Key Points
Multiple mechanisms drive CD22 antigen escape, including epitope loss (protein truncation and destabilization) and epitope alteration.
Hypermutation caused by error-prone DNA damage repair may serve as a driver of CD22 mutation and escape.
Visual Abstract
Inotuzumab ozogamicin (InO) is an antibody-drug conjugate that delivers calicheamicin to CD22-expressing cells. In a retrospective cohort of InO-treated patients with B-cell acute lymphoblastic leukemia, we sought to understand the genomic determinants of the response and resistance to InO. Pre- and post-InO–treated patient samples were analyzed by whole genome, exome, and/or transcriptome sequencing. Acquired CD22 mutations were observed in 11% (3/27) of post-InO-relapsed tumor samples, but not in refractory samples (0/16). There were multiple CD22 mutations per sample and the mechanisms of CD22 escape included epitope loss (protein truncation and destabilization) and epitope alteration. Two CD22 mutant cases were post-InO hyper-mutators resulting from error-prone DNA damage repair (nonhomologous/alternative end-joining repair, or mismatch repair deficiency), suggesting that hypermutation drove escape from CD22-directed therapy. CD22-mutant relapses occurred after InO and subsequent hematopoietic stem cell transplantation (HSCT), suggesting that InO eliminated the predominant clones, leaving subclones with acquired CD22 mutations that conferred resistance to InO and subsequently expanded. Acquired loss-of-function mutations in TP53, ATM, and CDKN2A were observed, consistent with a compromise of the G1/S DNA damage checkpoint as a mechanism for evading InO-induced apoptosis. Genome-wide CRISPR/Cas9 screening of cell lines identified DNTT (terminal deoxynucleotidyl transferase) loss as a marker of InO resistance. In conclusion, genetic alterations modulating CD22 expression and DNA damage response influence InO efficacy. Our findings highlight the importance of defining the basis of CD22 escape and eradication of residual disease before HSCT. The identified mechanisms of escape from CD22-targeted therapy extend beyond antigen loss and provide opportunities to improve therapeutic approaches and overcome resistance. These trials were registered at www.ClinicalTrials.gov as NCT01134575, NCT01371630, and NCT03441061.
Introduction
Inotuzumab ozogamicin (InO) is a highly active antibody-drug conjugate for the treatment of B-cell acute lymphoblastic leukemia (ALL). InO was designed to deliver the chemotherapeutic payload calicheamicin to cells expressing CD22, a type 1 transmembrane protein that is solely expressed in B-lineage cells.1,2 Calicheamicin is a potent toxin that binds to the DNA minor groove and induces double-strand breaks (DSBs).3 InO elicits high rates of remission and undetectable measurable residual disease and represents an advance in therapeutic options for relapsed/refractory (R/R) B-cell ALL.4-6 Despite the established survival advantage of InO over conventional chemotherapy, some patients are refractory to InO. Even among the responding patients, InO monotherapy is rarely curative. Elucidating the biological and genomic determinants of the response is, therefore, imperative to develop rational strategies to overcome this resistance.
The delivery of the calicheamicin payload requires CD22 binding and internalization. The loss or downregulation of target antigen is one way by which tumor cells escape targeted immunotherapy. For example, truncating mutations and alternative splicing in CD19 have been shown to lead to antigen loss after therapy with CD19–directed chimeric antigen receptor T cell and blinatumomab.7-9 CD22 modulation has been described as a mechanism of resistance to InO.10 Dysregulated CD22 splicing has been reported to be a mechanism of epitope downregulation and resistance to CD22-directed immunotherapies.11 In a phase 2 study of ALL in children, measurable residual disease negativity correlated with sensitivity to calicheamicin.12 However, other potential mechanisms of failure of InO therapy are poorly understood.
Upon the successful delivery of calicheamicin to target cells, the cytotoxicity of InO relies on its ability to produce DSBs, and this DNA damage can activate multiple apoptotic mechanisms.5 The known pathways of DSBs repair include the high-fidelity homologous recombination repair, and error-prone repairs such as nonhomologous end-joining (NHEJ), alternative end-joining (a-EJ), and single-strand annealing (SSA).13 When repairing nonblunt-ended DSBs, such as those induced by InO,14 error-prone repairs result in distinct types of genomic “scar”: NHEJ creates small insertions and deletions; a-EJ creates longer (>10 nucleotides) insertions; SSA is prone to generate deletions and translocations.13
There are multiple DNA damage checkpoints during the cell cycle including the G1/S checkpoint, G2/M checkpoint, and a less well-defined intra-S checkpoint.15 Of these checkpoints, the G1/S checkpoint is unique in depending primarily on the function of p53.15,16 A previous study on cell lines reported that cells surviving InO were mostly blocked in the G2/M phase, suggesting the reliance on the G2/M checkpoint in response to InO–induced DNA damage.17
In this study, we performed a comprehensive investigation of mechanisms of escape from InO therapy. We interrogated patient genomic data to examine CD22 antigen escape strategies, how tumor cells overcome InO-induced DSBs and the use of DNA damage checkpoints during InO resistance. We also used genome-wide CRISPR screens in vitro to identify novel targets of InO resistance.
Methods
Patients and clinical specimens
We studied 85 adult patients with B-cell ALL treated at the MD Anderson Cancer Center. The selection criteria were patients with pre-InO and/or post-InO tumor samples (banked cells/DNA/RNA from bone marrow or peripheral blood-derived tumor cells) available. A subset of the included patients were treated in several clinical studies (NCT01134575, NCT01371630, and NCT03441061), whereas the rest were treated with inotuzumab as the standard of care.
Genomic sequencing
Total stranded transcriptome sequencing (RNA sequencing) was performed using the TruSeq Stranded Total RNA library preparation kit. A low-input RNA library preparation kit (NuGen Ovation V2) was used for samples with limiting material (2-100 ng). The trimmed sequencing reads were mapped with Spliced Transcripts Alignment to a Reference (STAR, v2.7.9a)18 to Genome Reference Consortium Human Build 38 (GRCh38). Whole-exome sequencing of the genomic DNA was performed using the TruSeq DNA Exome library preparation kit (Illumina). Whole-genome sequencing libraries were prepared using a HyperPrep library preparation kit (Roche). The whole-genome and whole-exome sequencing reads were mapped with Burrows-Wheeler Aligner–maximal exact matches (BWA-MEM)19 to GRCh38. Genomic data are publicly available and have been deposited in the European Genome Phenome Archive (accession EGAS50000000067).
Mutation analysis
The variants were called using Mutect2 (v4.1.2.0)20 tumor-only calling approach. Variants passing the filtering steps fulfilled the following criteria: coverage depth of the variant >10, variant allele frequency (VAF) >0.01, alternative allele count ≥4, allele population frequency in public databases <0.01 (The Genome Aggregation Database [gnomadAD], 1000 genomes, The Exome Aggregation Consortium [ExAC], and Exome Sequencing Projects). The variant annotation was performed using ANNOtate VARiation (ANNOVAR).21 Oncoprints were generated using ProteinPaint.22 For each patient, acquired mutations were defined as mutations that were present in post-InO but not in pre-InO, by filtering all mutations identified in post-InO against pre-InO. Clonal evolution was visualized using fish plot.23 Post-InO–acquired mutations were used to analyze post-InO mutational signatures. Mutational signatures were profiled by fitting single nucleotide variation (SNV) and insertion/deletion (indel) counts per 96 trinucleotide contexts to Catalogue Of Somatic Mutations In Cancer (COSMIC) signatures version 3.2. Signatures with <5% overall contribution were excluded from the summary.
CD22 mutation modeling and characterization
The protein structural model of full-length CD22 was obtained from AlphaFold224 and mutations were introduced using FoldX25 to assess the effects on protein stability. The CMV-CD22-IRES-GFP vector was purchased from GeneCopoeia, Inc. CD22 SNV/indel mutations were generated using the Q5 Site-Directed Mutagenesis Kit (New England Biolabs) and the full mutation sequences were validated by Sanger sequencing. CD22 vectors were transfected into HEK-293T cells using FuGENE HD (Promega). Transfected HEK-293T cells were dissociated using TrypLE Express Enzyme (Gibco, Thermo Fisher Scientific) before staining. CD22 binding experiments were performed by flow cytometry using antibodies directed to different CD22 epitopes (InO, SHCL1, or RFB4; supplemental Table 1, available on the Blood website). The flow-gating strategy is shown in supplemental Figure 3. Western blotting was performed using antibodies specific to the N-terminus (clone E7L6Z, Cell Signaling) or C-terminus (polyclonal, Boster Bio catalog no. PB9691) of CD22 and imaged using an Odyssey CLx Imager (LI-COR Biosciences).
Additional details (including genetic subtyping, CD22 splicing analysis, and genome-wide CRISPR/Cas9 screen) are provided in supplemental Methods.
The study was approved by the institutional review boards of the MD Anderson Cancer Center and St. Jude Children's Research Hospital with informed consent.
Results
Patient characteristics and response to InO by subtype
We studied 85 adult patients with R/R (n=81, 95%) or newly diagnosed (n = 4, 4.7%) B-cell ALL, of whom 55 (65%) were treated with InO monotherapy and 30 (25%) with InO in combination with low-intensity chemotherapy (mini-hyper-CVD [low-intensity therapy administered on a hyperfractionated schedule];26 supplemental Table 2). For this retrospective study, the inclusion criterion was the availability of pre-InO and/or post-InO tumor samples, either as banked cells or nucleic acids from the bone marrow or peripheral blood-derived tumor cells. All patients had detectable disease (>5% blasts) in their bone marrow at the time of receiving treatment. Demographic and clinical features are summarized in Table 1. The median age was 42 years (range, 18-84). The median number of prior therapies was 2 (range, 0-5) and 55% of patients received InO as their second or subsequent course of salvage therapy. The cohort was divided into responders (those who achieved complete remission [CR; n = 26] or CR with incomplete hematologic recovery [CRi; n = 25]) and nonresponders (either no response [n = 31] or early death [n = 3]). Thirty-one (61%) responders subsequently underwent allogeneic hematopoietic stem cell transplantation (HSCT) after attaining remission with InO (Figure 1A; supplemental Table 2). Of the 35 patients with post-InO relapse, 11 (31%) relapsed during InO therapy, 7 (20%) after completion of InO without undergoing HSCT, and 17 (49%) after completion InO and after subsequent HSCT (Figure 1B; supplemental Table 2). Samples were obtained at pre-InO only (n = 42), post-InO only (n = 15), or at pre- & post-InO (n = 28) (Figure 1B; supplemental Table 2). The post-InO samples included samples from patients who achieved CR/CRi but subsequently relapsed (n = 27) and patients who were refractory to InO (n = 16).
Patient demographics and clinical characteristic
| . | n (%) . |
|---|---|
| Age, y | |
| Median | 42 |
| Range | 18-84 |
| Sex | |
| Male | 50 (59) |
| Female | 35 (41) |
| Race | |
| African American | 3 (3.5) |
| Asian | 7 (8.2) |
| Hispanic | 30 (35) |
| White | 45 (53) |
| Sample collection | |
| Pre-InO only | 42 |
| Pre- and post-InO | 28 |
| Post-InO only | 15 |
| WBC count, ×109/L | |
| Median | 4.2 |
| Range | 0.1-107.3 |
| Salvage-treatment phase | |
| 0 | 4 (4.7) |
| 1 | 34 (40) |
| 2 | 25 (29) |
| 3 | 9 (11) |
| 4 | 8 (9.4) |
| 5 | 5 (5.9) |
| BM blast percentage, n (%) | |
| Median (range) | 66 (10-97) |
| >5% to ≤50% | 28 (33) |
| >50% | 53 (62) |
| Unknown | 4 (4.7) |
| . | n (%) . |
|---|---|
| Age, y | |
| Median | 42 |
| Range | 18-84 |
| Sex | |
| Male | 50 (59) |
| Female | 35 (41) |
| Race | |
| African American | 3 (3.5) |
| Asian | 7 (8.2) |
| Hispanic | 30 (35) |
| White | 45 (53) |
| Sample collection | |
| Pre-InO only | 42 |
| Pre- and post-InO | 28 |
| Post-InO only | 15 |
| WBC count, ×109/L | |
| Median | 4.2 |
| Range | 0.1-107.3 |
| Salvage-treatment phase | |
| 0 | 4 (4.7) |
| 1 | 34 (40) |
| 2 | 25 (29) |
| 3 | 9 (11) |
| 4 | 8 (9.4) |
| 5 | 5 (5.9) |
| BM blast percentage, n (%) | |
| Median (range) | 66 (10-97) |
| >5% to ≤50% | 28 (33) |
| >50% | 53 (62) |
| Unknown | 4 (4.7) |
Data are presented for 85 patients.
BM, bone marrow; WBC, white blood cell.
Response to inotuzumab. (A) Swimmer plot for InO responders (n = 51). Each bar represents the start of the InO therapy to the last follow-up. Once attaining CR/CRi, patients were off InO therapy, and 31 responders subsequently received HSCT. (B) Flow chart summarizing the types of samples and the outcome of the patients. CR, complete remission; CRi, CR with incomplete hematologic recovery; HSCT, hematopoietic stem cell transplantation.
Response to inotuzumab. (A) Swimmer plot for InO responders (n = 51). Each bar represents the start of the InO therapy to the last follow-up. Once attaining CR/CRi, patients were off InO therapy, and 31 responders subsequently received HSCT. (B) Flow chart summarizing the types of samples and the outcome of the patients. CR, complete remission; CRi, CR with incomplete hematologic recovery; HSCT, hematopoietic stem cell transplantation.
Overall, of the 85 patients, 51 (60%) achieved CR/CRi with InO treatment. We investigated whether B-cell ALL genomic subtypes were associated with response to InO. Genetic subtypes were determined by the analysis of gene rearrangements, DNA copy number alterations, somatic SNV or indel mutations, and gene expression data (supplemental Table 2). The most common (>10%) subtypes were BCR::ABL1-like (29%), BCR::ABL1-positive (14%), low hypodiploid (13%), and KMT2A-rearranged (KMT2A-R; 11%), consistent with the known distribution of genomic subtypes observed in adult B-cell ALL.27 Less frequent subtypes (<5% each) were grouped together as “other subtypes” (n = 23) for outcome analysis and comprised TCF3::PBX1, DUX4-rearranged, BCL2/MYC, CDX2/UBTF, hyperdiploid, MEF2D-rearranged, ZNF384-rearranged, ETV6::RUNX1, ZEB2/CEBP, PAX5 P80R, and unclassified (B-other) cases. The “other subtypes” were predominantly standard-to-intermediate risk based on the molecular classification of risk assessment, whereas BCR::ABL1-like, low hypodiploid, and KMT2A-R were considered high risk.27 As expected, the CR/CRi rates were generally lower in the BCR::ABL1-positive, BCR::ABL1-like, low hypodiploid, and KMT2A-R subtypes (42%-56%) than in the “other subtypes” (83%; supplemental Table 3). Event-free survival (EFS) and overall survival rates varied among subtypes (supplemental Figure 1A-B). Compared with “other subtypes” group, EFS was shorter in BCR::ABL1-positive (P = .02) and low hypodiploid (P = .006), but the differences were not significant in BCR::ABL1-like (P = .5) or KMT2A-R (P = .1).
Given that KMT2A-R ALL may undergo lineage switch after CD19-directed therapy,28-30 we examined the expression of CD22, CD19, and myeloid lineage markers in 3 KMT2A-R patients with post-InO samples available. The KMT2A-R cases demonstrated the post-InO reduced CD22 RNA expression (47%-59% decrease) and the percentage of CD22+ blasts (75%-90% decrease), with no change in the level of CD19 (supplemental Figure 1C). One sample (SJALL079501) had elevated RNA expression of myeloid lineage genes (MPO, ITGAM [CD11b], ANPEP [CD13], CD33, and CD14) and the stem cell marker KIT (CD117), without detectable changes in protein expression by flow cytometry or lineage switch at post-InO relapse. This patient experienced lineage switch and relapse with acute myeloid leukemia after subsequent therapy with blinatumomab. No signs of lineage switch were observed in the other 2 KMT2A-R samples.
Multiple mechanisms of CD22 antigen escape
No somatic CD22 mutations were observed in the pre-InO baseline samples. After InO treatment, acquired CD22 mutations were present in 11% (3/27) of post-InO relapsed samples, but not in post-InO refractory samples (0/16). One patient with acquired CD22 mutations (SJALL058834) was treated with InO monotherapy and the other 2 (SJALL058975 and SJALL074541) with InO in combination with low-intensity chemotherapy. In all 3 cases, relapse occurred after completion of InO and after the subsequent HSCT. There were multiple CD22 mutations per sample (range, 3-4) with a total of 10 mutations identified (Table 2). Mutations occurred in the extracellular and cytoplasmic domains of CD22, with exon 2, which encodes the first of the 7 immunoglobin (Ig) domains, as the mutational hot spot (Figure 2A). The identified mutation types included frameshift indel (n = 4), nonsense SNV (n = 2), missense SNV (n = 3), and stoploss SNV (n = 1) (Figure 2B). The sum of the VAF of CD22 mutations in each sample was proportional to the blast percentage (Table 2), suggesting that all or nearly all cells harbored a CD22 mutation. We identified multiple mechanisms of CD22 antigen escape through genetic mutations as described below, including protein truncation, epitope alteration, and protein destabilization.
Post-InO–acquired CD22 mutations
| Patient . | Subtype . | Relapse day . | Sample day . | Blast % by morphology . | Blast % by MRD flow . | Sum of VAF . | Amino acid change . | Effect . | VAF . |
|---|---|---|---|---|---|---|---|---|---|
| SJALL058975 | DUX4-R | 303 | 303 | 31 | 21 | 0.15 | p.R75T | Missense | 0.03 |
| p.N101fs | Frameshift (truncating) | 0.02 | |||||||
| p.C529Y | Missense | 0.1 | |||||||
| SJALL058834 | Hyperdiploid | 185 | 214 | 80 | 31 | 0.64 | p.Q116X | Nonsense (truncating) | 0.07 |
| p.V136fs | Frameshift (truncating) | 0.07 | |||||||
| p.L178fs | Frameshift (truncating) | 0.41 | |||||||
| p.X848G | Stoploss (addition of 93 amino acids) | 0.09 | |||||||
| SJALL074541 | ETV6::RUNX1 | 191 | 191 | 25 | 10 | 0.13 | p.L104P | Missense | 0.05 |
| p.R433X | Nonsense (truncating) | 0.02 | |||||||
| p.G745fs | Frameshift (truncating) | 0.06 |
| Patient . | Subtype . | Relapse day . | Sample day . | Blast % by morphology . | Blast % by MRD flow . | Sum of VAF . | Amino acid change . | Effect . | VAF . |
|---|---|---|---|---|---|---|---|---|---|
| SJALL058975 | DUX4-R | 303 | 303 | 31 | 21 | 0.15 | p.R75T | Missense | 0.03 |
| p.N101fs | Frameshift (truncating) | 0.02 | |||||||
| p.C529Y | Missense | 0.1 | |||||||
| SJALL058834 | Hyperdiploid | 185 | 214 | 80 | 31 | 0.64 | p.Q116X | Nonsense (truncating) | 0.07 |
| p.V136fs | Frameshift (truncating) | 0.07 | |||||||
| p.L178fs | Frameshift (truncating) | 0.41 | |||||||
| p.X848G | Stoploss (addition of 93 amino acids) | 0.09 | |||||||
| SJALL074541 | ETV6::RUNX1 | 191 | 191 | 25 | 10 | 0.13 | p.L104P | Missense | 0.05 |
| p.R433X | Nonsense (truncating) | 0.02 | |||||||
| p.G745fs | Frameshift (truncating) | 0.06 |
MRD, measurable residual disease.
Multiple mechanisms of CD22 antigen escape. (A) Protein domain plot of all the post-InO–acquired CD22 mutations (n = 10) identified in this cohort. (B) WT and predicted mutant CD22 protein structures. Group by mutation type. (C) Clinical flow cytometry plots of paired pre- and post-InO patient samples. CD22 status (WT or mutations) was labeled below each flow plot. The relative abundance of alternative splicing of CD22 e1 was depicted as stacking plots at the bottom. e1_e2 was the canonical splicing junction, and e1_e3, e1_e3alt, e1_e4, e1_e5, e1_e6, and e1_e7 were alternative splicing junctions. (D) Antibodies directed to different CD22 epitopes. InO and SHCL1 that bind the first Ig domain of CD22, and RFB4 that bind the third. (E-F) Functional characterization of CD22 p.R75T and p.C529Y. CD22 protein expression (by western blot) and binding (by flow cytometry) were performed using antibodies directed to different CD22 epitopes. All controls and CD22 mutants were run in the same experiment of western blot or flow cytometry. The results for CD22 p.R75T and CD22 p.C529Y were presented in seperate panels with the same controls (EV, CD22 WT). (G) Stack plots depicting the relative abundance of alternative splicing of CD22 e1 in pre-InO baseline samples, comparing responders (EFS >12 m and EFS <12 m) and nonresponders. (H) Stack plots depicting the relative abundance of alternative splicing of CD22 e1, comparing pre- and post-InO samples. EV, empty vector; WT, wild-type; m, month.
Multiple mechanisms of CD22 antigen escape. (A) Protein domain plot of all the post-InO–acquired CD22 mutations (n = 10) identified in this cohort. (B) WT and predicted mutant CD22 protein structures. Group by mutation type. (C) Clinical flow cytometry plots of paired pre- and post-InO patient samples. CD22 status (WT or mutations) was labeled below each flow plot. The relative abundance of alternative splicing of CD22 e1 was depicted as stacking plots at the bottom. e1_e2 was the canonical splicing junction, and e1_e3, e1_e3alt, e1_e4, e1_e5, e1_e6, and e1_e7 were alternative splicing junctions. (D) Antibodies directed to different CD22 epitopes. InO and SHCL1 that bind the first Ig domain of CD22, and RFB4 that bind the third. (E-F) Functional characterization of CD22 p.R75T and p.C529Y. CD22 protein expression (by western blot) and binding (by flow cytometry) were performed using antibodies directed to different CD22 epitopes. All controls and CD22 mutants were run in the same experiment of western blot or flow cytometry. The results for CD22 p.R75T and CD22 p.C529Y were presented in seperate panels with the same controls (EV, CD22 WT). (G) Stack plots depicting the relative abundance of alternative splicing of CD22 e1 in pre-InO baseline samples, comparing responders (EFS >12 m and EFS <12 m) and nonresponders. (H) Stack plots depicting the relative abundance of alternative splicing of CD22 e1, comparing pre- and post-InO samples. EV, empty vector; WT, wild-type; m, month.
Frameshift or nonsense mutations lead to CD22 protein truncation and loss
Three frameshift mutations (p.N101fs, p.V136fs, and p.L178fs) and 2 nonsense mutations (p.Q116X and p.R433X) were predicted to generate truncated CD22 proteins lacking the transmembrane domain, and consequent a lack of membrane anchorage and loss of CD22 surface antigen (Figure 2B). Clinical flow cytometry of samples from 2 patients (SJALL074541 and SJALL058834) confirmed that the post-InO samples exhibited almost complete loss of CD22 surface protein (Figure 2C).
Mutational alteration of CD22-InO binding epitope without protein loss
The post-InO sample of SJALL058975 harbored 3 CD22 mutations (CD22 p.R75T, p.N101fs, and p.C529Y), but a loss of CD22 expression at post-InO was not evident by clinical flow cytometry (Figure 2C). We cloned the CD22 p.R75T missense mutation and expressed it in HEK-293T cells, and then measured CD22 protein expression and binding using antibodies directed to different CD22 epitopes (InO and SHCL1, which bind the first Ig domain of CD22, and RFB4, which binds the third Ig domain; Figure 2D). Using immunoblotting, there was no change in the total CD22 protein level, but on flow cytometry, CD22 p.R75T resulted in nearly complete abrogation of InO binding (Figure 2E). The binding of CD22 by the antibody SHCL1 was slightly reduced, and binding by the antibody RFB4 was unchanged (Figure 2E). Protein structure modeling revealed that CD22 p.R75 was surface-exposed on the first Ig domain and the p.R75T mutation was predicted to be not distablizing. Thus, rather than causing a loss of antigen expression, CD22 p.R75T is expected to alter the InO epitope and impair InO binding. Importantly, the antibody used for clinical flow (SHCL1) still recognizes CD22 p.R75T and thus, underestimated the degree of CD22 antigen escape to InO.
Mutational destabilization resulting in loss of CD22 protein
The protein expression of CD22 p.C529Y, also identified in SJALL058975, was much lower than that of wild-type CD22, despite similar transfection rates and vector expression level (IRES-mediated expression of GFP as an internal control; Figure 2F). Flow cytometry confirmed the loss of binding to InO and the antibodies SHCL1 and RFB4. The CD22 protein has a “bead-on-string” structure, with 7 Ig domains as 7 beads, and each of the Ig domains is stabilized by a disulfide bond.31 CD22 p.C529 formed a disulfide bond in the core of the sixth Ig domain and CD22 p.C529Y was predicted to be massively destabilizing using FoldX25 (wild-type Ig6 was predicted to have a free energy of folding of −5.2 kcal/mol and this value for the p.C529Y mutant was predicted to be +23.6 kcal/mol). This explains the low protein level as misfolded proteins are usually degraded by the cellular quality control machinery.
Alternative splicing associated with downregulation of CD22 protein expression
The pre-InO sample of SJALL058975 did not have any CD22 mutations but was partially CD22 positive (65%; Figure 2C), indicating that CD22 protein level was modulated by mechanisms other than genetic mutations. We performed splicing analysis as alternative splicing of exon 1 (e1) to alternative exons or junctions (exon3 [e1_e3], exon3-alt [e1_e3alt], exon4 [e1_e4], exon5 [e1_e5], exon6 [e1_e6], and exon7 [e1_e7]) with skipping of AUG-containing exon 2 has been reported to modulate CD22 protein expression in pediatric B-cell ALL.11 Alternative splicing of CD22 e1 was observed in all patients at the pre-InO baseline at various levels (16%-54%), although, at the cohort level, it did not appear to be a predictor of response to InO (Figure 2G). Compared with pre-InO, an increase in post-InO CD22 e1 alternative splicing was observed (median, 36% [range, 16%-54%] vs 41% [range, 16%-66%]; Figure 2H). Alternative spliced isoforms of e1 comprised 33% of the CD22 transcripts of SJALL058975 at pre-InO (Figure 2C), partly explaining the reduced CD22 positivity. The post-InO sample from patient SJALL058834 had increased alternative splicing (46% of CD22 transcripts) in addition to multiple acquired CD22 mutations (Figure 2C), all of which contributed to post-InO CD22 antigen escape.
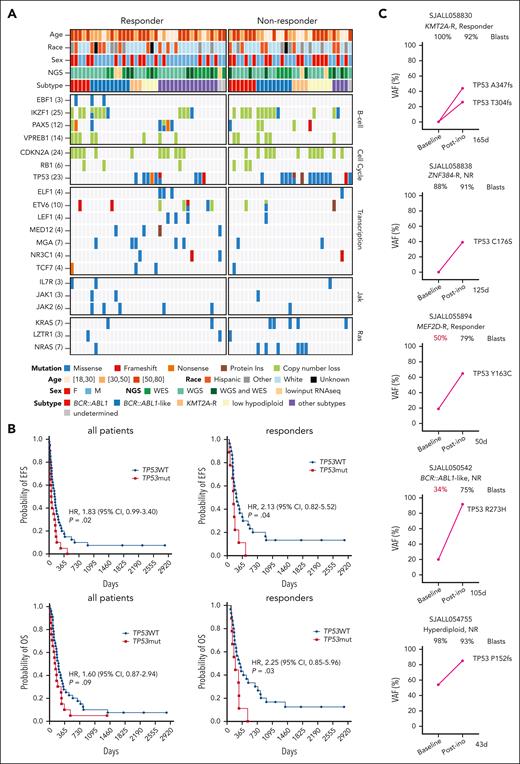
TP53 mutations were associated with primary and acquired resistance to InO
We characterized the pre-InO baseline genomic alterations in genes known to be mutated in leukemia (Figure 3A). Alterations in transcriptional regulators (excluding B-lineage transcription factors) were more common in responders than in nonresponders (54% vs 23%; P = .01). Samples from nonresponders were enriched with alterations in pathways of cell cycle (77% vs 54% in responders; P = .05) and Ras (35% vs 13%; P = .04). Notably, TP53 mutations were more common in nonresponders than in responders at the pre-InO baseline (48% vs 23%; P = .04; Figure 3A). TP53 mutations were identified in 34% (24/70) of the cases with pre-InO samples. Although 9 of TP53-mutated cases (37.5%) attained CR/CRi, 8 relapsed within 12 months, and TP53-mutated cases had shorter EFS and overall survival compared with cases without TP53 mutations (Figure 3B). Among patients who were refractory or relapsed after InO, TP53 mutant clones emerged or expanded, suggesting that TP53 mutations promoted acquired resistance to InO. Of the 14 cases with TP53 mutations in post-InO samples, 2 acquired TP53 mutations post-InO (1 was KMT2A-R, with 2 mutations and a sum of VAF of 0.7; 1 was ZNF384-rearranged, with VAF of 0.39; Figure 3C); 3 had TP53 mutation VAF increased post-InO (Figure 3C); and in 9 cases, mutations were hemizygous and/or maintained high VAF during disease progression (supplemental Figure 2).
TP53 mutations in primary and acquired resistance to InO. (A) Baseline genomic alterations in major leukemia genes, comparing responders and nonresponders. (B) EFS and overall survival in TP53WT vs TP53mut patients. The elapsed observation time is plotted on the curve as a circle. Symbols for both events and censored observations were plotted so that each subject was shown. The censored observations can be clearly seen as circles along the horizontal portion of the curve. The medians were estimated using the Kaplan-Meier method. P values were determined using the log-rank test. (C) Dynamic changes in TP53 VAF in 5 cases with paired pre- and post-InO samples. The VAF for each mutation is shown along with the sample blast count at the corresponding time point. The time elapsed from the start of InO to treatment failure (R/R) was is in days. CI, confidence interval; HR, hazard ratio; mut, mutation; NGS; next-generation sequencing; WES, whole-exome sequencing; WGS, whole-genome sequencing; WT, wild-type.
TP53 mutations in primary and acquired resistance to InO. (A) Baseline genomic alterations in major leukemia genes, comparing responders and nonresponders. (B) EFS and overall survival in TP53WT vs TP53mut patients. The elapsed observation time is plotted on the curve as a circle. Symbols for both events and censored observations were plotted so that each subject was shown. The censored observations can be clearly seen as circles along the horizontal portion of the curve. The medians were estimated using the Kaplan-Meier method. P values were determined using the log-rank test. (C) Dynamic changes in TP53 VAF in 5 cases with paired pre- and post-InO samples. The VAF for each mutation is shown along with the sample blast count at the corresponding time point. The time elapsed from the start of InO to treatment failure (R/R) was is in days. CI, confidence interval; HR, hazard ratio; mut, mutation; NGS; next-generation sequencing; WES, whole-exome sequencing; WGS, whole-genome sequencing; WT, wild-type.
Hypermutation–driven CD22 antigen escape from InO
For cases with paired pre- and post-InO samples, we examined the number and type of post-InO–acquired mutations to investigate which pathways were involved in overcoming InO-induced DNA damages. Single base substitutions (SBSs) were the primary mutation type in all but 1 patient (Figure 4A), suggesting that in most cases, the rapid but indel-prone DSB repair pathway (NHEJ, a-EJ, or SSA) was not primarily used for InO–induced DNA damage; instead, homologous recombination repair, a higher-fidelity mechanism, may have been used. Based on the density histogram of the number of acquired mutations per sample in our cohort (Figure 4A), we divided the patients into post-InO low-mutators (n = 21; 36-252 acquired mutations), moderate-mutators (n = 3; 447-759 acquired mutations), and hyper-mutators (n = 2; 1737-2433 acquired mutations). Patients who were refractory to InO and those who relapsed during InO therapy were all low-mutators; in contrast, patients who relapsed after completion of InO were low-, moderate-, or hyper-mutators (Figure 4A). Notably, 2 hyper-mutators (SJALL058834 and SJALL074541) and 1 moderate-mutator (SJALL058975) acquired multiple CD22 mutations post-InO (Figure 4A-B), suggesting that accelerated acquisition of mutations was a driving force behind CD22 antigen escape to InO.
Post-InO–acquired determinants of resistance. (A) Number (top plot) and OncoPrint (bottom plot) of post-InO–acquired mutations. For each patient, acquired mutations were mutations present in post-InO but not in pre-InO. The dotted lines (at 300 and 900, number of post-InO–acquired mutations) were used as cutoff to divide patients into post-InO low-mutators, moderate-mutators, and hyper-mutators. (B) Fish plots showing acquisition of mutations in CD22 and KMT2D. (C) Mutational signatures of post-InO–acquired mutations in the hyper-mutators SJALL058834 (>10 bp insertions) and SJALL074541 (SBS6 and MMR-deficient). (D) A simplified schematic of the compromised G1/S DNA damage checkpoint by acquired mutations in InO-treated patients. SBS, single base substitution; DBS, doublet base substitution; HSCT, hematopoietic stem cell transplantation; NR, nonresponder. Figure created with BioRender.com.
Post-InO–acquired determinants of resistance. (A) Number (top plot) and OncoPrint (bottom plot) of post-InO–acquired mutations. For each patient, acquired mutations were mutations present in post-InO but not in pre-InO. The dotted lines (at 300 and 900, number of post-InO–acquired mutations) were used as cutoff to divide patients into post-InO low-mutators, moderate-mutators, and hyper-mutators. (B) Fish plots showing acquisition of mutations in CD22 and KMT2D. (C) Mutational signatures of post-InO–acquired mutations in the hyper-mutators SJALL058834 (>10 bp insertions) and SJALL074541 (SBS6 and MMR-deficient). (D) A simplified schematic of the compromised G1/S DNA damage checkpoint by acquired mutations in InO-treated patients. SBS, single base substitution; DBS, doublet base substitution; HSCT, hematopoietic stem cell transplantation; NR, nonresponder. Figure created with BioRender.com.
Mutational signature analysis revealed that hypermutation was caused by InO-induced DSB repair or tumor-intrinsic mismatch repair (MMR) deficiency. The primary mutation type of post-InO–acquired mutations in the hyper-mutator SJALL058834 were indels (86%; Figure 4A), most commonly insertions of >10 nucleotides (74%; Figure 4C). These features were not present in the corresponding pre-InO sample (7% of mutations were indels and 1.7% were insertions >10 nucleotides), indicating that this post-InO hypermutation signature was treatment-induced. Of the 4 DSB repair pathways, long insertions are commonly created by a-EJ and can result from NHEJ in pre-B cells.13,32 Therefore, error-prone DSBs repair of InO–induced DNA damage by a-EJ and/or NHEJ led to hypermutation in SJALL058834. In contrast, the hyper-mutator SJALL074541 acquired SBS6 signature mutations attributable to a defective MMR (Figure 4C). The pre-InO sample harbored a loss-of-function mutation in MLH1 (MLH1 p.L155fs) and was, therefore, tumor-intrinsic MMR-deficient, which was recognized as the cause of hypermutation and the corresponding mutational signature.
Resistance to InO by compromised G1/S checkpoint or mitigation of replication stress
We observed acquired loss-of-function mutations post-InO in genes that regulate the G1/S DNA damage checkpoint in addition to the previously mentioned TP53 mutations (Figure 4D). SJALL058848 acquired a missense mutation, ATM p.V2424G, a known loss-of-function pathogenic variant that impairs ATM kinase activity.33 SJALL058975 acquired a nonsense p.R58X mutation in the CDKN2A tumor suppressor upstream of TP53. Because the G1/S checkpoint is often compromised during tumorigenesis,34 our observations suggested that acquired mutation-induced defects in the G1/S DNA damage checkpoint contribute to InO resistance. Importantly, as tumor cells with compromised G1/S checkpoint depend on the G2/M checkpoint,34 this vulnerability may be exploited through the use of selective molecularly targeted agents (eg, G2/M checkpoint inhibitors) in combination with InO.
Another gene recurrently mutated post-InO was KMT2D (MLL4; n = 3; Figure 4A). SJALL058838 (ZNF384-rearranged) had a pre-existing loss-of-function KMT2D mutation (p.R2678X) and acquired another mutation (KMT2D p.A3292fs) post-InO, resulting in disruption of both KMT2D alleles (Figure 4B). Loss of KMT2D inhibits recruitment of the MRE11 nuclease to stalled replication forks, resulting in the replication fork protection and cell survival by mitigation of replication stress; this is a known resistance mechanism to DNA-damaging agents such as PARP inhibitors.35
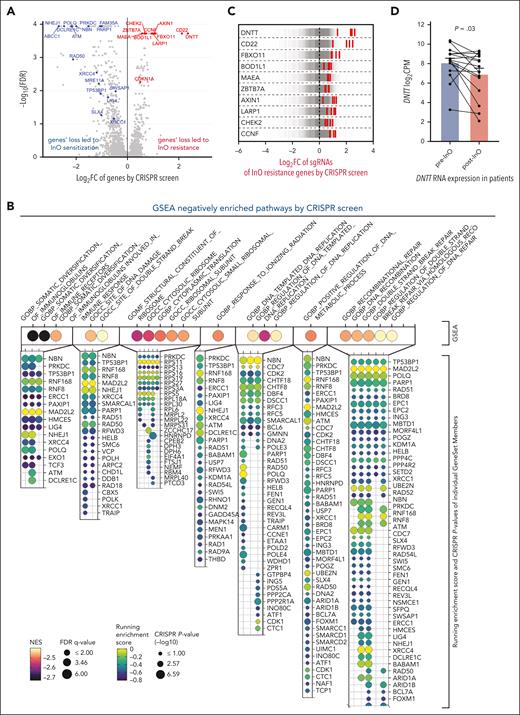
Genome-wide CRISPR screening has identified InO resistance and sensitization targets
Using genome-wide CRISPR/Cas9 screening in the NALM-6 (DUX4-rearranged) cell line, we identified 274 genes whose inactivation led to InO sensitization (negatively selected) and 102 genes whose inactivation led to InO resistance (positively selected; Figure 5A; supplemental Table 4). Gene set enrichment analysis identified multiple pathways relevant to InO sensitization, including DNA damage and DNA repair pathways (Figure 5B). In addition, ABCC1, encoding an ABC transporter protein, was among the top sensitizing targets (Figure 5A), suggesting ABCC1-mediated drug efflux was an important mechanism for InO clearance. CD22 was identified as a resistance gene as expected. Interestingly, DNTT (encoding terminal deoxynucleotidyl transferase [TdT]) came out as the top resistance gene (Figure 5A,C). The canonical function of TdT is to generate lymphocyte antigen receptor diversity by adding nontemplated nucleotides to the DNA 3′terminal ends during V(D)J recombination.36 Recent studies have demonstrated that TdT is a key factor determining the outcome of DSB repair in T-ALL and acute myeloid leukemia cell lines, and DNTT knockout in Jurkat cells shifted insertion-dominant repair to deletion-dominant repair.37 Another study reported that patients with TdT-negative T-cell ALL/lymphoblastic lymphoma had poor therapeutic responses; TdT loss decreased apoptosis, induced the accumulation of chromosomal abnormalities and tolerance of abnormal karyotypes.38DNTT loss might modulate the DSBs repair response to InO and result in increased tolerance of InO–induced DNA damage, leading to InO resistance. We observed decreased DNTT RNA expression in patient samples post-InO (P = .03; mean of different of −1.19 log2 [counts per million]; 95% confidence interval, [−2.23 to −0.11]; Figure 5D); no post-InO–acquired DNTT mutations were identified in this data set. TdT is commonly used in clinical flow and can be prospectively evaluated as a marker of the InO response. Moreover, the loss of CHEK2, the crucial G1/S checkpoint gene, led to InO resistance (Figure 5A,C). This supports the observation and hypothesis from patient data (Figure 4D) that tumor cells resisted InO by compromise of the G1/S DNA damage checkpoint.
Genome-wide CRISPR/Cas9 screening with InO in the NALM-6 cell line (passage 10). (A) Volcano plots showing InO sensitization (negatively selected, in blue) and resistance (positively selected, in red) genes by CRISPR/Cas9 screening. (B) GSEA of negatively enriched pathways. Magma colors and size of the dots represent the normalized enrichment score (NES) and −log10 false discovery rate (FDR) q-values of the top 20 negatively enriched pathways, respectively. The respective gene set members of each pathway are denoted by a bubble plot. Viridis colors and size of the dots represent running enrichment score and −log10 of CRISPR/Cas9 P value respectively. (C) Log2 FC values of sgRNAs targeting the top 10 InO resistance genes. Each gray line represents an individual sgRNA and the central dotted line represents the median log2 FC of the sgRNAs (∼0). Each red line represents the sgRNA of the target gene. Multiple sgRNAs targeting each of the top 10 genes (including CD22) were significantly enriched by inotuzumab, showing an on-target gene knockout by CRISPR/Cas9. (D) DNTT RNA expression in paired patient samples compared with pre- and post-InO. The plot represents the mean ± standard error of the mean for each group. P = .03 by two-tailed paired t test. Samples belonging to the same patient were connected using lines. CPM, counts per million FC, fold change; GSEA, gene set enrichment analysis; sgRNAs, single-guide RNAs.
Genome-wide CRISPR/Cas9 screening with InO in the NALM-6 cell line (passage 10). (A) Volcano plots showing InO sensitization (negatively selected, in blue) and resistance (positively selected, in red) genes by CRISPR/Cas9 screening. (B) GSEA of negatively enriched pathways. Magma colors and size of the dots represent the normalized enrichment score (NES) and −log10 false discovery rate (FDR) q-values of the top 20 negatively enriched pathways, respectively. The respective gene set members of each pathway are denoted by a bubble plot. Viridis colors and size of the dots represent running enrichment score and −log10 of CRISPR/Cas9 P value respectively. (C) Log2 FC values of sgRNAs targeting the top 10 InO resistance genes. Each gray line represents an individual sgRNA and the central dotted line represents the median log2 FC of the sgRNAs (∼0). Each red line represents the sgRNA of the target gene. Multiple sgRNAs targeting each of the top 10 genes (including CD22) were significantly enriched by inotuzumab, showing an on-target gene knockout by CRISPR/Cas9. (D) DNTT RNA expression in paired patient samples compared with pre- and post-InO. The plot represents the mean ± standard error of the mean for each group. P = .03 by two-tailed paired t test. Samples belonging to the same patient were connected using lines. CPM, counts per million FC, fold change; GSEA, gene set enrichment analysis; sgRNAs, single-guide RNAs.
Discussion
CD22 is broadly and uniquely expressed by B-lineage cells and is, therefore, an attractive candidate for targeted therapy in B-cell ALL. Our study shed light on the mechanisms of CD22 antigen escape. Similar to CD19,8,9 CD22 antigen escape may occur through the acquisition of truncating mutations; strikingly, however, this escape may also occur through the alternation of a single amino acid. CD22 missense mutations could be massively destabilizing and result in CD22 protein loss (eg, CD22 p.C529Y) or be epitope-specific without causing CD22 protein loss or affecting binding to other antibodies (eg, CD22 p.R75T). Thus, for a full understanding of the clinical response to CD22-targeted therapy, both epitope loss and epitope alteration need to be taken into consideration, by incorporating multiple CD22 antibodies in clinical flow cytometry and/or deep sequencing of the CD22 locus.
In our cohort, acquired CD22 mutations were only observed in cases that relapsed after InO (rather than being refractory), and CD22 mutations occurred at relatively a low prevalence (11% of the relapsed cases and 7% of all R/R cases), suggesting that alternative mechanisms drive resistance apart from direct mutational perturbation of the target antigen. First, hypermutation by tumor-intrinsic MMR deficiency or InO-induced rapid low-fidelity DNA damage repair drove the accelerated acquisition of mutations, leading to the selection of clones with survival advantages (including CD22 mutations). Second, mutations in crucial genes or pathways can abrogate DNA damage-induced apoptosis, resulting in calicheamicin resistance. TP53 mutations were associated with primary resistance to InO. Notably, new TP53 mutant clones emerged at post-InO treatment, suggesting that TP53 mutations also mediate acquired resistance to InO. Of the DNA damage checkpoints, the G1/S checkpoint is unique in depending primarily on the function of p53.15,16 Post-InO–acquired mutations in other G1/S DNA damage checkpoint genes (ATM and CDKN2A) were observed in patient tumor samples, and loss of CHEK2 resulted in InO resistance in the NALM-6 cell line, suggesting tumor cells evaded InO-induced apoptosis by compromising the G1/S checkpoint. These observations, although small in number, justified further investigations of DNA damage checkpoints for determinants of response to InO. We observed a striking association between DNTT loss and InO resistance in the CRISPR screen, advocating the prospective evaluation of DNTT as a new biomarker of InO resistance.
One limitation worth considering is that some patients received InO in combination with low-intensity chemotherapy; therefore, some relapses may not have been mediated by InO-based mechanisms of resistance (rather, the patients may have become resistant to the backbone chemotherapy). Nevertheless, we observed that 2 of these patients still acquired CD22 mutations after treatment, suggesting InO-mediated resistance during disease recurrence in patients treated with combinational therapy.
In summary, we report multiple mechanisms of mutation-induced CD22 antigen escape, including epitope loss and epitope alteration, identified the genomic features driving CD22 mutagenesis, and described the DNA damage response relevant to calicheamicin resistance. These observations contribute to our understanding of escape strategies within and beyond antigen loss in CD22-targeted therapy, which may lead to improved therapeutic approaches in the future.
Acknowledgments
The authors thank the staff of the Hartwell Center for Bioinformatics and Biotechnology, the Flow Cytometry and Cell Sorting Shared Resource, the Center for Applied Bioinformatics, and the Center for Advanced Genome Engineering at St. Jude Children’s Research Hospital. The authors also thank Michael R. Lieber at the University of Southern California for comments on the manuscript, Shane Marshall and William Greene at St. Jude Children’s Research Hospital for providing inotuzumab reagent for experiment, and Jairo Matthews at MD Anderson Cancer Center for shipping the patient samples.
This study was supported in part by Pfizer, the National Cancer Institute of the National Institutes of Health (award number R35 CA197695) (C.G.M.), Cancer Center Support Grants (P30 CA021765), and ALSAC. C.G.M. is the William E. Evans Endowed Chair at St. Jude Children’s Research Hospital.
Authorship
Contribution: C.G.M., K.G.R., M.K., and E.J.J. conceived and designed the study; N.J.S., E.J.J., M.K., H.M.K., W.M., N.J., B.T., J.K., G.G.-M.,H.Y., R.S.G., and L.F.N. provided patient samples and clinical data; Y.Z., and P.S.G. performed experiments; Y.Z., C.Q., T.-C.C., P.S.G., A.H.P., W.Z., Y.F., R.W.K., and K.G.R. analyzed the data; Y.Z., N.J.S., and C.G.M. prepared the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: N.J.S. received honoraria and consultancy from Pfizer. H.M.K. received research funding from AbbVie, Amgen, Ascentage, Bristol Myers Squibb (BMS), Daiichi Sankyo, ImmunoGen, Jazz, Novartis; and honoraria, advisory board membership, and consultancy from AbbVie, Amgen, Amphista Therapeutics, Ascentage, Astellas, Biologix, Curis, Ipsen Biopharmaceuticals, KAHR Medical, Labcorp, Novartis, Pfizer, Shenzhen Target Rx, Stemline, and Takeda. M.K. received research funding from AbbVie, Allogene, AstraZeneca, Cellectis, DaiichiSankyo, Forty Seven, Genentech, Gilead, MEI Pharma, Precision Bio, Rafael Pharmaceutical, Sanofi, and Stemline-Menarini; and honoraria from AbbVie, AstraZeneca, Auxenion Research, Genentech, Gilead, F. Hoffman-La Roche, Janssen, MEI Pharma, Sellas, and Stemline-Menarini. E.J.J. received research funding and consultancy from Amgen, Pfizer, BMS, Novartis, AbbVie, Kite, Autolous, Genentech, and Ascentage. C.G.M. received research funding from Loxo Oncology, Pfizer, and AbbVie; honoraria from Pfizer, Illumina, and Amgen; and royalty payments from Cyrus. The remaining authors declare no competing financial interests.
Correspondence: Elias J. Jabbour, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; email: ejabbour@mdanderson.org; and Charles G. Mullighan, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 342, Memphis, TN 38105; email: charles.mullighan@stjude.org.
References
Author notes
Y.Z. and N.J.S. are joint first authors.
E.J.J. and C.G.M. are joint senior authors.
Genomic data are publicly available and have been deposited in the European Genome Phenome Archive (accession number EGAS50000000067).
Original data are available upon request from the corresponding author, Charles G. Mullighan (charles.mullighan@stjude.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal