Abstract
Members of the Jak family play a critical role in signal transduction mediated by cytokine and hormone receptors. In this study, we report the cloning and characterization of human Jak2. The predicted amino acid sequence shows 91% homology to the described murine Jak2, but with a significant difference in the extreme C-terminal sequence. Using the human cDNA as a probe, we localized the gene for human Jak2 to chromosome 9p23-24. Human Jak2 mRNA is highly expressed in the spleen, lymph nodes, and peripheral blood lymphocytes (PBLs). A polyclonal antibody raised against the unique C-terminus of human Jak2 was used to characterize Jak2 protein. Levels of Jak2 protein expression increased significantly in mitogen- and anti-IgM–stimulated B cells and to a lesser degree in activated T cells. In addition, high levels of Jak2 protein were detected in pre-B leukemia cells.
CYTOKINES CONTROL proliferation and differentiation by binding to specific receptors expressed on hematopoietic cells.1,2 Because cytokine receptors do not possess a kinase domain, great effort has been made to identify the cytoplasmic protein tyrosine kinases (PTKs) responsible for the rapid increase in PTK activity seen upon ligand-receptor interaction.3 A commonly used technique has involved polymerase chain reaction (PCR) with degenerate oligodeoxynucleotide primers directed to conserved tyrosine kinase catalytic domain motifs. Many new PTKs have been discovered by this method, including members of the Jak family.4,5 It has been shown recently that in addition to src PTKs, cytokine receptors activate one or more Jak kinases. Cytokines using receptors consisting of a single chain, such as erythropoietin, prolactin, and growth hormone, primarily activate Jak2,6-8 whereas the interferons activate two members of the Jak family.9-11 The receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15 are functionally coupled to Jak1 and Jak3,12,13 where the common γ-chain (γc) selectively recruits Jak3. The cytokines ciliary neurotropic factor, leukemia-inhibiting factor, oncostatin M, and IL-6 comprise a unique cytokine subfamily on the basis of their predicted structural similarity and shared β signal-transducing receptor component (gp 130). This family of receptors is capable of recruiting different members of the Jak family (Jak1, Jak2, and Tyk2) in different cell types.14 15
The Jak kinase family is unusual in possessing two distinct kinase domains.5,16 The kinase domain proximal to the carboxyl terminal contains all of the recognized essential PTK motifs and is therefore likely to be catalytically active.17 However, the function of the second kinase domain remains unknown, as it lacks several residues believed to be essential for catalytic activity.16,17 Members of the Jak family lack src homology 2 (SH2) and src homology 3 (SH3) domains,18 but they show other homology regions of yet unknown significance.5,16Jak3 expression appears limited to the myeloid and lymphoid lineages,19,20 and it appears to increase in T cells and B cells after mitogenic stimulation.19-21 In contrast, Tyk2, Jak1, and Jak2 are believed to be widely expressed.4,5 22However, for Jak2, experiments were performed with murine reagents and the assumption was made that similar expression patterns hold for humans.
Recently, we have shown that pre-B leukemia cells express elevated levels of Jak2 that appears to be constitutively activated.23 Inhibition of Jak2 activity with a specific inhibitor resulted in cell death, suggesting a critical role for this enzyme in leukemia cell growth.23 Indeed, a mutation in the Jak homolog was found to cause leukemia inDrosophila.24,25 To study the role of human Jak2 in signaling, we cloned the human Jak2 cDNA. The sequence was found to be highly homologous to murine Jak2, but with an alternative 13 amino acids at the extreme carboxy terminus. Antibodies raised against this unique sequence were used to analyze the expression of human Jak2 in various cells and tissues. In lymphoid cells, the level of Jak2 protein expression was found to be low in peripheral resting B and T cells. However, there was a significant increase in the level of Jak2 protein in B cells upon ligation of the B-cell antigen receptor (BCR) and after mitogenic stimulation with SAC. In T cells, the increase in Jak2 levels following ligation of the T-cell receptor or activation with phytohemagglutinin (PHA) was less significant.
Currently, controversy exists as to the exact chromosomal localization of the human Jak2 gene. Using murine cDNA, Jak2 was localized to chromosome 9p24.26 However, the murine Jak2 gene is genetically linked to Fas on chromosome 19,16 which corresponds to human chromosome 10q23-q24.16 Using the human Jak2 cDNA as a probe, we localized the human Jak2 gene to chromosome 9p23-24, confirming that the murine and human Jak2 genes are indeed located on different chromosomes.
MATERIALS AND METHODS
Library screening.
A human thymus cDNA λgt11 library (Clontech, Palo Alto, CA) was screened according to standard protocols27 using the full-length murine Jak2 cDNA as a probe. Plaques were transferred to ICN (Plainview, NY) Biotrans nylon filters and screened by hybridization at 65°C in 5× SSC, 5× Denhardt solution, and 0.1% SDS. The final wash was performed at 55°C in 2× SSC and 0.1% SDS. Filters were autoradiographed overnight using Kodak (Rochester, NY) XAR-5 x-ray film. Phage DNA was prepared from positive plaques. cDNA inserts were excised, subcloned, and sequenced.
Reverse transcriptase–PCR cloning.
For reverse transcriptase (RT)-PCR cloning, total RNAs were extracted from relevant cell types according to Chomczynski and Sacchi.28 The RNAs were reverse-transcribed using standard protocols.27 The resulting cDNAs were used as substrates for PCRs using Elongase (GIBCO-BRL, Gaithersburg, MD) as the thermoresistant amplifying enzyme. The PCR products were subcloned into a pUC19 vector and sequenced with Sequenase (Amersham, Arlington Heights, IL).
To isolate the 5′ end of the cDNA, total RNA from peripheral blood lymphocytes (PBLs) was reverse-transcribed with oligo dT. A 5′ degenerate oligonucleotide primer to the mouse sequence beginning at the start codon [ATG GG(ACGT) ATG GC(ACGT) TG(CT) CT(ACGT) A] was used with an antisense nondegenerate primer to the human sequence obtained from the library Jak2 clones (nucleotides 1416 to 1394; GAA GTT CTT CTT TGT CCC ACT G). The reaction cycle conditions were as follows: 94°C (30 seconds), 50°C (30 seconds), and 68°C (90 seconds) for five cycles, followed by 35 cycles of 94°C (30 seconds), 62°C (30 seconds), and 68°C (90 seconds).
To isolate the 3′ end of the cDNA, total RNA from PBLs was reverse-transcribed using oligo (dT)12GC as a primer. We designed a nondegenerate primer to the human Jak2 sequence from nucleotide 3055 to 3085 (ATA TTC TGG TAT GCT CGA CAA TCA CTG ACA) and an antisense nondegenerate primer to the murine Jak2 untranslated sequence (TTC TGC CTA GCT AGC ATC ATG ATA AGG ATG). The reaction cycle conditions were as follows: five cycles at 94°C (30 seconds), 40°C (30 seconds), and 68°C (60 seconds), followed by 35 cycles at 94°C (30 seconds), 50°C (30 seconds), and 68°C (60 seconds).
To isolate the complete cDNA, total RNAs from thymocytes, PBLs, and G2 cells (pre-B leukemia cell line) were reverse-transcribed with oligo dT primer. A 5′ primer beginning at the start codon of human Jak2 (ATG GGG ATG GCT TGC CTT ACG ATG ACA GAA) was used with an antisense primer starting at the stop codon of human Jak2 (TCA TCC AGC CAT GTT ATC CCT TAC TTG ATC). The PCR cycling conditions were 30 cycles at 94°C (30 seconds), 60°C (30 seconds), and 68°C (5 minutes).
Chromosomal localization.
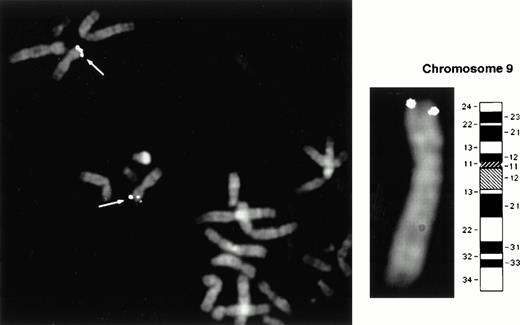
Positional mapping of the Jak2 gene was performed by fluorescence in situ hybridization (FISH)29 to normal human lymphocyte chromosomes counterstained with propidium iodide and 4′6-diamidin-2-phenylindole-dihydrochloride (DAPI). A 1.5-kb Jak2 cDNA probe and a corresponding genomic probe obtained by screening a P1-derived artificial chromosome (PAC) library were used. Both were labeled with biotin and detected with avidin-fluorescein isothiocyanate (FITC).29 Images of 20 well-spread metaphase preparations were captured by a thermoelectrically cooled charge-coupled camera (Photometrics, Tucson, AZ). Separate images of DAPI-banded chromosomes and FITC-targeted chromosomes were obtained.30Hybridization signals were acquired and merged using image-analysis software, and pseudocolored blue (DAPI) and yellow (FITC) and overlaid electronically.31
Northern blot analysis.
Membranes with 2 μg polyadenylated mRNA per lane from various human tissues were purchased from Clontech and probed with a 400-bp cDNA corresponding to the 3′ end of the human Jak2. The DNA probes were labeled with 32P by random priming (Boehringer, Mannheim, Germany) to a specific activity of 2 × 108cpm/μg DNA. Membranes were hybridized in ExpressHyb hybridization solution (Clontech) at 68°C for 1 hour, and washing was performed in 0.1× SSC and 0.1% SDS at 50°C twice for 40 minutes. Membranes were exposed to Kodak BMR-1 x-ray film at −70°C for 4 days. Subsequently, membranes were probed with β-actin to check RNA loading.
Antibody preparation.
The rabbit polyclonal anti-human Jak2 antibody was raised against a synthetic peptide, VLRVDQVRDNMAG, which is the unique C-terminus of human Jak2. This antibody was found to immunoprecipitate a 130-kD protein, which was recognized by three different commercially available anti-mouse Jak2 antibodies (made by PharMingen [San Diego, CA], UBI [Lake Placid, NY], and Santa-Cruz Biotech [Santa-Cruz, CA]). Because the anti-human Jak2 antibody we prepared was unable to recognize denaturated Jak2 protein in Western blots, we used anti-mouse Jak2 antibody (PharMingen) for immunoblotting after immunoprecipitation with our antibody.
Cell preparation and stimulation.
Peripheral blood mononuclear cells were obtained from healthy volunteers. Mononuclear cells were isolated by Ficoll-Hypaque gradient centrifugation. Adherent cells were removed by adherence to plastic dishes for 60 minutes at 37°C. Separation of T cells from B cells was performed by Ficoll-Hypaque centrifugation of cells rosetting with neuroaminidase-treated sheep erythrocytes.32 The purity of the B- and T-cell population was assessed by flow cytometry. The B-cell population was always 60% to 70% CD19+ with less than 5% CD3+ cells. The T-cell population was always 95% to 99% CD3+ with less than 2% CD19+ cells. T cells were stimulated with 10 μg/mL PHA or 20 μg/mL anti-CD3. B cells were stimulated with 2 mg/mL staphylococcus protein A cowan (SAC) or 20 μg/mL anti-IgM antibody for the indicated times at 37°C in RPMI with 10% fetal calf serum.
Immunoprecipitation and immunoblotting.
Immunoprecipitation was performed as described before.33Briefly, immunoprecipitates using the antibody to human Jak2 were prepared from lysates of 2 × 107 T or B cells in 1 mL lysis buffer containing 20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1% Triton X-100, and 1 mmol/L Na3VO4 buffer with antisera to human Jak2, and subjected to 6% SDS-PAGE. Where different cell types were used, the total amount of protein in the cell lysate was measured by the Lowry method and 750 μg was used for each immunoprecipitation. After transfer to supported nitrocellulose membrane (Amersham), immunoblotting was performed with anti-murine Jak2 antibody from PharMingen and then detected by ECL (Amersham).
In vitro transcription and translation.
Full-length Jak3 and Jak2 cDNAs were inserted into the pcDNA3 vector (Invitrogen, San Diego, CA) downstream from a T7 promoter. Approximately 1 μg luciferase control, Jak3, and Jak2 cDNAs in pcDNA3 were added to a combined transcription and translation reticulocyte lysate system (Promega, Madison, WI) in the presence of T7 polymerase. The protein products were labeled by inclusion of (35S) methionine in the reaction. Aliquots (5 μL) of each product were assayed on 6% SDS-PAGE, and the remaining 45 μL lysate from each reaction was diluted in 1 mL 1% Triton X-100 lysis buffer (as before) and immunoprecipitation was performed with antisera to human Jak2. The precipitates were washed with lysis buffer and separated on a 6% SDS-PAGE gel, followed by electrotransfer to nitrocellulose membrane and visualization by autoradiography.
RESULTS
Cloning of human Jak2 cDNA.
The murine Jak2 cDNA was used to screen a human thymus cDNA library to isolate the human Jak2 sequence. This screening yielded a series of overlapping fragments combining to form a 1.8-kb open reading frame (ORF) that was 89% homologous to the kinase domain of the translated murine Jak2 cDNA (from nucleotide 1398 to nucleotide 3207). Noting the extensive homology between our isolated clones and the murine Jak2 cDNA, we used RT-PCR with degenerate primers to murine Jak2 to complete the cloning of the 5′ and 3′ ends of the human cDNA. Figure1 summarizes the cloning strategy used. To obtain the 5′ end of human Jak2, we used a degenerate oligonucleotide primer to the murine start codon region and an antisense nondegenerate primer to human Jak2 in a PCR on human PBL first-strand cDNA. This produced a fragment of approximately 1.4 kb extending from the ATG and overlapping the 5′ end of our original 1.8-kb human Jak2 ORF. To obtain the 3′ end of Jak2, RNA extracted from human PBLs and reverse-transcribed with oligo(dT)12 GC was used as a substrate for PCR. We designed a nondegenerate primer on the 1.8-kb ORF and an antisense nondegenerate primer to murine Jak2 untranslated sequence. This amplification generated a sequence of approximately 450 bp containing a stop codon and overlapping the 3′ end of the 1.8-kb human Jak2 ORF. The RT-PCR–generated fragments and the 1.8-kb ORF combined to form a 3.4-kb ORF encoding a polypeptide of 1,134 amino acids with a predicted molecular weight of 130 kD.
Strategy used to clone human Jak2. A combination of library screening using the mouse Jak2 cDNA as a probe and RT-PCR was used to obtain the full-length cDNA of human Jak2. The screening yielded a series of overlapping fragments combining to form a 1.8-kb ORF (nucleotides 1398 to 3207). A RT-PCR with primers to the published murine sequence and to the 1.8-kb ORF obtained from the library screening was used to complete the cloning of the 5′ (nucleotides 1 to 1416) and 3′ (nucleotides 3055 to 3503) ends of the human Jak2. A stop codon was found in position 3402, as indicated by the arrow.
Strategy used to clone human Jak2. A combination of library screening using the mouse Jak2 cDNA as a probe and RT-PCR was used to obtain the full-length cDNA of human Jak2. The screening yielded a series of overlapping fragments combining to form a 1.8-kb ORF (nucleotides 1398 to 3207). A RT-PCR with primers to the published murine sequence and to the 1.8-kb ORF obtained from the library screening was used to complete the cloning of the 5′ (nucleotides 1 to 1416) and 3′ (nucleotides 3055 to 3503) ends of the human Jak2. A stop codon was found in position 3402, as indicated by the arrow.
This complete sequence demonstrated 87% homology with murine Jak2 cDNA. Although the predicted amino acid sequence of the human cDNA showed an overall homology of 91% with murine Jak2, a significant difference was noted at the extreme carboxy terminus. An insertion of one base in position 3361 causes a frameshift relative to the murine sequence that creates an alternate last 13 amino acids and with the addition of a further three amino acids. Using oligodeoxynucleotides to the 5′ and 3′ ends of the human sequence, we cloned (by RT-PCR) a full-length Jak2 cDNA from both human thymocytes and human G2 cells (pre-B leukemia cell line) and found them to be identical to the previously isolated human Jak2 sequence.
Figure 2 shows a comparison between the predicted amino acid sequence of human Jak1, Jak3, and Tyk2 and the human Jak2 sequence. The alignment shows that the human Jak2 exhibits the seven domains of homology (JH1 to JH7) that have been shown to exist among other members of the Jak family, confirming that this novel human cDNA is indeed a member of the human Jak family. The homology between the predicted amino acid sequence of our clone and that of murine Jak2 indicates that our clone represents the human homolog of the murine Jak2 gene.
Comparison of the predicted amino acid sequence of members of the human Jak family kinases. Alignment was performed with the PILEUP program (Genetics Computer Group, Madison, WI). Identical residues are shown on a black background, and related residues are shaded. Gaps were introduced for optimal alignment and are indicated by hyphens. Boundaries of the Jak homology (JH) domains are denoted by arrows.
Comparison of the predicted amino acid sequence of members of the human Jak family kinases. Alignment was performed with the PILEUP program (Genetics Computer Group, Madison, WI). Identical residues are shown on a black background, and related residues are shaded. Gaps were introduced for optimal alignment and are indicated by hyphens. Boundaries of the Jak homology (JH) domains are denoted by arrows.
Chromosomal localization.
A 1.5-kb DNA fragment corresponding to the 5′ end of the human Jak2 cDNA was used as a probe to localize the chromosomal position of the human Jak2 gene. This probe was chosen in a region of relatively low homology to other members of the Jak family. This probe was used in both FISH analysis and in obtaining a corresponding genomic PAC probe. Images of 20 well-spread metaphase preparations were captured by a thermoelectrically cooled charge-coupled camera, and separate images of DAPI-banded chromosomes and FITC-targeted chromosomes were obtained. Both probes mapped to chromosome 9p23-24 in greater than 95% of the cells.
The human Jak2 gene was previously localized on chromosome 9p24 using the murine Jak2 cDNA as a probe.26 However, the fact that the murine Jak2 gene itself was localized to chromosome 19, which corresponds to human 10q23-24, had cast doubt on the accuracy of the human localization. Our data obtained with a human sequence probe confirm localization of the human Jak2 gene to chromosome 9 (Fig3).
Chromosomal localization of the human Jak2 gene. Positional mapping was performed by FISH to normal human lymphocyte metaphase chromosomes. Both a 1.5-kb Jak2 cDNA probe and a corresponding genomic probe obtained by screening a PAC library showed positive signals in >95% of the cells on both chromatids of each homolog of chromosome 9p23-24, as indicated by the arrows.
Chromosomal localization of the human Jak2 gene. Positional mapping was performed by FISH to normal human lymphocyte metaphase chromosomes. Both a 1.5-kb Jak2 cDNA probe and a corresponding genomic probe obtained by screening a PAC library showed positive signals in >95% of the cells on both chromatids of each homolog of chromosome 9p23-24, as indicated by the arrows.
Human Jak2 mRNA expression in various tissues.
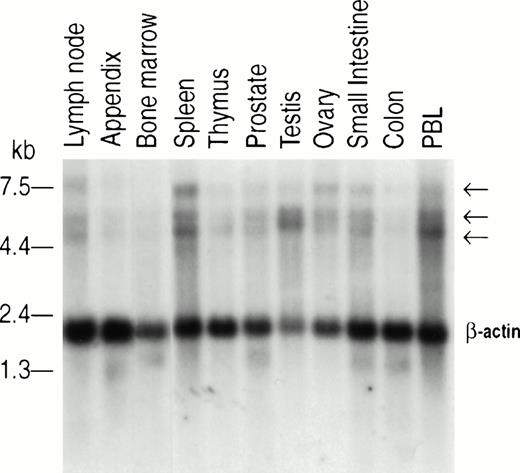
To assess human Jak2 tissue distribution, we used a 400-bp fragment of the human Jak2 cDNA to probe a series of membranes containing polyA+ mRNA from different tissues. This Northern blot analysis revealed the presence of three transcripts of approximately 7.0, 5.4, and 4.8 kb. These transcripts were found to be expressed ubiquitously (Fig 4). Densitometric scanning analysis corrected by reference to the β-actin signal showed that expression of the 7-kb mRNA is low (RNA to actin ratio, 0.01 to 0.05) in the thymus, prostate, testis, small intestine, colon, appendix, lymph node, and bone marrow, whereas the level of this transcript in the spleen and ovary is relatively high (RNA to actin ratio, 0.20 and 0.12, respectively). Similarly, densitometric scanning of the 5.4- and 4.8-kb transcripts showed the highest expression in the testis, spleen, lymph node, and PBLs (RNA to actin ratio, 1.26, 0.63, 0.64, and 0.28, respectively). The thymus and bone marrow showed a RNA to actin ratio of 0.25 and 0.16, respectively. The results suggest there is a higher expression of Jak2 in organs such as the spleen, PBLs, and lymph nodes that contain mature lymphocytes, whereas the thymus and bone marrow, which contain predominately immature lymphocytes, show lower levels of Jak2. Neither the structure nor the significance of the three human Jak2 transcripts are currently known. They may represent the products of alternative splicing or, alternatively, the use of different polyadenylation sites.
Northern blot analysis of human Jak2 expression. Commercial membranes containing 2 μg Poly A+ mRNA per lane of various human tissues (Clontech) were probed with a 400-bp cDNA corresponding to the 3′ end of human Jak2. Hybridization and washing of the membranes were performed according to the manufacturer's specifications (Clontech). β-Actin was used for comparison of RNA loading.
Northern blot analysis of human Jak2 expression. Commercial membranes containing 2 μg Poly A+ mRNA per lane of various human tissues (Clontech) were probed with a 400-bp cDNA corresponding to the 3′ end of human Jak2. Hybridization and washing of the membranes were performed according to the manufacturer's specifications (Clontech). β-Actin was used for comparison of RNA loading.
Jak2 protein expression in hematopoietic cells.
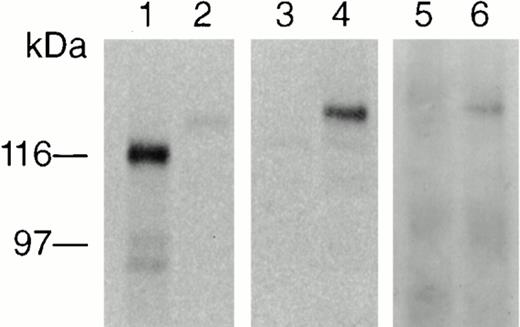
To examine Jak2 protein expression, we generated a polyclonal antibody against a peptide consisting of the 13 amino acids (VLRVDQVRDNMAG) located at the carboxy terminal of the Jak2 protein. This portion of human Jak2 does not exhibit homology with any other member of the human Jak family (Fig 2). The antibody was found to immunoprecipitate a 130-kD protein, which was recognized by commercially available anti-mouse Jak2 antisera from PharMingen (peptide 758), UBI (peptide 758), and Santa-Cruz Biotech (peptide 758). Because the anti-human Jak2 antibody was unable to recognize the denaturated Jak2 protein in Western blots, we used anti-mouse Jak2 (PharMingen) to detect the immunoprecipitated protein. To further confirm the specificity of the anti-human Jak2 antibody, we tested its ability to recognize an in vitro synthesized Jak2 protein. Jak3 and Jak2 cDNAs were added to a combined transcription and translation reticulocyte lysate system, and the products were labeled with (35S) methionine. Aliquots (5 μL) of each product were assayed on SDS-PAGE gel and visualized by autoradiography to examine translation. The reactions yielded two major proteins of 120 and 130 kD corresponding to Jak3 and Jak2 (lanes 1 and 2, Fig5), respectively. Anti-human Jak2 antibody was added to the remaining (45 μL) lysate from each reaction, and the immunoprecipitate was resolved on SDS-PAGE followed by transfer to nitrocellulose membrane and visualization by autoradiography. Only the Jak2 protein was immunoprecipitated by the anti-human Jak2 antibody (Fig 5, lane 4) with no cross-reactivity to Jak3 (Fig 5, lane 3). The immunoprecipitated Jak2 could then be recognized by immunoblotting with the anti-mouse Jak2 antibody (PharMingen) (Fig 5, lane 6).
In vitro translation and detection of Jak2. Approximately 1 μg Jak3 and Jak2 cDNAs were translated in a combined transcription and translation reticulocyte lysate system (Invitrogen) including (35S) methionine. Aliquots of each product were resolved on SDS-PAGE gel, followed by transfer to nitrocellulose and visualization by autoradiography. The reactions yielded proteins of 120 and 130 kD, corresponding to Jak3 and Jak2, respectively (lanes 1 and 2). The remaining lysate of each reaction was precleared with Protein A–Sepharose CL-4B and subsequently immunoprecipitated with anti-human Jak2 antibody, resolved by SDS-PAGE gel, electrotransferred to nitrocellulose membrane, and visualized by autoradiography. Only Jak2 was immunoprecipitated by the anti-human Jak2 antibody with no cross-reactivity to Jak3 (lanes 3 and 4). Only the immunoprecipitated Jak2 in lane 4 was detected by ECL (lane 6) after the same membrane was probed with an anti-mouse Jak2 antibody (PharMingen).
In vitro translation and detection of Jak2. Approximately 1 μg Jak3 and Jak2 cDNAs were translated in a combined transcription and translation reticulocyte lysate system (Invitrogen) including (35S) methionine. Aliquots of each product were resolved on SDS-PAGE gel, followed by transfer to nitrocellulose and visualization by autoradiography. The reactions yielded proteins of 120 and 130 kD, corresponding to Jak3 and Jak2, respectively (lanes 1 and 2). The remaining lysate of each reaction was precleared with Protein A–Sepharose CL-4B and subsequently immunoprecipitated with anti-human Jak2 antibody, resolved by SDS-PAGE gel, electrotransferred to nitrocellulose membrane, and visualized by autoradiography. Only Jak2 was immunoprecipitated by the anti-human Jak2 antibody with no cross-reactivity to Jak3 (lanes 3 and 4). Only the immunoprecipitated Jak2 in lane 4 was detected by ECL (lane 6) after the same membrane was probed with an anti-mouse Jak2 antibody (PharMingen).
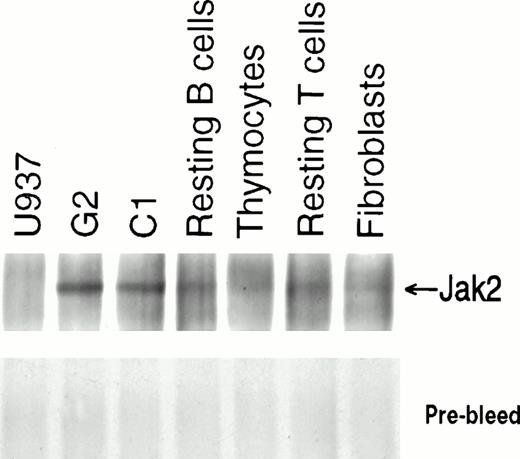
Using the anti-human Jak2 antibody for immunoprecipitation, we demonstrated low levels of Jak2 expression in resting B and T cells separated from PBLs, and even lower levels in thymocytes. As expected, Jak2 expression in pre-B ALL cell lines was high,23 serving here as a positive control, while the monocytic leukemia cell line U937 showed virtually undetectable levels of Jak2, providing a negative control (Fig6). We showed here that the anti-human Jak2 antibody that was produced against the unique C terminus of this enzyme specifically recognizes human Jak2.
Expression of human Jak2 protein. Immunoprecipitates were prepared from lysates containing 750 μg protein from each type of cell with antisera to human Jak2 and subjected to 6% SDS-PAGE. After transfer to nitrocellulose membrane, immunoblotting was performed with anti-Jak2 antibody (PharMingen) and detection was made by ECL.
Expression of human Jak2 protein. Immunoprecipitates were prepared from lysates containing 750 μg protein from each type of cell with antisera to human Jak2 and subjected to 6% SDS-PAGE. After transfer to nitrocellulose membrane, immunoblotting was performed with anti-Jak2 antibody (PharMingen) and detection was made by ECL.
Expression of Jak2 is increased in stimulated lymphocytes.
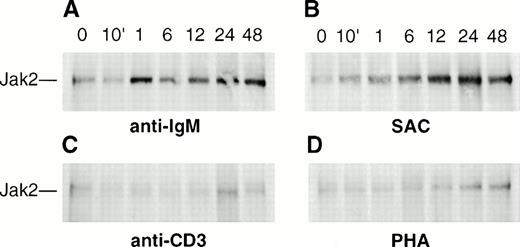
Northern blot analysis of hematopoietic and lymphoid organs suggested relatively high expression of Jak2 in tissues that contain activated lymphocytes. We have therefore examined whether human Jak2 is inducible in stimulated PBLs. There was a significant increase in Jak2 expression in B lymphocytes stimulated with anti-IgM (Fig7A). The time course of anti-IgM–induced B-cell activation showed (by densitometric scanning of the autoradiographs from four separate experiments) that the maximum expression of Jak2, an increase of fourfold, was reached within 1 hour (Fig 7A) with no significant change over the course of 48 hours. These results are in contrast to previous observations by Saouaf et al,38 who showed a threefold to fourfold increase in the level of Jak2 within 5 to 10 minutes of B-cell antigen receptor (BCR) ligation in murine WEHI 231 cells but reported no expression of Jak2 in mature lymphoma B-cell lines. These differences may be explained by a possible aberrant expression of Jak2 in malignant cell lines or interspecies differences between mouse and human tissues. B cells stimulated for 12 hours with SAC showed a sixfold to sevenfold increase in the level of Jak2, with no significant change in this level over the course of 48 hours (Fig 7B). Although there is an increased expression of Jak2 protein, this does not appear to be tyrosine-phosphorylated in either T or B cells (data not shown). The results suggest that the increased expression of Jak2 protein in stimulated B cells might reflect the use of this kinase by growth and differentiation factor receptors needed for the terminal maturation of these cells. In contrast, the time course of Jak2 expression in peripheral T cells stimulated with anti-CD3 or PHA showed a modest twofold increase after 24 hours, with no further change apparent up to 48 hours (Fig 7C and D).
Induction of Jak2 expression in activated peripheral blood B and T lymphocytes. Human B or T cells (2 × 107cells per lane) were incubated for the indicated times with (A) 20 μg/mL anti-IgM antibody, (B) 2 mg/mL SAC, (C) 20 μg/mL anti-CD3 antibody, or (D) 10 μg/mL PHA, respectively. Cell lysates were prepared, followed by immunoprecipitation with anti-human Jak2 and immunoblotting with anti-mouse Jak2 antibody (PharMingen).
Induction of Jak2 expression in activated peripheral blood B and T lymphocytes. Human B or T cells (2 × 107cells per lane) were incubated for the indicated times with (A) 20 μg/mL anti-IgM antibody, (B) 2 mg/mL SAC, (C) 20 μg/mL anti-CD3 antibody, or (D) 10 μg/mL PHA, respectively. Cell lysates were prepared, followed by immunoprecipitation with anti-human Jak2 and immunoblotting with anti-mouse Jak2 antibody (PharMingen).
DISCUSSION
We have cloned the human Jak2 cDNA by a combination of library screening and RT-PCR. The human Jak2 cDNA demonstrates an overall homology of 87% with murine Jak2, and the predicted amino acid sequences are 91% identical. As previously observed for the three other known Jak kinases, human Jak2 contains kinase and kinase-like domains (JH1 and JH2, respectively) at its C terminus along with five other conserved domains (JH3 to JH7). This close homology to the murine sequence suggests that we have isolated the human Jak2 gene. Using the human cDNA as a probe, we localized the human Jak2 to chromosome 9p23-24. Although previous data using the murine Jak2 as a probe had localized the human Jak2 on chromosome 9p24,26 the accuracy of this location had been questioned, as the mouse Jak2 gene is genetically linked to Fas on chromosome 19, which corresponds to human chromosome 10q23-24.1.16 Our data confirm that the human Jak2 gene is indeed located on chromosome 9.
Northern blot analysis of murine Jak2 had previously demonstrated the presence of two transcripts of 5.0 and 5.3 kb in a wide variety of tissues, with the relative level of the two transcripts differing in some tissues.22 Northern blot analysis of human tissues reveals three polyA+ transcripts of 7.0, 5.4, and 4.8 kb in a variety of tissues. At present, it is not possible to explain the biologic significance of the three transcripts. In the absence of further data, it is not clear whether the transcripts are the result of alternative splicing or the use of different promoters or different polyadenylation sites. Analysis of immune relevant tissues showed that Jak2 appears to be primarily expressed in the spleen, lymph nodes, and PBLs, but to a lesser degree in the thymus and bone marrow. This is in sharp contrast to results obtained in mouse tissues, where expression of murine Jak2 mRNA was found to be high in the thymus and much lower in the spleen.22 Our findings suggest that expression of human Jak2 is likely to be more important in mature rather than immature lymphocytes and in lymphoid organs that contain activated lymphocytes.
To analyze Jak2 protein expression in resting and activated lymphocytes, we have generated a polyclonal antibody against the C terminus of Jak2, an area that does not exhibit homology to the other known members of the human Jak family. We examined Jak2 protein expression in peripheral resting T and B cells before and after stimulation with anti-CD3 or PHA and with anti-IgM or SAC, respectively. Using this antibody, we found a striking difference in the pattern of Jak2 expression in mature T and B cells. In both resting T and B cells, Jak2 protein appears to be expressed at low levels. However, whereas activated T cells show a small increase of Jak2 expression, activated B cells exhibit a dramatic increase of Jak2 expression. The increase of Jak2 is probably required to accommodate signal transduction through growth and differentiation factor receptors. Indeed, the cytokine receptors for IL-2, IL-4, IL-5, IL-6, and IL-10 act at various stages in the growth and differentiation of mature B cells and require Jak kinases for signal transduction.12,14,16,34-36 Although most PTKs are not inducible by mitogens, recently yet another Jak kinase, Jak3, was found to be markedly upregulated following stimulation of B cells with SAC or anti-CD40 antibodies,21 and also in PHA-stimulated T cells.19 20
We confirm here that Jak2 is highly expressed in human pre-B leukemia cells (Fig 6). We have previously shown that inhibition of Jak2 activity by a specific tyrosine kinase blocker selectively killed leukemic cells, suggesting that Jak2 activity is essential for the survival of these cells.23 Similar suggestions for the contribution of Jak kinases to transformation were obtained in other systems such as T cells infected with HTLV-I37 and also in hematopoietic malignancy (fly leukemia) inDrosophila.24,25
We report here the cloning and characterization of human Jak2. Although the predicted amino acid sequence of the human cDNA showed 91% overall homology with the murine Jak2, a significant difference in the extreme C-terminal sequence was noted. The gene was localized to chromosome 9p23-24. Northern blot analysis revealed that Jak2 mRNA was highly expressed in the spleen and lymph nodes, which contain activated lymphocytes. In complete agreement with these results, Jak2 protein levels were markedly increased in mitogen-stimulated B lymphocytes and to a lesser degree in T cells, highlighting the need for an enhanced presence of Jak2 to accommodate the lymphokine receptor signal transduction required for terminal differentiation of these cells.
ACKNOWLEDGMENT
We are indebted to Nigel Sharfe for helpful discussion and for reviewing the manuscript, and to Bill Fox for the illustrations. The GenBank accession no. for human Jak2 cDNA is AF001362.
Supported by the Medical Research Council of Canada and the National Cancer Institute of Canada.
Address reprint requests to Chaim M. Roifman, MD, Division of Immunology/Allergy, The Hospital for Sick Children, 555 University Ave, Toronto, Ontario, M5G 1X8.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal