Abstract
The late stages of megakaryocytopoiesis, consisting of the terminal processes of cytoplasmic maturation and platelet shedding, remain poorly understood. A simple liquid culture system using CD34+ cells in serum-free medium has been developed to study the regulation of platelet production in vitro. Platelets produced in vitro were enumerated by flow cytometry. A truncated form of human Mpl-Ligand conjugated to polyethylene glycol (PEG-rHuMGDF) played a crucial role in both proplatelet formation and platelet production. A combination of stem cell factor (SCF), interleukin-3 (IL-3), and IL-6 was as potent as PEG-rHuMGDF for the growth of megakaryocytes (MKs). However, the number of proplatelet-displaying MKs and platelets was increased 10-fold when PEG-rHuMGDF was used. Peripheral blood mobilized CD34+ cells gave rise to a threefold augmentation of platelets compared with marrow CD34+ cells. This finding was related to the higher proliferative capacity of the former population because the proportion of proplatelet-displaying MKs was similar for both types of CD34+ cells. The production of platelets per MK from CD34+ cells was low, perhaps because of the low ploidy of the cultured MKs. This defect in polyploidization correlated with the degree of proliferation of MK progenitors induced by cytokines. In contrast, ploidy development closer to that observed in marrow MKs was observed in MKs derived from the low proliferative CD34+CD41+ progenitors and was associated with a twofold to threefold increment in platelet production per MK. As shown using this CD34+ CD41+ cell population, PEG-rHuMGDF was required throughout the culture period to potently promote platelet production, but was not involved directly in the process of platelet shedding. IL-3, SCF, and IL-6 alone had a very weak effect on proplatelet formation and platelet shedding. Surprisingly, when used in combination, these cytokines elicited a degree of platelet production which was decreased only 2.4-fold in comparison with PEG-rHuMGDF. This suggests that proplatelet formation may be inhibited by non-MK cells which contaminate the cultures when the entire CD34+ cell population is used. Cultured platelets derived from PEG-rHuMGDF– or cytokine combination-stimulated cultures had similar ultrastructural features and a nearly similar response to activation by thrombin. The data show that this culture system may be useful to study the effects of cytokines and the role of polyploidization on platelet production and function.
IN CONTRAST TO the early stages of megakaryocyte (MK) differentiation, the ultimate step of megakaryocytopoiesis, that of platelet shedding, is incompletely understood. Most investigators consider that platelet shedding, both in vitro and in vivo, requires the formation of long cytoplasmic extensions termed proplatelets.1-5 Another hypothesis concerning the mechanism of platelet release proposes that MK demarcation membranes delineate platelet territories and that future platelets are released subsequent to membrane fragmentation.6,7 The late stages of megakaryocytopoiesis have remained poorly understood because of the difficulty of acquiring large populations of MKs capable of shedding platelets in vitro. Culture systems permitting all stages of megakaryocytopoiesis might be particularly valuable for understanding platelet formation and its regulation by cytokines. Several culture systems have been designed to study the late stages of thrombocytopoiesis. In mice, guinea pigs and humans, MKs have been purified and cultured on a short-term basis to study proplatelet formation.8-10 In the rat, MK progenitors have been purified based on the expression of glycoprotein (GP) IIb/IIIa and proplatelet formation was analyzed after 2 to 3 days of culture in the presence of cytokines.8,10-12 Recently, Choi et al13 14 described an in vitro system in which platelet-sized fragments that are morphologically and functionally similar to blood-derived platelets were generated from human MKs derived from CD34+ cells cultured in liquid medium.
The role of cytokines in proplatelet formation and platelet shedding is also poorly understood. It has been suggested that MPl-Ligand (Mpl-L), also called thrombopoietin or megakaryocyte growth and development factor (MGDF, a nonglycosylated truncated form of Mpl-L),15-17 which plays a crucial role in MK maturation, is not directly involved in the process of platelet shedding and at high concentrations might even inhibit proplatelet formation.18,19 Among the other cytokines, it has been shown in rodents that IL-6, IL-11, and erythropoietin can induce proplatelet formation.8,20,21 The effect of IL-6 on proplatelet formation in humans is controversial.18 19Unlike Mpl-L, the other cytokines such as IL-6 are regulators of platelet production in stress conditions such as inflammation.
In this report we describe the effects of culture conditions and several cytokines on MK proplatelet formation, together with the ultrastructural and functional characteristics of the platelet-like particles produced in vitro.
MATERIALS AND METHODS
Blood and Bone Marrow (BM) Cells
Blood CD34+ cells were isolated from leukapheresis samples performed on patients undergoing autologous peripheral blood stem cell transplantation. BM cells were obtained from normal adult donors undergoing hip surgery. Informed consent was obtained from all donors.
BM cells were collected by vigorous shaking of bone fragments in Iscove's modified Dulbecco medium (IMDM; GIBCO, Paisley, UK) supplemented with 100 ng/mL of DNAse (Sigma, St Louis, MO). Blood cells were diluted to a concentration of ∼50 × 103/μL in a phosphate-buffered saline (PBS; GIBCO) solution containing 5 ng/mL EDTA (Sigma). Mononuclear cells were separated on a Ficoll gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway). Low-density cells (<1.077 g/cm3) were recovered, washed, and then used for isolation of CD34+ cells.
Antibodies
Directly conjugated monoclonal antibodies (MoAbs) R-phycoerythrin-(PE)-HPCA2 (anti-CD34) and fluorescein isothiocyanate (FITC) anti-CD41a (Becton Dickinson, Mountain View, CA; and Pharmingen, San Diego, CA; respectively) were used for cell sorting. FITC-TAB (anti-CD41b; TAB provided by Dr R. McEver, Oklahoma Medical Research Foundation), R-PE anti-CD41a MoAb (Pharmingen), and an R-PE anti-CD62 MoAb (anti–P-selectin; Becton Dickinson) were used for analysis of MKs and platelets by flow cytometry. FITC- and R-PE–conjugated immunoglobulin (Ig) G1 MoAb controls were obtained from Becton Dickinson.
Isolation of CD34+ Cells
Mononuclear cells were separated using a magnetic cell sorting system (mini MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), in accordance with the manufacturer's recommendations. The purity of CD34+ cells recovered was determined by flow cytometry using PE-HPCA2 and was greater than 80%.
Cell Sorting
BM CD34+ cells were treated with neuraminidase to prevent binding of platelets.22 Cells (1 × 106) were incubated in 100 μL PBS and 0.2% bovine serum albumin (BSA) with 0.2 U/mL neuraminidase for 1 hour at 37°. Cells were washed and incubated with R-PE-HPCA2 (20 μL) and FITC anti-CD41a (20 μL) in 100 μL volume at 4°C for 45 minutes. After one wash, cells were suspended in IMDM at a concentration of 5 × 105cells/mL and separated by cell sorting. Cells were sorted on a FACS Vantage flow cytometer (Becton Dickinson). A morphologic gate including 80% of the events and all the CD34+ cells was determined on two-parameter histograms (side scatter [SSC] versus forward scatter [FSC]). Compensation for two-color labeled samples was set up with singly stained samples. Positivity or negativity for the CD41 antigen among the CD34+ cells was determined using control cells labeled with PE-HPCA2 and an irrelevant IgG1 MoAb. Cells were sorted into a CD34+ CD41− (∼96% of the CD34+ cells) and a CD34+ CD41+ cell fraction (∼2% of the CD34+ cells).
Human Cytokines and Cytokine Receptors
Recombinant human (rHu)IL-3 (a gift from Immunex, Seattle, WA) and rHuIL-6 were both used at a final concentration of 100 U/mL (3 and 5 ng/mL, respectively). Recombinant human stem cell factor (rHuSCF) and polyethylene glycol (PEG)-rHuMGDF (gifts of Amgen Corp, Thousand Oaks, CA) were usually used at a final concentration of 50 ng/mL and 10 ng/mL, respectively. CD34+ CD41+ cells were cultured with increasing concentrations of PEG-rHuMGDF ranging from 0.01 to 500 ng/mL. In some experiments, a murine soluble Mpl receptor (gifts of ZymoGenetics, Seattle, WA) and full length rHuMpl-L (TPO; Genzyme, Cambridge, MA) were added at a concentration of 5 to 10 μg/mL and 10 ng/mL, respectively.
Cell Cultures
CD34+ cells were cultured in IMDM with penicillin/streptomycin/glutamine and 11.5 μmol/L α-thioglycerol (Sigma). Cultures were usually performed in serum-free conditions in which IMDM was supplemented with 1.5% BSA (Cohn's fraction V; Sigma), sonicated lipids, and iron-saturated human transferrin.23,24 Two types of serum-containing cultures were used as controls. In the first, IMDM was supplemented with 1% BSA and 10% normal human AB serum, and in the second with 1% BSA and 10% normal human serum derived from platelet-poor plasma (PPP).24 In some experiments, heparin (3 U/mL, Fragmine; Pharmacia, Orsay, France) or an anti–TGF-β antibody (R&D Systems, Minneapolis, MN) were added to the medium. CD34+ cells were cultured in 6- or 24-well tissue culture plates, in 3 or 1 mL volumes, respectively. CD34+CD41+ cells were grown in 96-well plates in a 100 μL volume. Cultures were incubated at 37°C in a fully-humidified atmosphere containing 5% CO2 in air. Two O2concentrations (21% or 7%) were compared. The cultures were examined with an inverted microscope at 40× and 100× magnification.
Determination of MKs and Platelet Numbers Produced in Culture
The total numbers of MKs were determined by flow cytometry or cytology. Platelet numbers were determined by flow cytometry. Flow cytometry quantification was performed after FITC-TAB (anti-CD41) or R-PE anti-CD41a MoAb labeling. After collecting and rinsing with PBS/EDTA, cultured cells were centrifuged at 350g for 15 minutes, incubated with the MoAb for 30 minutes, and fixed with 0.5% paraformaldehyde (Serva, Heidelberg, Germany) for 20 minutes. Cells from each culture condition were distributed in the same volume (400 μL). For each sample, the acquisition rate was 1 μL/second for 100 seconds. For quantitation of MKs, a linear scale was used for FCS and SSC. MKs were defined as brightly positive CD41 cells with scatter properties of nucleated cells. For quantitation of platelets, events were collected without gating using a log scale for FSC and SSC. An analytical gate was determined based on scatter properties of normal blood platelets treated similarly. This gate excluded large contaminating cells (MKs) and small debris or microparticles. Culture-derived platelets were enumerated as CD41+ events with the same scatter properties as blood platelets. Samples were analyzed with a FACSort flow cytometer (Becton Dickinson).
Proplatelet-displaying MKs were defined as cells exhibiting one or more cytoplasmic processes with areas of constriction. The percentage of MKs with such processes was quantitated with a hemocytometer at various times during the culture period depending on the starting cell population (blood or marrow CD34+ cells or marrow CD34+ CD41+ cells).
Determination of MK Ploidy
MK ploidy was measured by a double-staining technique and flow cytometry.25 Cultured cells were counted, fixed with 0.5% paraformaldehyde for 20 minutes at 4°C, and then washed in calcium and magnesium-free PBS. Cells were kept at 4°C until analysis (<2 days). MKs were identified after labeling with FITC-TAB, while DNA staining was performed by incubating the cells in a solution of propidium iodide (50 μg/mL in isotonic NaCl containing 100 μg/mL RNAse [Merck, Darmstadt, Germany] and 0.1% Tween 20 [Sigma]) for 1 hour. Control cells were stained with an irrelevant FITC-IgG1 MoAb and propidium iodide. The flow rate used was 500 to 1,000 cells/s. MKs were identified by the expression of CD41b. The ploidy distribution was determined by setting markers at the nadirs between peaks, whereas the frequency of MKs in different ploidy classes was evaluated on 5,000 cells.
Detection of the Activation-Dependent Antigen P-Selectin (CD62) on Platelets
Cultured cells were stimulated for 10 minutes at 37°C with 2 U/mL thrombin (Stago) added directly to the culture well. Activated and nonactivated platelets were incubated for 30 minutes with both R-PE-anti-CD62 (2 μg/mL) and FITC-TAB. The cells were then fixed for 1 hour with an equal volume of 0.5% paraformaldehyde. Control cells were fixed in the same manner without prior activation. Cells were subsequently resuspended in PBS.
Activation of normal plasma-derived platelets was performed in parallel; blood platelets were purified by gel filtration on Sepharose 2B in a buffer (containing NaCl, 129 mmol/L; Na3 citrate, 13.6 mmol/L; glucose, 11.1 mmol/L; KH2PO4, 1.6 mmol/L; and NaH2PO4, 8.6 mmol/L, pH 7.3) and then treated as above. Activated and nonactivated blood and culture-derived platelets were analyzed by flow cytometry.
Ultrastructural Studies
Cultured cells were examined by electron microscopy. The cells were fixed with 1.25% glutaraldehyde in 0.1 mmol/L phosphate buffer for 1 hour at 22°C washed, postfixed with osmium tetroxide, dehydrated, and embedded in Epon (TAAB, Aldermaston, Berkshire, UK). Thin sections were examined with a Philips CM 10 electron microscope (Philips, Eindoven, The Netherlands) after lead citrate staining.
Statistical Analysis
The numbers of MKs, of MKs bearing proplatelets, and of platelets were expressed as the mean ± 1 SD. In each experiment, cultures were performed in parallel in different conditions (medium or cytokines). Results of these different conditions were compared with those obtained in serum-free medium or with PEG-rHuMGDF–stimulated cultures, respectively. Statistical analysis was thus performed using a pairedt-test.
RESULTS
Optimization of Culture Conditions
Various approaches to improve proplatelet formation and platelet production were evaluated. Reproducible proplatelet formation required a relatively pure population of CD34+ cells. This was attainable using an immunobead selection technique with which a purity of greater than 85% CD34+ cells was routinely observed. As free oxygen radicals produce toxicity on maturing cells in many culture systems,26-28 the effects of low O2concentration (7%) on platelet production were compared with ambient O2 concentrations (21%) in six experiments. The average number of proplatelet-bearing MKs and platelets observed was not significantly different at these two O2 concentrations but was always higher at 7%. In these experiments, we did not remove the antioxidant (α-thioglycerol) which may have minimized differences.28 Finally, initial densities of 5 × 104 cells/mL ensured satisfactory MK proliferation, with only one refeeding performed during the 14- to 16-day culture period. Based on these preliminary data, all subsequent cultures were established using purified populations of CD34+ cells at an initial density of 5 × 104 cells/mL cultured at 7% O2.
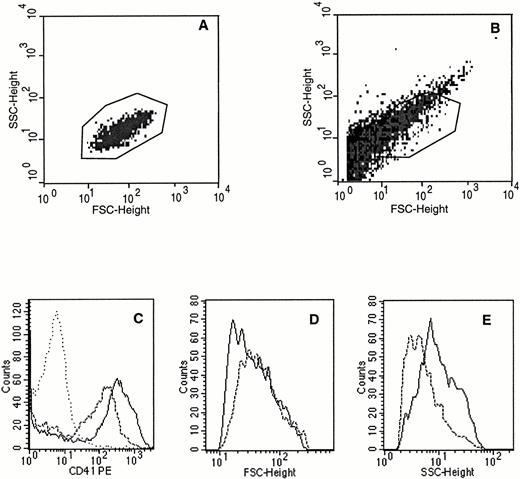
Platelets produced in culture were enumerated by flow cytometry as particles having the same scatter properties as blood platelets and expressing a high level of CD41 (Fig 1A, B, and C). This definition seems the more appropriate to define and quantitate platelets produced in vitro. In addition, as previously shown,29 there are numerous other CD41+ elements with much lower forward scatter which may correspond to platelet microparticules or cell fragments (Fig 1B).
Flow cytometric characteristics of platelets produced in culture. Culture-produced platelets were defined as CD41+elements with the same scatter properties as blood platelets. (A) Scatter properties of blood platelets after FITC-TAB (anti-CD41b) labeling. (B) Scatter properties of culture platelets labeled in the same conditions and the gate chosen for the analysis. Black points are CD41− elements, grey points CD41+ elements. (C) CD41 expression of platelets produced in culture before (---) and after (—) thrombin activation. The isotype control (....) is shown. (D) Forward scatter height of platelets produced in culture before (---) and after (—) thrombin activation. (E) Side scatter height of platelets produced in culture before (---) and after (—) thrombin activation.
Flow cytometric characteristics of platelets produced in culture. Culture-produced platelets were defined as CD41+elements with the same scatter properties as blood platelets. (A) Scatter properties of blood platelets after FITC-TAB (anti-CD41b) labeling. (B) Scatter properties of culture platelets labeled in the same conditions and the gate chosen for the analysis. Black points are CD41− elements, grey points CD41+ elements. (C) CD41 expression of platelets produced in culture before (---) and after (—) thrombin activation. The isotype control (....) is shown. (D) Forward scatter height of platelets produced in culture before (---) and after (—) thrombin activation. (E) Side scatter height of platelets produced in culture before (---) and after (—) thrombin activation.
Kinetics of MK growth, proplatelet formation, and platelet production (Fig 2) were first determined. A plateau in MK growth was observed between day 9 and day 14 for marrow CD34+ cells. This plateau was delayed for MK derived from blood CD34+ cells and was reached at day 11 to day 12 (Fig2A). Proplatelet formations were observed from day 6 to day 14 for marrow-derived MKs whereas they were detected from day 12 to day 14 for blood-derived MKs (Fig 2B). Platelet production paralleled proplatelet formation and was maximum at day 14 for blood CD34+cell-derived cultures (Fig 2C). The vast majority of cells began to lyze after day 14. Therefore, proplatelet formation and platelet production were studied between day 6 and day 12 for marrow CD34+ cell-derived cultures and at day 13 or day 14 for those derived from blood CD34+ cells.
Kinetics of MK growth (A), proplatelet formation (B), and platelet production (C) from marrow or blood CD34+ cells. CD34+ cells from marrow (◊) and blood CD34+ cells (□) were grown in serum-free conditions in the presence of PEG-rHuMGDF (10 ng/mL). MKs were enumerated as CD41+ cells by flow cytometry at different days of culture. In this and all subsequent figures, the number of MKs bearing proplatelets was determined using an inverted microscope and hemocytometer as MKs showing one or more cytoplasmic expansions with constriction areas. In this and all subsequent figures, culture platelets were enumerated by flow cytometry as CD41+events with the same scatter properties as blood platelets shown in Fig1. For the kinetics of MK growth and proplatelet formation, results are the average of three experiments. For determination of platelet production, the entire kinetics was only performed in one experiment from a blood CD34+ cell-derived culture.
Kinetics of MK growth (A), proplatelet formation (B), and platelet production (C) from marrow or blood CD34+ cells. CD34+ cells from marrow (◊) and blood CD34+ cells (□) were grown in serum-free conditions in the presence of PEG-rHuMGDF (10 ng/mL). MKs were enumerated as CD41+ cells by flow cytometry at different days of culture. In this and all subsequent figures, the number of MKs bearing proplatelets was determined using an inverted microscope and hemocytometer as MKs showing one or more cytoplasmic expansions with constriction areas. In this and all subsequent figures, culture platelets were enumerated by flow cytometry as CD41+events with the same scatter properties as blood platelets shown in Fig1. For the kinetics of MK growth and proplatelet formation, results are the average of three experiments. For determination of platelet production, the entire kinetics was only performed in one experiment from a blood CD34+ cell-derived culture.
The effects of the serum on platelet production were evaluated. Six experiments comparing serum-free medium and medium supplemented with 10% serum or PPP were performed. Blood- and marrow-derived CD34+ cells (n = 3 for each) were cultured with 10 ng/mL PEG-rHuMGDF. MK growth was twofold to threefold higher in serum-free medium compared with serum and PPP (Fig3A). The percentage of MKs displaying proplatelet was similar in the different conditions, ranging from 42% to 51%. Therefore, the differences in the number of proplatelet-displaying MKs paralleled those of MK growth (4.6 × 103/103 CD34+ cells in cultures without serum compared with 2 × 103/103 CD34+ cells or 1.1 × 103/103 CD34+ cells;P = .04, in cultures containing serum or PPP; Fig 3B). In these experiments, platelet number was 50-fold the number of proplatelet-bearing MKs (Fig 3C).
Effects of culture conditions on MK growth (A), proplatelet formation (B), and platelet production (C). CD34+ cells from marrow (n = 3, ▧) or blood (n = 3, ▧) were grown during 14 days in the presence of PEG-rHuMGDF (10 ng/mL) in serum-free conditions, in serum derived from PPP, or normal serum in the absence or presence of heparin (H) or an anti–TGF-β antibody. MKs and platelet bearing MKs were enumerated using an inverted microscope and hemocytometer. The results represent the mean ± SD of three independent experiments, and are expressed per 1 × 103 plated CD34+ cells. Asterisks denote a significative difference (P < .05) in comparison with culture in serum-free conditions.
Effects of culture conditions on MK growth (A), proplatelet formation (B), and platelet production (C). CD34+ cells from marrow (n = 3, ▧) or blood (n = 3, ▧) were grown during 14 days in the presence of PEG-rHuMGDF (10 ng/mL) in serum-free conditions, in serum derived from PPP, or normal serum in the absence or presence of heparin (H) or an anti–TGF-β antibody. MKs and platelet bearing MKs were enumerated using an inverted microscope and hemocytometer. The results represent the mean ± SD of three independent experiments, and are expressed per 1 × 103 plated CD34+ cells. Asterisks denote a significative difference (P < .05) in comparison with culture in serum-free conditions.
We next tested if addition of heparin or an anti–TGF-β antibody might improve these serum or PPP-containing cultures. Addition of heparin (3 U/mL) to PPP-containing medium increased the number of MKs and proplatelet-bearing MKs by twofold to threefold, as well as increasing the number of platelets produced. In contrast, addition of heparin to serum-containing medium or of an anti–TGF-β antibody in media containing serum or PPP increased the number of MKs and platelets produced, but not significantly. Nevertheless, the numbers of MKs, proplatelet-bearing MKs, and platelets observed were much lower than in serum-free medium (Fig 3A, B, and C). The mean ploidy remained nearly identical in all tested culture conditions, but was low (range, 3.9 to 4.5; data not shown).
In these various conditions, the percentage (about 50%) of proplatelet-bearing MKs was similar using either blood- or marrow-derived CD34+ cells, but as shown in Fig 3, their absolute numbers per CD34+ cell and the number of platelets produced were on average threefold higher for peripheral blood CD34+ cells (P < .01). The mean MK ploidy was similar (in serum-free medium, 5.1 and 4.9 for blood- and marrow-derived cells, respectively; data not shown).
Effects of Cytokines on the Production of Proplatelet-Bearing MKs and Platelets
The effects of cytokines on the entire CD34+ cell population, and on CD34+ CD41+ cells, highly enriched in mature MK progenitors, were assessed in serum-free medium at 7% O2.
Effects of cytokines on CD34+ cells.
Cytokines were tested on purified populations of blood- and marrow-derived CD34+ cells. In a first experiment, we determined that full-length rHu Mpl-L–stimulated cultures supplemented with the same molarity of PEG as Mpl-L– and PEG-rHuMGDF–stimulated cultures gave the same platelet production (91, 100, 101 × 103 platelets/103 BM CD34+ cells, respectively, average from one experiment). This confirms previous experiments showing that PEG does not modify the in vitro biological activity of a molecule.30 Subsequently, PEG-rHuMGDF was used alone or in different combinations with IL-3 plus SCF, or SCF plus IL-6. These effects of PEG-rHuMGDF were compared with those of different combinations of IL-3, SCF, and IL-6 without PEG-rHuMGDF (Fig 4). PEG-rHuMGDF had a major effect on the production of MKs, proplatelet-bearing MKs, and platelets associated with a high proportion (mean, 79%) of MKs in the cultures. From blood CD34+ cells, PEG-rHuMGDF induced an average of 4.3 × 103 proplatelet-bearing MKs (54% of cultured MKs) and 199 × 103 platelets/103CD34+ cells. Addition of IL-3, SCF, or the combination of SCF and IL-6 to PEG-rHuMGDF increased the number of MKs by 2.2, 1.6, and 1.5; proplatelet-bearing MKs by 1.9, 1.9, and 1.7; and the number of platelets by 1.7, 1.5, and 1.2; respectively (Fig 4A, B, and C). Of interest, addition of IL-6 to the combination of SCF and PEG-rHuMGDF had no effect on the number of proplatelet-bearing MKs. Other than PEG-rHuMGDF, IL-3 was the only cytokine which induced MK differentiation from CD34+ cells. The number of MKs obtained from blood CD34+ cells was only 1.8-fold lower than with rHuMGDF, but MKs represented only 6% of the output. The number of proplatelet-bearing MKs and platelets was ∼14-fold lower using IL-3 than with PEG-rHuMGDF (P = .01). This difference with PEG-rHuMGDF–stimulated cultures remained significant despite addition of SCF or of a combination of SCF plus IL-6 to the IL-3, which induced the growth of a similar number of MKs than with PEG-rHuMGDF. Therefore, the more significant effect of PEG-rHuMGDF concerns the transition from MKs to proplatelet-bearing MKs. The mean ploidy on day 9 in PEG-rHuMGDF–stimulated cultures was 5, and was not significantly different from that observed with IL-3 alone (4.6). Ploidy was lower on day 12 (4.4 and 4.3, respectively). A combination of several cytokines increased MK numbers but led to a significant decrease in ploidy (Fig5). As previously observed, marrow CD34+ cells showed a threefold lower capacity to produce MKs than their blood counterparts.
Effects of PEG-rHuMGDF and of SCF, IL-3, and IL-6 on MK growth (A), proplatelet formation (B), and platelet production (C). Marrow (n = 3, ▧) or blood (n = 6, ▩) CD34+cells were grown from 11 to 14 days in serum-free conditions, in the presence of PEG-rHuMGDF (10 ng/mL), IL-3 (100 U/mL), and different combinations of these two different cytokines with SCF (50 ng/mL) and IL-6 (100 U/mL). Asterisks denote a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone.
Effects of PEG-rHuMGDF and of SCF, IL-3, and IL-6 on MK growth (A), proplatelet formation (B), and platelet production (C). Marrow (n = 3, ▧) or blood (n = 6, ▩) CD34+cells were grown from 11 to 14 days in serum-free conditions, in the presence of PEG-rHuMGDF (10 ng/mL), IL-3 (100 U/mL), and different combinations of these two different cytokines with SCF (50 ng/mL) and IL-6 (100 U/mL). Asterisks denote a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone.
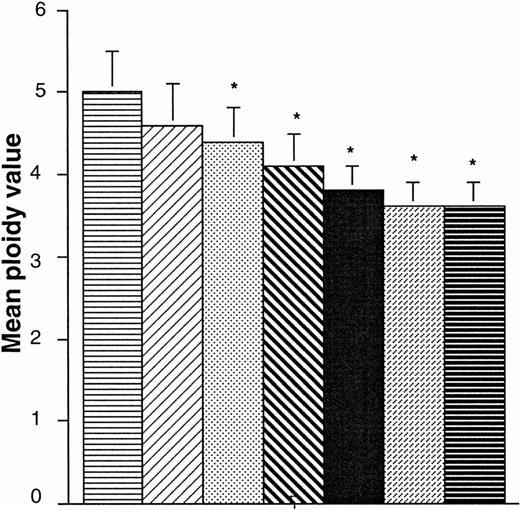
Effects of PEG-rHuMGDF and of SCF, IL-3, and IL-6 on MK ploidy. MK ploidy was measured by a double staining technique with an FITC–anti-CD41b MoAb and propidium iodide solution using flow cytometry. The mean ploidy was calculated. The results are the average of six experiments performed starting with blood CD34+cells. Asterisks denote a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone. (▤), MGDF; (▨), IL3; (▧), MGDF + SCF; (□), MGDF + SCF + IL6; (▪), MGDF + IL3; (▩), IL3 + SCF; (⊟), IL3 + SCF + IL6.
Effects of PEG-rHuMGDF and of SCF, IL-3, and IL-6 on MK ploidy. MK ploidy was measured by a double staining technique with an FITC–anti-CD41b MoAb and propidium iodide solution using flow cytometry. The mean ploidy was calculated. The results are the average of six experiments performed starting with blood CD34+cells. Asterisks denote a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone. (▤), MGDF; (▨), IL3; (▧), MGDF + SCF; (□), MGDF + SCF + IL6; (▪), MGDF + IL3; (▩), IL3 + SCF; (⊟), IL3 + SCF + IL6.
In subsequent experiments, cultures were initially stimulated in two different manners (either with a combination of IL-3 plus IL-6 and SCF, or with PEG-rHuMGDF) and after 7 days, cultures initially stimulated with the cytokine combination were switched to PEG-rHuMGDF. The data indicated that the presence of PEG-rHuMGDF in the second phase of the culture (corresponding to the terminal phase of MK maturation) did not induce a high proportion of proplatelet-displaying MKs or the production of a large quantity of platelets (15% of MKs displaying proplatelets and 68 × 103 platelets/103CD34+ cells in the switch conditions v 41% and 220 × 103, for cultures initiated with PEG-rHuMGDF). Nevertheless, the proportion of MKs displaying proplatelets and the number of platelets was threefold higher than in cultures stimulated with IL-3, SCF, and IL-6 throughout the culture period. These data indicated that in this culture system, the presence of PEG-rHuMGDF at all phases of megakaryocytopoiesis maximizes platelet production.
The CD34+ cell population is highly heterogenous and contains all types of hematopoietic progenitors. Depending on the culture conditions, the percentage of MKs was extremely variable, from 79% in PEG-rHuMGDF stimulated cultures to 4% with a combination of cytokines. Thus, we investigated the effects of cytokines on proplatelet formation and platelet shedding using a more homogenous population of MK progenitors. In this purpose, the CD34+CD41+ cells were purified. This cell fraction is highly enriched in late MK progenitors which synchronously differentiate in 5 to 7 days. However, the majority of the MK progenitors responsible for platelet production at day 12 are present in the CD34+CD41− cell population.
Effects of cytokines on CD34+ CD41+ cells.
To reduce the risk of false positivity due to platelet binding to CD34+ cells, the purification of CD34+CD41+ cells from BM cells was performed in the presence of PBS/EDTA and the cells were treated with neuramidinase.
CD34+ CD41+ cells (∼2% of CD34+cells) were cultured in the presence of increasing PEG-rHuMGDF concentrations (0.01 to 500 ng/mL). Without PEG-rHuMGDF, CD34+ CD41+ cells disintegrated after 2 days. MK differentiation and maturation were more rapid at PEG-rHuMGDF concentrations greater than 1 ng/mL, with development of the first proplatelet-bearing MKs observed at day 4 using 10 ng/mL, at day 5 at 1 ng/mL, and at day 7 at lower PEG-rHuMGDF concentrations. As shown in Fig 6, MK proliferation and the percentage of proplatelet-bearing MKs increased with PEG-rHuMGDF concentrations up to 1 ng/mL; there was no significant inhibitory effect at higher PEG-rHuMGDF concentrations up to 500 ng/mL, although some diminution was observed. The frequency of proplatelet-bearing MKs was 1%, 4%, 44%, 45%, 43%, and 38% at a concentration of 0.01, 0.1, 1, 10, 100, and 500 ng/mL, respectively. Platelet production precisely paralleled the number of proplatelet-bearing MKs and reached a plateau at 1 ng/mL PEG-rHuMGDF. The production of platelets per MK was threefold higher in cultures initiated with CD34+ CD41+ cells than in those commencing with the total CD34+ population (Fig4). The ploidy of MK grown from CD34+ CD41+cell population was much higher (mean ploidy value = 8; Fig7A) than in cultures derived from CD34+ cells (mean = 5).
Effects of increasing concentrations of PEG-rHuMGDF on proplatelet formation (A) and platelet production (B) from CD34+ CD41+ cells. CD34+CD41+ cells sorted from marrow were grown in serum-free conditions in the presence of increasing concentrations of PEG-rHuMGDF (0.01 to 500 ng/mL). A murine soluble Mpl receptor was added at day 4 (time of differentiation into proplatelet-bearing MKs, ⧫) or day 6 (time of platelet shedding, •) to cultures stimulated with 1 ng/mL PEG-rHuMGDF. PEG-rHuMGDF (500 ng/mL) was also added at day 4 to cultures initiated with 1 ng/mL of this cytokine (▴). MKs bearing proplatelets and platelets were enumerated at days 6 and 7, respectively, as in Figs 3 and 4. Asterisks denote a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone.
Effects of increasing concentrations of PEG-rHuMGDF on proplatelet formation (A) and platelet production (B) from CD34+ CD41+ cells. CD34+CD41+ cells sorted from marrow were grown in serum-free conditions in the presence of increasing concentrations of PEG-rHuMGDF (0.01 to 500 ng/mL). A murine soluble Mpl receptor was added at day 4 (time of differentiation into proplatelet-bearing MKs, ⧫) or day 6 (time of platelet shedding, •) to cultures stimulated with 1 ng/mL PEG-rHuMGDF. PEG-rHuMGDF (500 ng/mL) was also added at day 4 to cultures initiated with 1 ng/mL of this cytokine (▴). MKs bearing proplatelets and platelets were enumerated at days 6 and 7, respectively, as in Figs 3 and 4. Asterisks denote a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone.
Comparison of ploidy distribution of MKs and the expression of CD41b in different ploidy classes in the MKs obtained in the presence of PEG-rHuMGDF or the combination of IL-3, SCF, and IL-6 from CD34+ CD41+ cells. Marrow CD34+ CD41+ cells were grown from 6 days in serum-free conditions, in the presence of PEG-rHuMGDF (10 ng/mL) or IL-3 (100 U/mL) plus SCF (50 ng/mL) plus IL-6 (100 U/mL). Ploidy of the CD41+ cells obtained in the presence of PEG-rHuMGDF (A) and IL-3 plus SCF plus IL-6 (B). Comparison of the expression of CD41b in MK cultivated in the presence of PEG-rHuMGDF (solid line) and the combination of IL-3, SCF and IL-6 (broken lines) in the 2N (C), 4N (D), 8N (E), 16N (F), and 32N MKs (G). MKs were considered as CD41b+ cells and analysis was performed in each ploidy class.
Comparison of ploidy distribution of MKs and the expression of CD41b in different ploidy classes in the MKs obtained in the presence of PEG-rHuMGDF or the combination of IL-3, SCF, and IL-6 from CD34+ CD41+ cells. Marrow CD34+ CD41+ cells were grown from 6 days in serum-free conditions, in the presence of PEG-rHuMGDF (10 ng/mL) or IL-3 (100 U/mL) plus SCF (50 ng/mL) plus IL-6 (100 U/mL). Ploidy of the CD41+ cells obtained in the presence of PEG-rHuMGDF (A) and IL-3 plus SCF plus IL-6 (B). Comparison of the expression of CD41b in MK cultivated in the presence of PEG-rHuMGDF (solid line) and the combination of IL-3, SCF and IL-6 (broken lines) in the 2N (C), 4N (D), 8N (E), 16N (F), and 32N MKs (G). MKs were considered as CD41b+ cells and analysis was performed in each ploidy class.
The effect of PEG-rHuMGDF on platelet production was investigated further in three experiments by adding a soluble Mpl receptor or a high concentration (500 ng/mL) of PEG-rHuMGDF after 4 to 5 days of culture in the presence of 1 ng/mL PEG-rHuMGDF (these additions were made at the time of differentiation into proplatelet-bearing MKs). A high dose of PEG-rHuMGDF had no effect on proplatelet formation and the number of platelets produced, whereas a soluble Mpl receptor markedly inhibited their production. In contrast, a soluble Mpl receptor added at day 6 (at the time of platelet shedding) had no effect (Fig 6).
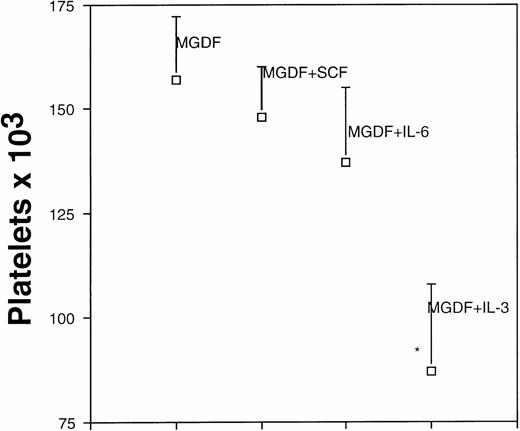
The effects of the three other tested cytokines (IL-3, SCF, and IL-6) were also determined on this population of mature MK progenitors (n = 7). The only other cytokine promoting MK proliferation and differentiation into proplatelet-bearing MKs was IL-3, but its effect was weak compared with that of PEG-rHuMGDF. In contrast, a combination of two cytokines gave a higher number of proplatelet-bearing MKs and platelets with IL-3 + SCF > IL-3 + IL-6 > SCF + IL-6. The combination of the three cytokines (SCF, IL-3, and IL-6) gave the best results by inducing the growth of a number of MKs quite similar to that obtained with PEG-rHuMGDF (1.2 or 0.8 × 103proplatelet-bearing MKs in presence of PEG-rHuMGDF or combination of the three cytokines, respectively). Platelet production was decreased but only 2.4-fold in comparison with PEG-rHuMGDF–stimulated cultures (46 or 111 platelets per 1 × 103 CD34+CD41+ cells, respectively). Ploidy distribution (Fig 7A and B) and expression of CD41 in each ploidy class (Fig 7C through G) were also identical to that noted in PEG-rHuMGDF-stimulated cultures.
As SCF and IL-6 do not permit MK proliferation when used alone, we studied their effect on MK differentiation and platelet production by adding them to a suboptimal concentration (1 ng/mL) of PEG-rHuMGDF. Both cytokines did not significantly modify the number of MK and platelets produced. In contrast, addition of IL-3 to PEG-rHuMGDF inhibited proplatelet formation (Fig 8).
Effects of PEG-rHuMGDF associated with other cytokines on platelet production from CD34+ CD41+ cells. Marrow-derived CD34+ CD41+ cells were grown in serum-free conditions in the presence of 1 ng/mL PEG-rHuMGDF alone or combined with IL-3 (100 U/mL), SCF (50 ng/mL), and IL-6 (100 U/mL). Platelets were enumerated at day 7 of culture by flow cytometry. Asterisk denotes a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone.
Effects of PEG-rHuMGDF associated with other cytokines on platelet production from CD34+ CD41+ cells. Marrow-derived CD34+ CD41+ cells were grown in serum-free conditions in the presence of 1 ng/mL PEG-rHuMGDF alone or combined with IL-3 (100 U/mL), SCF (50 ng/mL), and IL-6 (100 U/mL). Platelets were enumerated at day 7 of culture by flow cytometry. Asterisk denotes a significative difference (P < .05) in comparison with culture with 1 ng/mL PEG-rHuMGDF alone.
Structure and Function of Platelets Produced in Culture
We previously described the ultrastructure of platelet formation by human MKs cultured with PEG-rHuMGDF.29 No substantial differences were observed in the ultrastructure of the platelet-shedding MKs produced in the presence of either PEG-rHuMGDF or the combination of IL-3, SCF, and IL-6. In the two conditions, platelet shedding MKs showed several cytoplasmic expansions which correspond to proplatelets (Figs 9a and10a); constriction zones already individualize distinct platelet fields. In the same way, platelets derived from culture with either PEG-rHuMGDF or the combination of IL-3, IL-6, and SCF were of similar size and exhibited α- and dense granules. Many of these platelets were adherent to MKs; however, they did not exhibit ultrastructural evidence of activation (Figs 9b and 10b). By fluorescent labeling, circumferential microtubule coils and actin filaments were observed with an antitubulin MoAb and phalloidin in the vast majority of the culture-derived platelets (data not shown).
Ultrastructure of platelet shedding MKs and platelets obtained in the presence of PEG-rHuMGDF from CD34+CD41+ cells. (a) A mature MK presenting signs of platelet formation. This MK displays dilatation of the demarcation membranes (dm) located at the periphery. They individualize a zone of cytoplasm (arrows) which will form a future proplatelet (N, nucleus). (b) Platelet-sized-fragments (P) exhibit the usual cytoplasmic organelles: α granules (A), smooth connected canalicular system (sccs), and endoplasmic reticulum (er).
Ultrastructure of platelet shedding MKs and platelets obtained in the presence of PEG-rHuMGDF from CD34+CD41+ cells. (a) A mature MK presenting signs of platelet formation. This MK displays dilatation of the demarcation membranes (dm) located at the periphery. They individualize a zone of cytoplasm (arrows) which will form a future proplatelet (N, nucleus). (b) Platelet-sized-fragments (P) exhibit the usual cytoplasmic organelles: α granules (A), smooth connected canalicular system (sccs), and endoplasmic reticulum (er).
Ultrastructure of platelet shedding MKs and platelets obtained in the presence of the combination of SCF, IL-3, and IL-6 from CD34+ CD41+ cells. (a) A similar mature MK as in Fig 9a with dilatation of demarcation membranes (dm) at the periphery (arrows) (N, nucleus). (b) Platelet-sized-fragments (P) are present with the combination of three cytokines. They are similar to those obtained with PEG-rHuMGDF (Fig 9b). On a proplatelet (PP), a constriction zone area is disposed along the cytoplasmic extension individualizing a distinct platelet field with a vacuole at the level of the future rupture (arrow) (A, α granules; sccs, smooth connected canalicular system; er, endoplasmic reticulum).
Ultrastructure of platelet shedding MKs and platelets obtained in the presence of the combination of SCF, IL-3, and IL-6 from CD34+ CD41+ cells. (a) A similar mature MK as in Fig 9a with dilatation of demarcation membranes (dm) at the periphery (arrows) (N, nucleus). (b) Platelet-sized-fragments (P) are present with the combination of three cytokines. They are similar to those obtained with PEG-rHuMGDF (Fig 9b). On a proplatelet (PP), a constriction zone area is disposed along the cytoplasmic extension individualizing a distinct platelet field with a vacuole at the level of the future rupture (arrow) (A, α granules; sccs, smooth connected canalicular system; er, endoplasmic reticulum).
Expression of CD62 on culture-derived platelets produced in various culture conditions was subsequently investigated. Before activation, less than 10% of platelets expressed CD62 on their surfaces (Fig11A). A majority were capable of being activated. Activation was associated with slight changes in the scatter properties of culture-derived platelets (Fig 1D and E). The percentage of activated platelets in cultures using PEG-rHuMGDF (92%) was similar to that of cultures using a combination of SCF, IL-3, and IL-6 (70%) (Fig 11B).
Expression of CD62 on the surface of platelets produced in vitro from CD34+ cells (A) or CD34+CD41+ (B). MKs were cultured either from CD34+ in the presence of PEG-rHuMGDF (A) or CD34+ CD41+ in the presence of PEG-rHuMGDF or the combination of IL-3, SCF, and IL-6 (B). At day 13 or 7 for cultures deriving from CD34+ or CD34+CD41+, respectively, cells were stimulated for 10 minutes at 37°C with 2 U/mL thrombin. Activated and nonactivated platelets were incubated with phycoerythrin (PE) anti-CD62 and FITC-TAB and analyzed relative to PE-conjugated IgG1MoAb control by flow cytometry in a morphological gate corresponding to blood platelets. (A) Serum-free cultures in the presence of PEG-rHuMGDF (10 ng/mL) from CD34+ cells. Expression of CD62 (solid line) in thrombin-activated platelets in comparison with nonactivated platelets (broken line). (B) Serum-free cultures in the presence of PEG-rHuMGDF (10 ng/mL) or rHuSCF (50 ng/mL), rHuIL-6 (100 U/mL), and rHuIL-3 (100 U/mL) from CD34+ CD41+ cells. Expression of CD62 in thrombin-activated platelets from PEG-rHuMGDF (thick solid line) or SCF, IL-3, and IL-6 (thin solid line). Isotype control is shown (broken line).
Expression of CD62 on the surface of platelets produced in vitro from CD34+ cells (A) or CD34+CD41+ (B). MKs were cultured either from CD34+ in the presence of PEG-rHuMGDF (A) or CD34+ CD41+ in the presence of PEG-rHuMGDF or the combination of IL-3, SCF, and IL-6 (B). At day 13 or 7 for cultures deriving from CD34+ or CD34+CD41+, respectively, cells were stimulated for 10 minutes at 37°C with 2 U/mL thrombin. Activated and nonactivated platelets were incubated with phycoerythrin (PE) anti-CD62 and FITC-TAB and analyzed relative to PE-conjugated IgG1MoAb control by flow cytometry in a morphological gate corresponding to blood platelets. (A) Serum-free cultures in the presence of PEG-rHuMGDF (10 ng/mL) from CD34+ cells. Expression of CD62 (solid line) in thrombin-activated platelets in comparison with nonactivated platelets (broken line). (B) Serum-free cultures in the presence of PEG-rHuMGDF (10 ng/mL) or rHuSCF (50 ng/mL), rHuIL-6 (100 U/mL), and rHuIL-3 (100 U/mL) from CD34+ CD41+ cells. Expression of CD62 in thrombin-activated platelets from PEG-rHuMGDF (thick solid line) or SCF, IL-3, and IL-6 (thin solid line). Isotype control is shown (broken line).
In some experiments, it was observed that CD41+ elements with high scatter properties and which were excluded from the analytical gate expressed CD62 before activation. The CD62 level was not increased by thrombin stimulation, suggesting that a proportion of the platelets quickly activate or become permeable to anti-CD62 during or following production in vitro.
DISCUSSION
We have developed a culture system capable of producing large numbers of MKs which release platelets after terminal differentiation. This culture system is similar to that described by Choi et al,13,18,31 and is initiated with CD34+ cells. However, the cultures reported here were performed in only one step. The present culture model is quite easy and can be used to study the terminal stages of megakaryocytopoiesis and platelet production. One pitfall of these cultures is the definition of platelets produced in vitro. Circulating platelets have precise cytological and ultrastructural features. Most workers define platelets by their discoid form, the presence of circumferentially arranged microtubules (relative to the plasma membrane), and specific organelles (α-granules and an open canalicular system). In cultures containing a majority of mature MKs, it is more difficult to define platelets. Such cultures can contain (1) functional platelets with typical ultrastructural characteristics, (2) detached proplatelets or giant platelets that may further fragment to evolve into functional platelets, (3) lytic, nonfunctional fragments of MKs, and (4) platelet microparticles. Apart from the studies of Choi et al,13there are no published reports on platelet production in vitro. Those investigators used a double-centrifugation procedure to isolate cell fragments produced in culture that have features of circulating platelets, including size (6 to 10 fL). Using this size criterion, they enumerated culture-derived platelets with an electrical impedance device. Employing the same conditions, we were unable to reproduce their results, because most of the cultured platelets could not be separated from MKs by centrifugation. At the ultrastructural level, we observed that a portion of the platelets produced in culture adhere to MKs during centrifugation, although there was no sign of activation. Therefore, in this study cultured platelets were defined on the basis of cytometric criteria: scatter properties and GPIIb expression identical to blood platelets. This definition has the advantage of not requiring centrifugation, and platelets produced in varying culture conditions can be readily quantitated. Moreover, these platelet-like particles were capable of being activated by thrombin, providing an additional criterion for a “viable” platelet. According to this definition, nonviable platelets were also present, and exhibited high side scatter properties and constitutive CD62 expression.
During optimization of the culture conditions, two main observations were made. First, substantial numbers of proplatelet-displaying MKs and platelets were noted only in serum-free conditions. In the guinea pig, it has been shown that serum prothrombin was responsible for inhibition of proplatelet formation and its effects could be reversed with glycosaminoglycans.11,12 Addition of heparin to the serum-containing cultures improved proplatelet formation and platelet production. Only a slight improvement was noted when an anti–TGF-β antibody was added. TGF-β is involved in the negative regulation of megakaryocytopoiesis,24,32,33 but does not seem to inhibit proplatelet formation.12 Therefore, other serum or plasma molecules which remain to be identified may inhibit platelet production in vitro. Second, blood CD34+ cells obtained from cytapheresis after mobilization gave rise to a threefold higher number of MKs and platelets than their marrow counterparts, but with a delay in their kinetics of growth. This may be caused by either a higher percentage or greater proliferative capacities of the CD34+colony-forming unit–MK contained in the blood in comparison with the marrow.
Although this culture system was quite efficient in the production of large numbers of MKs (4 MKs/CD34+ cell) and proplatelet-displaying MKs (up to 60% of the MKs), the number of platelets produced per MK is low (<50 per proplatelet-bearing MK). This is partly due to an underestimation of the number of platelets produced because cultures are not synchronous and platelets are not released simultaneously. Consequently, a portion of the produced platelets are not viable at the time of analysis. However, it is likely that another important factor explaining this platelet production defect is the low ploidy of MKs produced in vitro. This is not directly caused by the cytokines employed because the ploidy was identical using either PEG-rHuMGDF or IL-3, but appears to be related to the degree of progenitor cell proliferation. The combinations of cytokines which increased MK number to the greatest degree markedly diminished polyploidization. Moreover, we did not observe an increase in ploidy with time, but rather a marked decrease, in contrast to a previous report.34 A reasonable hypothesis is that mature MK progenitors which have low proliferative ability give rise to high ploidy MKs within several days, whereas more primitive progenitors will proliferate at the expense of endomitosis during terminal differentiation. This hypothesis is supported further by the fact that CD34+ CD41+ cells gave rise to MKs with an 8N modal ploidy in 5 to 7 days. An inverse relationship between proliferation and polyploidization has been described previously for IL-3–stimulated cultures.35-37 These data may have consequences for in vitro approaches to expand MKs from CD34+ cells for clinical purposes. Although use of multiple cytokines augments the total number of CD41+ or CD61+ cells,38-40 these cytokine combinations may expand low ploidy MK with limited platelet production capacity. Choi et al14,31 reported a higher number (>200) of platelets produced per MK. This difference can be explained by the use of velocity sedimentation for MK purification. That procedure depletes the 2, 4, and 8N cell populations and thus enriches 16N cells.41
PEG-rHuMGDF has a critical effect on the in vitro production of proplatelet-displaying MKs and of platelets. IL-3 or a combination of SCF plus IL-6 and IL-3 were capable of giving rise to a similar or greater number of MKs than PEG-rHuMGDF. However, stimulation with PEG-rHuMGDF yielded a greater percentage of MKs bearing proplatelets and a higher total number of platelets than did stimulation using other cytokines. Differences in platelet production between PEG-rHuMGDF and this combination of three cytokines were much less when CD34+ CD41+ cells were used instead of the entire CD34+ cell population. The combination of SCF, IL-3, and IL-6 stimulated primarily the growth of non-MK cells from CD34+ cells, whereas a nearly pure population of MKs was derived from CD34+ CD41+ cells. It is likely that these contaminating cells inhibit proplatelet formation and platelet shedding. In favor of this hypothesis, it has been recently described that accessory cells synthesize proteases which inhibit growth of hematopoietic progenitors in serum-free conditions.42 However, this study clearly shows that Mpl-L is not absolutely required in culture to observe full MK development as previously suggested,43 since “viable” platelets capable of being activated by thrombin are produced in culture in its absence. Whether the platelets produced in the presence of Mpl-L or other cytokines have similar functions remains to be determined. These in vitro results are comparable to those observed in c-mpl or Mpl-L knockout mice which have a 90-fold decrease in their number of platelets.44-46 Also valuable for interpreting the effects of cytokines on MK differentiation may be p45 NF-E2 knockout mice. Mice lacking p45 NF-E2 have a lethal thrombocytopenia with an increased number of MKs. These MKs exhibit abnormal demarcation membranes and a decreased number of granules.47 Because no binding site for NF-E2 is found in the promoter region of MK-specific genes, Shivdasani48 has hypothesized that NF-E2 regulates another set of genes involved in the regulation of MK cytoplasmic maturation. At the level of the MK progenitor, expression of MK-restricted genes could be regulated by several cytokines including Mpl-L, IL-3, or IL-6, whereas later in differentiation genes involved in MK cytoplasmic maturation may be regulated essentially by Mpl-L. However unlike the in vivo approach, our in vitro system using IL-3 and IL-6 may more mimick stress conditions of platelet regulation than a physiological regulation.
By testing the dose-response characteristics of PEG-rHuMGDF on CD34+ CD41+ cells, it was noted that PEG-rHuMGDF accelerated MK maturation and increased platelet formation up to 1 ng/mL. Higher doses did not significantly modify these results and only a slight decrease (<10%) was observed when 500 ng/mL was added at the stage of proplatelet formation. Others have reported a more marked inhibitory effect of PEG-rHuMGDF on proplatelet formation at high concentrations18,19 and it has been hypothesized that this paradoxical effect of PEG-rHuMGDF is due to an inhibition of MK apoptosis.49 As in the present report, Horie et al50 recently described a crucial effect of PEG-rHuMGDF vis-à-vis subsequent proplatelet production at the level of the rat MK progenitor, but with an optimal dose of 0.037 ng/mL. Larger MKs subsequently forming prominent proplatelets were observed at higher concentrations. The difference between the present study and that of Horie et al50 may be related to the species. Addition of SCF and IL-6 to PEG-rHuMGDF–stimulated cultures did not modify proplatelet formation. In contrast, in the presence of PEG-rHuMGDF, addition of IL-3 inhibited this process. A negative effect of IL-3 was also observed in the rat; however, it was found that IL-6 or IL-11 were very potent inducers of proplatelet formation.50 This effect of IL-6 on proplatelet formation seems to be restricted to rodents and is not observed with human cells.49
Finally, we investigated whether PEG-rHuMGDF is necessary for the final stages of thrombocytopoiesis. Addition of a soluble Mpl receptor to a homogenous population of mature proplatelet-displaying MKs derived from CD34+ CD41+ cells did not inhibit platelet production 1 or 2 days later. However, when the same experiments were performed on unseparated CD34+-derived MKs which were at different stages of maturation, or on CD34+CD41+ cells prior to proplatelet formation, addition of the soluble Mpl receptor inhibited proplatelet formation and induced apoptosis of maturing MKs. This difference may be explained by the fact that Mpl-L is not necessary for platelet shedding, which may be an intrinsic property of mature MKs,18 49 but is absolutely required for survival and terminal differentiation of immature MKs.
In conclusion, we have developed a simple liquid culture method to study the late stages of platelet production. This culture technique may permit testing the effects of cytokines or stromal cells on platelet formation. It may also facilitate the characterization of the genes involved in platelet formation by investigating cultures from patients with congenital thrombocytopenia.
ACKNOWLEDGMENT
We are grateful to J.-L. Nichol (Amgen, Thousand Oaks, CA) for providing the rHuSCF and PEG-rHuMGDF, to D. Foster (ZymoGenetics, Seattle, WA) for the murine soluble Mpl receptor, to D. Cosman (Immunex, Seattle, WA) for rHuIL-3, and to A. Katz and M. Zohar for cell sorting. We are indebted to J.-M. Massé for photographic assistance.
Supported by the Institut National de la Santé et de la Recherche médicale, the Institut Gustave Roussy, and by grants from the Association de la Recherche contre le Cancer (ARC), la Ligue Nationale contre le Cancer, and a Fogarty International Fellowship (to S.A.B.).
Address reprint requests to Najet Debili, PhD, INSERM U 362, PR1, Institut Gustave Roussy, Villejuif, 94800, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal