aliquots (1 mL) of the cell suspensions were denatured and processed for measurement of Ado content by liquid chromatography-mass spectrometry. The data are the means ± SEM of triplicate incubations from 1 experiment representative of 3 similar experiments.
Time course of Ado accumulation in neutrophil suspensions.
Neutrophils were resuspended in HBSS at the concentration of 2 × 107 cells/mL and incubated at 37°C for 13 minutes before the addition of 0.001, 0.01, and 0.1 U/mL ADA. At selected times, aliquots (1 mL) of the cell suspensions were denatured and processed for measurement of Ado content by liquid chromatography-mass spectrometry. The data are the means ± SEM of triplicate incubations from 1 experiment representative of 3 similar experiments.
Occupancy of Ado A2a receptors modulates fMLP-stimulated PLD activity
Neutrophils express Ado A1 and A2 receptors that respectively modulate positively or negatively their functional responses.2 To determine which receptor type is responsible for suppressing neutrophil PLD activity, we studied the effects of 3 Ado receptor agonists, NECA, CGS21680, and CCPA. These Ado analogues differ greatly in their affinities for receptor subtypes as determined by inhibition of ligand binding43: NECA is not specific for A1 or A2 receptors, whereas CGS21680 and CCPA are 170- and 770-fold more selective for the A2a and A1 receptor subtypes, respectively.44 Figure 4illustrates the inhibitory effect of NECA, CGS21680, and CCPA on fMLP-induced [3H]PEt formation. In these experiments, neutrophil suspensions were pretreated with 0.1 U/mL ADA to prevent competitive binding by extracellular Ado for cell surface Ado receptors. Both CGS21680 and NECA reduced fMLP-induced PLD activity in a concentration dependent manner. The amounts of [3H]PEt formed were inhibited by 19.9% ± 4.9% (P = .056), 61.4% ± 5.4% (P = .0075), 80% ± 3.8% (P = .0023), and 84.7% ± 3.9% (P = .0021) in the presence of 1, 10, 100, and 1000 nmol/L CGS21680, respectively. NECA was as potent as CGS21680 in reducing fMLP-induced PLD activity, with half maximal inhibitory effects observed at approximately 3 nmol/L. The selective A1 receptor agonist CCPA was less effective than NECA and CGS21680. No reduction in the levels of [3H]PEt formation was observed with up to 10 nmol/L CCPA. However, 49% ± 5% and 73.3% ± 3.9% reduction in the levels of PEt formation were observed in presence of 0.1 and 1 μmol/L CCPA, respectively. CGS21680 and CCPA had no effects on the basal levels of [3H]PEt formation in unstimulated neutrophils (data not shown). Together, the data indicate that A2a receptors are involved in the inhibition of fMLP-stimulated PLD activity by Ado receptor agonists.
Effect of Ado receptor agonists on fMLP-induced PLD activity.
1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were preincubated at 37°C for 5 minutes. The cell suspensions were then pretreated with 10 μmol/L CB, 0.1 U/ml ADA, and increasing concentrations of CGS21680, NECA, or CCPA for an additional 5 minutes. Neutrophils were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.
Effect of Ado receptor agonists on fMLP-induced PLD activity.
1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were preincubated at 37°C for 5 minutes. The cell suspensions were then pretreated with 10 μmol/L CB, 0.1 U/ml ADA, and increasing concentrations of CGS21680, NECA, or CCPA for an additional 5 minutes. Neutrophils were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.
Effects of Ado receptor antagonists on fMLP-induced PLD activity
Because Ado accumulates in incubation media, we hypothesized that blocking its action with Ado receptor antagonists will increase fMLP-stimulated PLD activity, mimicking the effect of Ado removal by ADA. The effects of 2 Ado receptor antagonists, CSC and CGS15943, were investigated. CSC is more selective for the A2a than for the A1 receptor,45 whereas CGS15943 is a nonselective A1 and A2 receptor antagonist.1 CB-treated neutrophils were incubated with or without 0.1 U/mL ADA before stimulation with fMLP in the presence of increasing concentrations of CGS15943 (Figure5A) or CSC (Figure 5B). As expected, the addition of CGS15943 enhanced fMLP-induced PLD activity with a characteristic bell-shape curve (Figure 5A). As little as 0.1 nmol/L CGS15943 increased [3H]PEt formation by 28.7% ± 14%, but statistical significance was not reached. However, 1 and 10 nmol/L CGS15943 enhanced by 1.78- ± 0.13-(P = .029) fold and 1.87- ± 0.16-(P = .032) fold fMLP-stimulated PLD activity, respectively. Increasing CGS15943 concentrations up to 1 μmol/L resulted in a progressive reduction of PLD activity, but the levels of [3H]PEt formed were still larger (1.52- ± 0.07-fold) than those elicited by fMLP alone. These stimulatory effects of the A1/A2 antagonist CGS15943 were abolished by the removal of extracellular Ado with ADA. The A2a selective antagonist CSC also enhanced fMLP-induced [3H]PEt formation in a concentration-dependent manner (Figure 5B). The levels of [3H]PEt formed averaged 116% ± 5.8%, 152% ± 8.7%, and 170% ± 7% of the fMLP-induced response in the presence of 0.01, 0.1, and 1 μmol/L CSC, respectively. As observed using CGS15943, the stimulatory effects of CSC on PLD activation were totally abolished by a pretreatment of the cell suspension with ADA before fMLP stimulation. The ability of MSU crystals to stimulate PLD was not enhanced by the addition of 1 μmol/L CSC to neutrophil suspensions. The levels of [3H]PEt formed in response to MSU crystals were 0.951% ± 0.212% and 0.885% ± 0.365% of total counts in the absence or presence of CSC, respectively (data not shown). By using the same cell suspensions, we found that the basal levels of [3H]PEt formed (0.039% ± 0.006% of total counts) were not affected by the addition of 1 μmol/L CSC to neutrophil suspensions (0.036% ± 0.005% of total counts), whereas the amount of [3H]PEt formed in response to fMLP increased from 0.755% ± 0.107% in the absence to 1.98% ± 0.62% of total counts (n = 3) in the presence of CSC. Thus, in contrast to our observation on fMLP-stimulated neutrophils, Ado receptor occupancy does not impact on MSU crystal-induced PLD activity.
Effect of Ado receptor antagonists on fMLP-induced PLD activity.
1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were preincubated at 37°C for 5 minutes. The cell suspensions were then pretreated with 10 μmol/L CB and increasing concentrations of either CGS15943 (A) or CSC (B) and incubated in the presence or absence of 0.1 U/mL ADA for 5 minutes. Neutrophils were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.
Effect of Ado receptor antagonists on fMLP-induced PLD activity.
1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were preincubated at 37°C for 5 minutes. The cell suspensions were then pretreated with 10 μmol/L CB and increasing concentrations of either CGS15943 (A) or CSC (B) and incubated in the presence or absence of 0.1 U/mL ADA for 5 minutes. Neutrophils were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.
Ado receptor antagonists suppress the inhibitory effect of CGS21680 on fMLP-induced PLD activity
To confirm that Ado modulates PLD activity by engaging A2a receptors, we determined whether the potent A1 and A2 competitive antagonists CGS15943 and the selective A2a receptor antagonist CSC could reverse the inhibitory effects of CGS21680 (0.1 μmol/L) on fMLP-induced [3H]PEt formation. The addition of CGS15943 reversed the CGS21680-induced inhibition of fMLP-stimulated PLD activity (Figure 6A). When cells were incubated with ADA, CGS15943 was without effect on fMLP-induced [3H]PEt formation, but it abrogated significantly CGS21680-mediated inhibition of PLD activity (Figure 6A). The addition of 0.1 and 1 μmol/L CGS15943 restored the magnitude of [3H]PEt formed to levels very similar to those measured in fMLP-stimulated and ADA-treated neutrophils (79.4% ± 15.9% and 97.6% ± 5.2%, respectively). Compared with fMLP-stimulated but otherwise untreated neutrophils (no ADA), the amounts of [3H]PEt formed increased from 25.4% ± 4.4% (CGS21680 alone) to 98.8% ± 0.7%, and 129.8% ± 11.2% in the presence 0.1 and 1 μmol/L CGS15943, respectively (data not shown). Similarly, the A2a antagonist CSC reversed in a concentration-dependent manner formation (not shown) the inhibition by the A2a selective agonist (CGS21680) of fMLP-induced [3H]PEt. The data obtained with the highest concentration of CSC tested (10 μmol/L) and ADA are shown in Figure 6B. CSC reversed significantly the inhibitory effect of CGS21680, increasing the levels of [3H]PEt formed from 23.6% ± 2.3% to 67.1% ± 3.5% of fMLP-stimulated neutrophils. In the absence of ADA, CGS21680 reduced PLD activity to 44.7% ± 3.5%, compared with fMLP-stimulated cells and this inhibition was overcome by the addition of 10 μmol/L CSC to the cell suspension (131.9% ± 12.8% of control fMLP, data not shown).
The Ado receptor antagonists CGS15943 and CSC reverse the suppressive effect of CGS21680 on fMLP-stimulated PLD activity.
1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were prewarmed at 37°C for 5 minutes. (A) The cell suspensions were pretreated with 10 μmol/L CB and 0.1 U/mL ADA in the presence or absence of 0.1μmol/L CGS21680 for an additional 5 minutes. Where indicated, 0.1 and 1 μmol/L CGS15943 was also added to neutrophil suspensions 5 minutes before stimulation with 0.1 μmol/L fMLP for 10 minutes in the presence of 1% ethanol. (B) The experimental conditions were similar to A. Where indicated, 10 μmol/L CSC was added to neutrophil suspensions 5 minutes before stimulation with 0.1 μmol/L fMLP for 10 minutes in the presence of 1% ethanol. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.
The Ado receptor antagonists CGS15943 and CSC reverse the suppressive effect of CGS21680 on fMLP-stimulated PLD activity.
1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were prewarmed at 37°C for 5 minutes. (A) The cell suspensions were pretreated with 10 μmol/L CB and 0.1 U/mL ADA in the presence or absence of 0.1μmol/L CGS21680 for an additional 5 minutes. Where indicated, 0.1 and 1 μmol/L CGS15943 was also added to neutrophil suspensions 5 minutes before stimulation with 0.1 μmol/L fMLP for 10 minutes in the presence of 1% ethanol. (B) The experimental conditions were similar to A. Where indicated, 10 μmol/L CSC was added to neutrophil suspensions 5 minutes before stimulation with 0.1 μmol/L fMLP for 10 minutes in the presence of 1% ethanol. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.
Ado receptor antagonists CSC and CGS15943 reverse CGS21680-induced inhibition of fMLP-mediated translocation of PLD cofactors to membranes
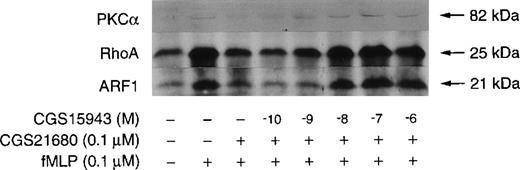
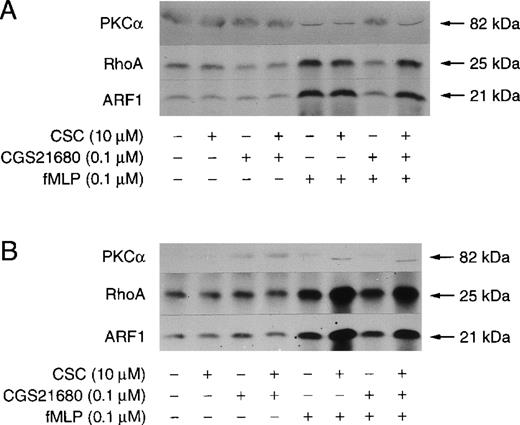
We next assessed whether the A2 antagonists also impact on the membrane translocation of ARF, RhoA, and PKCα. The effects of the addition of the A1/A2 antagonist CGS15943 before stimulation of CGS21680-treated neutrophils with fMLP are shown in Figure7. CGS15943 had no effect on the membrane levels of ARF, RhoA, and PKCα in unstimulated neutrophils as estimated by immunoblotting (data not shown). However, the addition of CGS15943 to cell suspensions enhanced the fMLP-induced recruitment to membrane of the small GTPases and PKC (data not shown), mimicking the effect of extracellular removal of Ado by ADA (Figure 2). The A2a selective agonist CGS21680 totally abolished the membrane recruitment of PKCα and decreased the amounts of membrane-associated RhoA and ARF in fMLP-stimulated neutrophils (Figure 7, lane 3). These inhibitions by CGS21680 were reversed by the antagonist CGS15943 in a concentration-dependent manner (Figure 7, lanes 4-8). The inhibitory effect of 0.1 μmol/L CGS21680 was not affected by 0.1 nmol/L CGS15943 (lane 4) but was fully reversed by a 10 nmol/L concentration of the nonselective Ado receptor antagonist (lane 6). Higher CGS15943 concentrations (0.1 and 1 μmol/L) increased the amount of membrane-associated ARF, RhoA, and PKCα above the levels detected in fMLP-stimulated but otherwise untreated neutrophils as estimated by immunoblotting (compare lanes 7 and 8 with lane 3). We next investigated the effects of the more selective A2a antagonist, CSC. As shown in Figure 8 the addition of CGS21680 and CSC alone or in combination had no effect on the basal levels of membrane-associated ARF, RhoA, and PKCα. As observed for fMLP-stimulated PLD activity, the inhibitory effects of CGS21680 on fMLP-induced ARF, RhoA, and PKCα membrane translocation were fully reversed by the addition of 10 μmol/L CSC either in the presence (Figure 8A, lane 8) or absence (Figure 8B, lane 8) of ADA, suggesting that the 2 events may be causally related. In the absence of ADA, the translocation of PLD cofactors induced by fMLP was potentiated by CSC (Figure 8B, lane 6). This stimulatory effect was abolished by the removal of extracellular Ado with ADA (Figure 8A, lane 6) indicating that Ado A2a receptor occupancy exerts suppressive effects on fMLP-induced ARF, RhoA, and PKCα membrane translocation.
Effect of CGS15943 on CGS21680-induced inhibition of fMLP-stimulated translocation of PKC, RhoA, and ARF1 to membranes.
Unlabeled neutrophils were prewarmed 5 minutes at 37°C. Then 10 μmol/L CB was added and the cell suspensions incubated for an additional 5 minutes in the absence of ADA. Where indicated, 0.1 μmol/L CGS21680 (lanes 3-8) and increasing concentrations of CGS15943 (lanes 4-8) were added to the cell suspensions. Neutrophils were stimulated with 0.1 μmol/L fMLP for 2 minutes (lanes 2-8). Incubations were stopped and neutrophil membranes were prepared as described in “Materials and Methods.” The levels of PKCα, RhoA, and ARF1 in samples were analyzed by immunoblotting. The data are from 1 experiment representative of 3 similar experiments.
Effect of CGS15943 on CGS21680-induced inhibition of fMLP-stimulated translocation of PKC, RhoA, and ARF1 to membranes.
Unlabeled neutrophils were prewarmed 5 minutes at 37°C. Then 10 μmol/L CB was added and the cell suspensions incubated for an additional 5 minutes in the absence of ADA. Where indicated, 0.1 μmol/L CGS21680 (lanes 3-8) and increasing concentrations of CGS15943 (lanes 4-8) were added to the cell suspensions. Neutrophils were stimulated with 0.1 μmol/L fMLP for 2 minutes (lanes 2-8). Incubations were stopped and neutrophil membranes were prepared as described in “Materials and Methods.” The levels of PKCα, RhoA, and ARF1 in samples were analyzed by immunoblotting. The data are from 1 experiment representative of 3 similar experiments.
Effect of CSC on CGS21680-mediated inhibition of fMLP-stimulated translocation of PKC, RhoA, and ARF1 to membranes.
Unlabeled neutrophils were prewarmed 5 minutes at 37°C. Neutrophils were then pretreated with 10 μmol/L CB in the presence (A) or absence (B) of 0.1 U/ml ADA for 5 minutes. Where indicated, 0.1 μmol/L CGS21680 (lanes 3, 4, 7, 8) and 10 μmol/L CSC (lanes 2, 4, 6, 8) were also added to the cell suspensions. Neutrophils were stimulated with 0.1 μmol/L fMLP (lanes 5-8) or DMSO (lanes 1-4) for 2 minutes. Incubations were stopped and neutrophil membranes prepared as described in “Materials and Methods.” The levels of PKCα, RhoA, and ARF1 in samples were analyzed by immunoblotting. The data are from 1 experiment representative of 3 similar experiments.
Effect of CSC on CGS21680-mediated inhibition of fMLP-stimulated translocation of PKC, RhoA, and ARF1 to membranes.
Unlabeled neutrophils were prewarmed 5 minutes at 37°C. Neutrophils were then pretreated with 10 μmol/L CB in the presence (A) or absence (B) of 0.1 U/ml ADA for 5 minutes. Where indicated, 0.1 μmol/L CGS21680 (lanes 3, 4, 7, 8) and 10 μmol/L CSC (lanes 2, 4, 6, 8) were also added to the cell suspensions. Neutrophils were stimulated with 0.1 μmol/L fMLP (lanes 5-8) or DMSO (lanes 1-4) for 2 minutes. Incubations were stopped and neutrophil membranes prepared as described in “Materials and Methods.” The levels of PKCα, RhoA, and ARF1 in samples were analyzed by immunoblotting. The data are from 1 experiment representative of 3 similar experiments.
Discussion
In this study, we demonstrated that endogenous Ado is a potent autocrine regulator of PLD activity in ligand-activated neutrophils. Ado exerts its biologic effects through the pharmacologically distinct receptor subtypes, A1, A2a, A2b, and A3.43,44 Both A1 and A2 receptors are expressed on neutrophil plasma membranes.2Several lines of evidence identify the Ado receptor that diminishes fMLP-stimulated PEt formation in neutrophils as the A2a receptor. First, NECA (nonselective) and CGS21680 (A2a selective) inhibited ligand-activated neutrophils with the same efficacy and they were 30-fold more potent than the selective A1 agonist CCPA. Moreover, inhibition of fMLP-induced PLD activity by CCPA only occurred at concentrations known to activate the A2a receptor.46 The order of agonist potencies observed, NECA = CGS21680 > CCPA is characteristic of the involvement of A2a receptors.43,47Secondly, the involvement of the A2 receptor is further supported by the ability of CGS15943, which has similar affinity for both the A1 and the A2 receptors, to fully reverse the inhibition by CGS21680 of ligand-stimulated PLD activity. Furthermore, our data strongly suggest the involvement of the A2a receptor, because the highly selective A2a antagonist CSC efficiently blocked the suppressive effect of CGS21680. In addition, the selective A1 antagonist DPCPX (see the) did not reverse the inhibitory effect of Ado or CGS21680 at concentrations up to 100 nmol/L (ie, 30 to 300 times higher than the Kd of DPCPX for the A1 receptor).1 With respect to neutrophils, pharmacologic studies with Ado receptor agonists and antagonists support the involvement of A2a receptors in the inhibition of neutrophil functional responses.12,41 The expression of A2a receptors on neutrophils was supported by reverse transcriptase polymerase chain reaction detection of its mRNA.11
The mechanism by which Ado suppresses neutrophil functional responses remains to be defined. Although the occupancy of A2 receptors has been suggested to uncouple chemoattractant receptors from their downstream signal transduction pathways,48 Ado does not interfere with the generation of inositol 1,4,5-triphosphate by phospholipase C,38 the mobilization of intracellular Ca++, or the early transient in diacylglycerol formation induced by fMLP.16,37 Ado has however been shown to inhibit the influx of Ca++ induced by fMLP,39 and to diminish the late and sustained increase in diacylglycerol that follows fMLP stimulation.16 We previously attributed this late and sustained increase in diacylglycerol formation to the sequential actions of a phosphatidylcholine-specific PLD and of lipid phosphohydrolases.40 These observations led us to hypothesize that Ado might exert a negative regulation of PLD activity in ligand-activated neutrophils. Accordingly, we found that the addition of Ado receptor agonists to neutrophil suspensions diminished fMLP-induced PLD activity. It is recognized that Ado accumulates in neutrophil suspensions in a time- and cell concentration-dependent manner.41 Measurements of Ado in neutrophil suspensions clearly indicate that its extracellular concentration rapidly increases after only a few minutes of incubation up to 30 to 120 nmol/L. Given the reported IC50 (50-100 nmol/L) for the inhibition of neutrophil adherence to endothelial cells,5 of the respiratory burst,9,11,49 and of leukotriene synthesis,8 such concentrations are likely to alter neutrophil functions, including PLD activation. Accordingly, the addition of ADA was sufficient to suppress the accumulation of Ado in the extracellular milieu and to reverse the inhibitory effect of endogenous Ado on stimulated PLD activity. The concentration of Ado in ADA-treated cell suspensions (< 20 nmol/L) is very similar to the levels reported in plasma or in blood.41,50 Ado uptake by red blood cells contributes to maintain the plasma Ado concentration at low levels but pharmacologic agents such as the inhibitor of ADA deoxycorformycin,50 the inhibitor of adenosine kinase GP515,51 or the inhibitor of Ado uptake dipyridamole41 have been reported to increase its concentration in the micromolar range. Several studies have established that GP515,52 methotrexate, and sulfasalazine53share the capacity to increase Ado release at sites of inflammation. Furthermore, these studies showed that increased Ado concentration at inflammatory sites suppresses inflammation by diminishing leukocyte accumulation in the air pouch model. These anti-inflammatory effects could be completely reversed by the addition of ADA or inhibition of Ado action by receptor antagonists.13,15 53 Similarly, we observed that endogenous Ado exerts a negative constraint on the PLD activation pathway that could be nullified by ADA or the receptor antagonists CSC and CGS15943. Thus, endogenous Ado suppresses fMLP-induced PLD activation and, at least in vitro, decreases the ability of neutrophils to respond to agonists.
It is well established that A2a receptors are linked to Gαs.54 Like other Gαs-linked receptors, Ado receptors on neutrophils stimulate an increase in the intracellular levels of cAMP.16,37,48 Although this was not investigated in this study, it is tempting to speculate that the suppression of fMLP-induced PLD activation by Ado is related to changes in the intracellular concentrations of cAMP that follow the occupancy of A2a receptors. Indeed, enhanced intracellular cAMP accumulation as the result of ligand-induced activation, or treatment of neutrophil suspensions with cell membrane permeable cAMP analogues have previously been shown to reduce fMLP-stimulated PLD activity.55,56 Interestingly, elevation of intracellular cAMP levels has been shown to inhibit chemoattractant-stimulated RhoA activation,57 a small GTPase that regulates human neutrophil PLD.58 The hypothesis that inhibition of fMLP-stimulated PLD activity by Ado is mediated by cAMP accumulation and activation of protein kinase A will be the subject of future investigations.
Ado does not affect Ca++ mobilization from intracellular stores37 but diminishes Ca++ influx stimulated by fMLP.39 Because the calcium ionophore A23 187 increased PLD activation by increasing Ca++ entry and intracellular Ca++ concentration, it seems possible that the decreased Ca++ influx and the reduction in the concentration of cytosolic Ca++ that follow the occupancy of A2a receptors impact on fMLP-stimulated PLD activity. In accord with such a role for calcium, the addition of EGTA in the incubation media (to prevent Ca++ influx) or the buffering of the elevation of cytosolic Ca++ with the intracellular calcium chelator BAPTA have been shown to reduce fMLP-stimulated PLD activity.59,60However, the putative effect of Ca++ entry on PLD activation in intact cells is likely to be indirect, because in vitro Ca++ itself has no impact on PLD activation by ARF, RhoA, and PKCα.31
Two mammalian PLDs, PLD1,23 and PLD224 have recently been cloned. Two spliced isoforms of PLD1 named PLD1a and PLD1b exist.31 The levels of expression of PLD1a and PLD1b proteins are cell-type and tissue-specific.61,62 We reported using immunoblot analyses that PLD1a but not PLD1b could be detected in human neutrophils.35 Both PLD1 and PLD2 require PIP2 for activity.24,25 PLD1 is activated by ARF, RhoA, and PKCα in vitro.58 In contrast, PLD2 shows high basal activity and is not activated by the same cofactors. Neutrophil PLD is activated by RhoA, ARF, and PKCα.17 These cytosolic proteins are recruited to membranes of fMLP-stimulated cells and there is strong evidence suggesting that they mediate fMLP-induced PLD activity in human granulocytes.33 36 Our results demonstrate that the removal of extracellular Ado using ADA significantly increased the levels of RhoA, ARF, and PKCα recovered in the membrane fractions obtained from ligand-activated human neutrophils. These were the first indications that endogenous Ado may suppress fMLP-stimulated PLD activity by inhibiting the recruitment of RhoA, ARF, and PKCα to membranes. This hypothesis is further supported by the experiments showing that blockade of Ado action by the antagonists CSC or CGS15943 produced very similar increases in the levels of small GTPases and PKCα associated with membranes. As shown for stimulated PLD activity, ADA and A2 receptor antagonists have no additive effect on the magnitude of translocation of these PLD cofactors. In contrast, we found that the selective A2a agonist CGS21680 reduced fMLP-stimulated translocation of ARF, RhoA, and PKCα. This suppressive effect of Ado on PLD cofactors recruitment to neutrophil membranes in ligand-activated human neutrophil was also characteristic of the involvement of A2 receptor. This is demonstrated by the ability of CGS15943 to reverse in a concentration-dependent manner the suppression by GGS21680 of fMLP-induced membrane translocation of PLD cofactors. Furthermore, the Ado receptor involved is likely to be the A2a subtype because the selective A2a antagonist CSC efficiently abolished the inhibition by CGS21680 of ARF, RhoA, and PKCα translocation elicited by fMLP. Taken together, the results strongly suggest that the engagement of A2a receptor inhibits PLD activation by limiting the recruitment of ARF, RhoA, and PKCα to membranes of fMLP-stimulated neutrophils. Interestingly, the magnitude of the inhibitory effects of Ado and Ado analogues on PLD activity correlated with the inhibition of PLD1 cofactor association to membranes, supporting a causal link.
Our studies demonstrate a unique role for Ado in inhibiting the PLD signaling cascade in fMLP-activated human neutrophils. To our knowledge, the PLD signaling pathway is the only one reported to be inhibited by Ado. The ability of the A2a receptor to suppress PLD activity is therefore of pathophysiologic interest. Many studies have shown that the occupancy of the A2 receptor suppresses several neutrophil functional responses,4 including actin polymerization,63 adherence to endothelium,5expression of CD11b/CD18,6,7 secretion,51leukotriene synthesis,8,41 and fMLP-stimulated respiratory burst.3,9-12 Inhibition of PLD is likely to reduce the generation of PLD-derived phosphatidic acid (and lysophosphatidic acid and diacylglycerol) and the functional responses associated with the generation of these intracellular mediators. There is increasing evidence suggesting that PLD-derived second messengers regulate particle phagocytosis,22 the expression of β2 integrins,21 secretion,20 and the respiratory burst.64 The suppression of neutrophil functions by Ado is therefore compatible with an inhibition of the PLD1 isoform by occupancy of A2a receptors. Moreover, the suppressive effect of Ado and Ado analogues on fMLP-stimulated PLD activity is agonist specific and cannot be generalized because PLD activation induced by MSU crystals was not inhibited; this observation suggests that the PLD isoform activated by MSU crystals is unlikely to be the PLD1 isoform.35
In summary, this study demonstrates the ability of endogenous Ado and Ado analogues to inhibit the activation of PLD in neutrophil suspensions through engagement of A2a receptors. Our results indicate that the inhibition of the fMLP-induced PLD activity is functionally linked to inhibition of fMLP-stimulated recruitment of ARF, RhoA, and PKCα to membranes. Taken together, these results indicate that Ado exerts suppressive effects on human neutrophil functions by uncoupling chemoattractant receptors from PLD1 activation.
Acknowledgments
We thank Dr David Brindley for valuable discussions and critical reading of the manuscript. In addition, we thank Serge Picard for expert technical assistance.
The addition of 0.1 and 1 μmol/L DPCPX to cell suspensions increased the amount of PEt formed in response to fMLP by 1.73- ± 0.12-fold and 1.98- ± 0.27-fold, respectively. The inhibition by CGS21680 of fMLP-induced PLD activity was reversed by DPCPX. The amounts of PEt formed increased from 34% ± 9.2% to 37.5% ± 12.9%, 53.2% ± 16.5%, and 77.2% ± 25% of control fMLP in the presence of 0.1, 1, and 10 μmol/L DPCPX, respectively. DPCPX (10 μmol/L) was able to reverse the inhibitory effect of CGS21680 on fMLP-stimulated membrane translocation of ARF, RhoA, and PKCα, an effect characteristic of the involvement of A2 receptor.1
Supported by a Senior Scholarship from the Arthritis Society of Canada and grants from the Medical Research Council of Canada (MT-14 790) and the Arthritis Society. Nathalie Thibault is the recipient of a studentship from the “Fonds pour la Formation de Chercheurs et l'Aide à la Recherche”.
Reprints:Centre de recherche en Rhumatologie et Immunologie, C.H.U.Q., Pavillon C.H.U.L, 2705 Blvd Laurier, Sainte-Foy, Québec, G1V 4G2, Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Effect of ADA on fMLP- and MSU-crystals–induced PLD activity. / Neutrophils labeled with 1-O-[3H]alkyl-2-lysophosphatidylcholine were prewarmed at 37°C for 5 minutes. (A) The cell suspensions were pretreated with 10 μmol/L CB and incubated for an additional 5 minutes in the absence or presence of 0.1 U/mL ADA. Cells were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into PEt was measured as described in “Materials and Methods.” The levels of [3H]PEt formed are expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 6 separate experiments. (B) Cell suspensions were stimulated with 3 mg/mL MSU crystals in the presence of 1% ethanol for 15 minutes, without CB. The data are the means ± SEM of 4 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.519/6/m_bloo00203001x.jpeg?Expires=1763667911&Signature=ZaRf88nso83Mf89DUx357vid8rCLnwEqfuY83s7~sCL3x8BUFKZHEGQch-LmuZYMiZ1Yv~zLZewF81xmbuvItPiiiasKaDDzsKDhoHUCQGb3D8l-QVUKn4vc1r9ojVpztcRngJErb2KiD6XJrtx9PN8dBy6uaUMgKT4dlp5f30hGQ4IQIyaucxbRlGu1y27kFghLjzyGI~6wlBIKdG-0ccU96ZsL7p9wcLoUSpMvTcCiMBYRgKq7QotZ5UEwyFyaKnsYHQdb58jc4oRxhiVkPq-T-JUGyg4ae3HK6lph9WOXuJhg6IpedsMOsHIKoH-Tv~Ckf1ywtMb2htZRKADePg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of Ado receptor agonists on fMLP-induced PLD activity. / 1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were preincubated at 37°C for 5 minutes. The cell suspensions were then pretreated with 10 μmol/L CB, 0.1 U/ml ADA, and increasing concentrations of CGS21680, NECA, or CCPA for an additional 5 minutes. Neutrophils were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.519/6/m_bloo00203004x.jpeg?Expires=1763667911&Signature=42O40thZYUtzwGf2CrkSU-f5RvQ7RDBcMNRPVjQrZq3MBN8-iuQBUYDMSQHZ3gGVXNf~e~ynO-WcjS5rZjqGxPy6DIRvDZhzMU3k0FvhGDmKKmgVnJyuupOSiUHOK50nquOvlwEVb43DR9ZTGQKJVpZcs~50iipy~RAGN5TtCqbUvuhadvORiaUD6Y~a4ShHpNUHGe9TkkWRNDl5H1CJz5FimWvIAOKlfYjqGZXKwiw6dyZiCEIXM-Fugg3hnMDrn66TLHnsApXNdKcEbbF06rLwf~pNnq7R-LhtgApxNyh6Oro7pagK2sQCdK5Pj1-u1sc9l2S6IHUzYynlFp8jLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effect of Ado receptor antagonists on fMLP-induced PLD activity. / 1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were preincubated at 37°C for 5 minutes. The cell suspensions were then pretreated with 10 μmol/L CB and increasing concentrations of either CGS15943 (A) or CSC (B) and incubated in the presence or absence of 0.1 U/mL ADA for 5 minutes. Neutrophils were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.519/6/m_bloo00203005ax.jpeg?Expires=1763667911&Signature=VO2hN5YeXGruUKQZAoZNxM8btQJpFYkGeKyg0qCUr2n5xZAN9o8gyA3XK8-8SG6zmQF7lLNJSixMA~-~82Gxm2cAdWNq2rPiYK3eR0MuoG-5TTxTTU~lMDjCgJk~TFAc1q9bWS7teAJRF-BD8Ji1XzaW3w9g0uy-7M0lncQiMX7Z-c6YU5nLN0tBXnSL1h7i~Xjzt7wN8ytd6Mz3dnUcztNOKlwJNZji~5zdYvYi~5mE5kx~u04WAntWHQ24qXKCIPeNdMa0jtExmkbOZwbT~6XfRCLp1wrxEDE6XnGgTYiGSKuVjCUKAbBCOv41uXqlScGVwTEVku0gsYA5dp-D1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effect of Ado receptor antagonists on fMLP-induced PLD activity. / 1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were preincubated at 37°C for 5 minutes. The cell suspensions were then pretreated with 10 μmol/L CB and increasing concentrations of either CGS15943 (A) or CSC (B) and incubated in the presence or absence of 0.1 U/mL ADA for 5 minutes. Neutrophils were stimulated with 0.1 μmol/L fMLP in the presence of 1% ethanol for 10 minutes. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.519/6/m_bloo00203005bx.jpeg?Expires=1763667911&Signature=XI~bfo9AA9FpuzOz0LU2TrnIu-863D1yETZRPLpaOXk0xlKY5HhTnsM0EvM7NghTEt48llZGMbwjNBERRApJpxGLkodT5tJ8JSwqpNU00DnjZDSF~ncw3h2HbAygdEcf~z2uNkzrCBRCUCjJppJSPnXZCobecvoqBtBJTuO9QXh8FyP9RQ~RtVr6I7dMlkyPde8CvnPzml6ETdriMZVYXysNixXlLHmJuPzDZa0uO95OjMm0cJvWhGo-0PgBN52Q~EW-9IkCmwfQ8-pWl5HIpjATc9jBk9g~ozfFgNdZNutZnlto-3oPpZ-3-CISiRFosp38~L6GmWbWQ5Cz5K6xjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. The Ado receptor antagonists CGS15943 and CSC reverse the suppressive effect of CGS21680 on fMLP-stimulated PLD activity. / 1-O-[3H]alkyl-2-lysophosphatidylcholine-labeled neutrophils were prewarmed at 37°C for 5 minutes. (A) The cell suspensions were pretreated with 10 μmol/L CB and 0.1 U/mL ADA in the presence or absence of 0.1μmol/L CGS21680 for an additional 5 minutes. Where indicated, 0.1 and 1 μmol/L CGS15943 was also added to neutrophil suspensions 5 minutes before stimulation with 0.1 μmol/L fMLP for 10 minutes in the presence of 1% ethanol. (B) The experimental conditions were similar to A. Where indicated, 10 μmol/L CSC was added to neutrophil suspensions 5 minutes before stimulation with 0.1 μmol/L fMLP for 10 minutes in the presence of 1% ethanol. The amount of radioactivity incorporated into [3H]PEt was assessed as described in “Materials and Methods” and is expressed as the percentage of the radioactivity in the total lipid extracts. The data are the means ± SEM of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.519/6/m_bloo00203006x.jpeg?Expires=1763667911&Signature=b7SLcaTMBY~19kQSLlYY61iepKtNlYHMvMUJI4p8KE2iwllzDyg-1FjYho1M4ZaudXAKor6gcLM~rJOq7uLkuvbJI~Bodtq~VDeB~gez3Fkdt2LxcGEVWi-9eto0r5m3QPaQ3jQqD2kvmtTLOt~fjCZorPMureKlfKR37VELRlpIVoeQ8WwaYjmsUzx9WVq7jtDLjSHxDDat0C6h6ga1Uf~HFv7Ffi5P0R58XzYE01LMovVH43LYokEyoG0c2r2AljbgnOw5qKF9rUm7J-zHhNJyqiFgpziyJyicmFudDzTprk5ISflsEwsJF62VxuKP6wgbWdbFNhxYsZOaEE4HCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal