FLC-MS can detect persistent light chains in a significant proportion of patients in a conventional hematologic CR.
Patients with no detectable FLC by FLC-MS have significantly better OS and organ response irrespective of conventional hematologic response.
Visual Abstract
Amyloidogenic serum free light chains (sFLCs) drive disease progression in AL amyloidosis. Matrix-assisted laser desorption/ionization time of flight mass spectrometry–based FLC assay (FLC-MS) has greater sensitivity than conventional sFLC assays allowing for the detection of serological residual disease. We report the utility of FLC-MS in a large series of patients with AL amyloidosis assessing the impact of FLC-MS negativity after treatment on overall survival (OS) and organ response rates. Serum samples were analyzed using FLC-MS at diagnosis and at 6 and 12 months after treatment. The impact of FLC-MS negativity over standard hematologic responses on survival and organ response was assessed. A total of 487 patients were included; 290 (59%) and 349 (71.5%) had cardiac and renal involvement, respectively. There was 100% concordance between the light chain (LC) fibril type and LC isotype identified by FLC-MS. At 6 and 12 months, 81 (16.6%) and 101 (20.7%) were FLC-MS negative. Of those achieving a conventional hematologic complete response (CR) at 6 and 12 months, 45 (27.7%) and 64 (39%) were FLC-MS negative. At 12 months, median OS for CR + FLC-MS negative was not reached vs 108 months in CR + FLC-MS positive (P = .024). At 12 months, 70% of patients with FLC-MS negativity (vs 50% FLC-MS positive) achieved a cardiac response (P = .015). In a multivariate analysis, FLC-MS negativity at 12 months was an independent predictor of better outcomes. FLC-MS can detect persistent monoclonal light chains in a significant proportion of patients in a conventional hematologic CR. FLC-MS assessment promises to be a new standard for response assessment in AL amyloidosis.
Introduction
Light chain amyloidosis (AL amyloidosis) is a condition in which abnormal light chains, produced by an underlying clonal plasma cell/B-cell disorder, misfold and form insoluble amyloid fibrils. Deposition and accumulation of amyloid fibrils in multiple organs results in progressive organ dysfunction.1 The abnormal amyloidogenic free light chains (FLCs) drive the disease in AL amyloidosis. In the absence of therapies targeting the removal of amyloid fibrils, treatment is directed toward the amyloid-producing clonal disorder with the aim of reducing or eliminating FLC production, reducing proteotoxicity, and allowing for natural macrophage-led amyloid regression.2 The depth of hematologic response directly correlates with outcomes in AL amyloidosis.3 Patients who achieve a complete response (CR) have better outcomes, and lately, minimal residual disease (MRD) negativity on bone marrow has been correlated with even better outcomes.4
The greatest step change in the diagnosis and monitoring of AL amyloidosis was the development of assay(s) to measure serum FLC. This continues to be the backbone of response assessment in AL.3 The main limitation of all FLC assays is the lack of ability to directly measure the monoclonal FLC; this is inferred indirectly by a skew in the kappa/lambda ratio. The interpretation of FLC levels is further limited by the impact of a loss of linearity at low concentrations and the impact of renal dysfunction,5 which causes polyclonal retention of FLC, a common problem in AL amyloidosis. With the availability of agents that achieve very deep responses, these flaws become critically limiting in AL amyloidosis. A persistent low level monoclonal FLC, undetectable by current FLC assays, have the potential to cause significant proteotoxicity and lead to ongoing amyloid deposition with progressive organ dysfunction, despite the response being assessed as a conventional hematologic CR.
Recently, detection of monoclonal proteins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) has been a major advance in monoclonal protein detection and monitoring in plasma cell disorders. MALDI-TOF MS was adopted by the International Myeloma Working Group as a potential surrogate for immunofixation electrophoresis (IFE).6-9 The standard intact light chain MALDI-TOF MS assays that have been developed (MASS-FIX and EXENT) to detect total light chains (ie, light chains bound to intact immunoglobulins as well as the FLC together), which will have reduced sensitivity compared with FLC-specific assays.9,10 Therefore, these assays may have limited utility in AL amyloidosis in which the FLC is the disease driver.
We have previously reported a small series of patients analyzed by MALDI-TOF MS using FLC specific reagents (FLC-MS) for detecting the presence of a monoclonal FLC.11-13 The Mayo clinic group found that residual disease was detectable in 2 of 33 (6.1%) and 4 of 33 patients (12.1%) in hematologic CR by MASS-FIX (an assay that does not include FLC specific reagents) and liquid-chromatography MS (LC-MS), respectively. Both these studies reported patients in CR by standard criteria, who still showed evidence of a monoclonal light chain by MS, highlighting persistent residual disease.11,14 The impact of this low-level persistent disease on survival and organ response was not assessed, although the presence of residual disease by MS was associated with a poorer time to progression at 50 months of 75% vs 13% (P = .003), respectively.14
We report here the utility of FLC-MS in serial assessments for a large series of patients with systemic AL amyloidosis with long follow-up showing the impact of achieving FLC-MS negativity after treatment on overall survival (OS) and organ-specific response rates.
Methods
All patients from a prospective observational study of newly diagnosed AL amyloidosis (ALCHEMY), seen at the UK National Amyloidosis Centre, who had serum samples stored at baseline and 6 and 12 months after diagnosis were included. The diagnosis of AL amyloidosis was confirmed by central review of histological material. Amyloid subtype was identified by immunohistochemistry with specific antibodies or by laser capture microdissection and tandem MS, as appropriate. All patients underwent a protocolized serial assessment including assessment of organ function, clonal parameters, and serial echocardiography. Serum FLC were analyzed with the Freelite assay (The Binding-Site, Birmingham, UK) at diagnoses and 6 and 12 months. Amyloidotic organ involvement, hematologic responses to chemotherapy, and organ responses were defined as per the International Society of Amyloidosis (ISA) consensus criteria.3,15-17 A CR was defined as the absence of a monoclonal protein in serum/urine by protein electrophoresis/immunofixation as well as the normalization of the FLC ratio or when the uninvolved FLC was greater than the involved FLC (iFLC). A further analysis based on the CR definition used in the Andromeda trial was also undertaken. This was defined as an iFLC level less than the upper limit of the normal range with negative serum and urine immunofixation.18 All patients were treated with a bortezomib-based chemotherapy regimen (none with daratumumab upfront).
FLC-MS
Serum samples were analyzed by FLC-MS at baseline and 6 and 12 months after diagnosis. Commercially available paramagnetic microparticles were covalently coated with polyclonal sheep antibodies specific for human kappa FLCs (anti-free κ) and lambda FLCs (anti-free λ) (The Binding-Site). The microparticles were incubated with patient sera, washed, and then eluted with acetic acid (5% volume-to-volume ratio), containing tris(2-carboxyethyl)phosphine (20 mM). Mass spectra were acquired on a Microflex LT/SH smart MALDI-TOF mass spectrometer (Bruker, GmbH) over a mass range of 5000 to 32 000 Da. Spectra were visually inspected in the mass-to-charge (m/z) region for the doubly charged light chain (m/z, 11 000-13 000) and singly charged light chain (m/z, 22 000-26 000). Reviewers were blinded to the results of the assessments using standard techniques at the time of analyzing the mass spectra. The presence of N-linked glycosylation was identified by the detection of polytypic peaks with mass differences between the peaks consistent with N-linked glycans. Confirmation that these polytypic peaks represented glycosylated FLC was also obtained by incubating samples from 5 of the patients with peptide N-glycosidase F, which is an enzyme which cleaves N-linked glycans.19 Patients with evidence of a monoclonal spike were denoted as ‘FLC-MS positive’ and those without a monoclonal spike were denoted as “FLC-MS negative.”
Statistical analysis
Statistical analysis was performed using SPSS version 27. Approval for analysis and publication was obtained from the National Health Service institutional review board; written consent was obtained from all patients in accordance with the Declaration of Helsinki. The Kaplan-Meier method was used to analyze survival outcomes. The analysis in this series is automatically a landmark analysis because only patients with baseline and 2 follow-up samples at 6 and 12 months were included. Multivariate modeling by Cox regression analysis was performed on factors found to significantly affect survival on univariate analysis. All factors that were significant in univariate modeling were studies in various multivariate models. Mayo staging system (European modification of 2004 and Mayo 2012) were studied in separate models. Two tailed unpaired t tests were used to compare continuous variables, whereas analysis of variance was used when >2 variables were included. All P values were 2-sided with a significance level of <.05.
Results
Baseline characteristics
A total of 487 patients were included in this study. The baseline characteristics are outline in Table 1. A total of 291 patients (59.6%) were male with median age at diagnosis of 67 (range, 36-88) years. There was cardiac and renal involvement in 290 (59.4%) and 349 (71.5%) patients, respectively, with 256 (52.4%) having ≥2 organs involved. Cardiac disease stage (by European Modification of Mayo 2004 staging20) was 1, 2, 3a, and 3b in 101 (20.7%), 177 (36.5%), 168 (34.5%), and 39 patients (8%), respectively. The Eastern Cooperative Oncology Group performance status was ≥2 in 108 patients (22.2%). Median iFLC at diagnosis was 197 mg/L (range, 11.6-15 900 mg/L) and median difference between the involved and uninvolved FLC (dFLC) was 177.7 mg/L (range, 0-15 898 mg/L).
Baseline characteristics
| . | Total cohort (n = 487), (%)/median (range) . | FLC-MS negative (n = 112) . | FLC-MS positive (n = 375) . | P value . |
|---|---|---|---|---|
| Male | 291 (59.6%) | 55 (49.1%) | 236 (62.9%) | .009 |
| Age, y | 67 (36-88) | 65 (40-88) | 67 (36-86) | .62 |
| Detectable M-protein by SPEP/IFE | ||||
| None | 126 (26%) | 44 (39.3%) | 82 (21.9%) | |
| Immunofixation only | 122 (25%) | 19 (17.0%) | 103 (27.5%) | |
| SPEP, g/L, median (range) | 239 (49.0%); 8 g/L (1-45) | 49 (43.8%) 8 g/L (2-31) | 190 (50.7%) 8 g/L (1-45 g/L) | .907 |
| M-protein type | ||||
| A | 70 (14.3%) | 10 (8.9%) | 60 (16%) | .06 |
| D | 3 (0.6%) | 0 (0%) | 3 (0.8%) | |
| G | 163 (33.4%) | 41 (36.6%) | 122 (32.5%) | .422 |
| M | 13 (2.7%) | 2 (1.8%) | 11 (2.9%) | .509 |
| LC | 111 (22.7%) | 15 (13.4%) | 96 (25.6%) | .007 |
| Serum light chain type | ||||
| Kappa | 89 (18.2%) | 35 (31.3%) | 55 (14.6%) | |
| Lambda | 396 (81.1%) | 76 (67.9%) | 319 (85.1%) | ≤.0001 |
| Involved light chain, mg/L | 197 (11.6-15 900) | 145 (11.8-2 211) | 208 (11.6-15 900) | .065 |
| dFLC, mg/L | 177.7 (0-15 898) | 125 (0-2203) | 186 (2.1-15 898) | .061 |
| Organ involvement | ||||
| Renal | 349 (71.5%) | 83 (74.1%) | 266 (70.9%) | .513 |
| Creatinine, μmol/L | 94 (27-777) | 104 (33-609) | 91 (27-777) | .243 |
| eGFR, mL/min per 1.73 m2 | 67 (15 to >90) | 61.5 (15 to >90) | 69 (15 to >90) | .017 |
| 24 h urinary protein, g/24 h | 3.1 (0.1-31.6) | 3.4 (0.1-19.6) | 3.1 (0.1-31.6) | .581 |
| Heart | 290 (59.4%) | 61 (54.5%) | 229 (61.1%) | .212 |
| NTProBNP, pg/ml | 1548 (12-44 611) | 1841 (12-34 082) | 1522 (34-44 611) | .121 |
| Bilirubin, mg/dL | 5 (2-61) | 5 (2-25) | 6 (2-61) | .246 |
| Alkaline phosphatase, U/L | 85 (26-1 035) | 138 (39-734) | 82 (26-1 035) | .195 |
| LV septum, mm | 13 (6-22) | 12 (7-21) | 13 (8-22) | .032 |
| LVEF, % | 60% (11-80) | 59 (11-77) | 60 (16-80) | .278 |
| Cardiac disease stage European Modification of Mayo 2004 | ||||
| 1 | 99 (20.3%) | 29 (25.9%) | 72 (19.2%) | .125 |
| 2 | 178 (36.5%) | 30 (26.8%) | 146 (38.9%) | .019 |
| 3A | 167 (34.2%) | 41 (36.6%) | 126 (33.6%) | .556 |
| 3B | 39 (8%) | 11 (9.8%) | 28 (7.4%) | .420 |
| Missing | 4 (0.8%) | 1 (0.9%) | 3 (0.8%) | .924 |
| Cardiac disease stage Mayo 2012 | ||||
| 1 | 71 (14.5%) | 24 (21.4%) | 47 (11.7%) | .019 |
| 2 | 123 (25.2%) | 26 (23.2%) | 97 (25.8%) | .571 |
| 3 | 138 (28.3%) | 25 (22.3%) | 113 (20.1%) | .107 |
| 4 | 116 (23.8%) | 26 (23.2%) | 90 (24%) | .864 |
| Missing | 39 (8.0%) | 11 (9.8%) | 28 (7.5%) | .420 |
| Other organs | ||||
| Liver | 50 (10.2%) | 19 (17.0%) | 31 (8.3%) | .008 |
| Peripheral neuropathy | 29 (5.9%) | 6 (5.4%) | 23 (6.1%) | .761 |
| Autonomic neuropathy | 29 (5.9%) | 4 (3.6%) | 25 (6.7%) | .224 |
| Soft tissue | 80 (16.4%) | 14 (12.5%) | 66 (17.6%) | .201 |
| GI | 15 (3.1%) | 1 (0.8%) | 14 (0.4%) | .127 |
| Other | 4 (0.8%) | 0 | 4 (0.01%) | .272 |
| Number of organs involved | ||||
| 1 | 231 (47.3%) | 56 (50%) | 175 (46.7%) | .535 |
| 2 | 170 (34.8%) | 39 (34.8%) | 131 (34.9%) | .983 |
| 3 | 71 (14.5%) | 15 (13.4%) | 56 (14.9%) | .685 |
| 4 | 15 (3.1%) | 2 (1.7%) | 13 (3.5%) | .366 |
| . | Total cohort (n = 487), (%)/median (range) . | FLC-MS negative (n = 112) . | FLC-MS positive (n = 375) . | P value . |
|---|---|---|---|---|
| Male | 291 (59.6%) | 55 (49.1%) | 236 (62.9%) | .009 |
| Age, y | 67 (36-88) | 65 (40-88) | 67 (36-86) | .62 |
| Detectable M-protein by SPEP/IFE | ||||
| None | 126 (26%) | 44 (39.3%) | 82 (21.9%) | |
| Immunofixation only | 122 (25%) | 19 (17.0%) | 103 (27.5%) | |
| SPEP, g/L, median (range) | 239 (49.0%); 8 g/L (1-45) | 49 (43.8%) 8 g/L (2-31) | 190 (50.7%) 8 g/L (1-45 g/L) | .907 |
| M-protein type | ||||
| A | 70 (14.3%) | 10 (8.9%) | 60 (16%) | .06 |
| D | 3 (0.6%) | 0 (0%) | 3 (0.8%) | |
| G | 163 (33.4%) | 41 (36.6%) | 122 (32.5%) | .422 |
| M | 13 (2.7%) | 2 (1.8%) | 11 (2.9%) | .509 |
| LC | 111 (22.7%) | 15 (13.4%) | 96 (25.6%) | .007 |
| Serum light chain type | ||||
| Kappa | 89 (18.2%) | 35 (31.3%) | 55 (14.6%) | |
| Lambda | 396 (81.1%) | 76 (67.9%) | 319 (85.1%) | ≤.0001 |
| Involved light chain, mg/L | 197 (11.6-15 900) | 145 (11.8-2 211) | 208 (11.6-15 900) | .065 |
| dFLC, mg/L | 177.7 (0-15 898) | 125 (0-2203) | 186 (2.1-15 898) | .061 |
| Organ involvement | ||||
| Renal | 349 (71.5%) | 83 (74.1%) | 266 (70.9%) | .513 |
| Creatinine, μmol/L | 94 (27-777) | 104 (33-609) | 91 (27-777) | .243 |
| eGFR, mL/min per 1.73 m2 | 67 (15 to >90) | 61.5 (15 to >90) | 69 (15 to >90) | .017 |
| 24 h urinary protein, g/24 h | 3.1 (0.1-31.6) | 3.4 (0.1-19.6) | 3.1 (0.1-31.6) | .581 |
| Heart | 290 (59.4%) | 61 (54.5%) | 229 (61.1%) | .212 |
| NTProBNP, pg/ml | 1548 (12-44 611) | 1841 (12-34 082) | 1522 (34-44 611) | .121 |
| Bilirubin, mg/dL | 5 (2-61) | 5 (2-25) | 6 (2-61) | .246 |
| Alkaline phosphatase, U/L | 85 (26-1 035) | 138 (39-734) | 82 (26-1 035) | .195 |
| LV septum, mm | 13 (6-22) | 12 (7-21) | 13 (8-22) | .032 |
| LVEF, % | 60% (11-80) | 59 (11-77) | 60 (16-80) | .278 |
| Cardiac disease stage European Modification of Mayo 2004 | ||||
| 1 | 99 (20.3%) | 29 (25.9%) | 72 (19.2%) | .125 |
| 2 | 178 (36.5%) | 30 (26.8%) | 146 (38.9%) | .019 |
| 3A | 167 (34.2%) | 41 (36.6%) | 126 (33.6%) | .556 |
| 3B | 39 (8%) | 11 (9.8%) | 28 (7.4%) | .420 |
| Missing | 4 (0.8%) | 1 (0.9%) | 3 (0.8%) | .924 |
| Cardiac disease stage Mayo 2012 | ||||
| 1 | 71 (14.5%) | 24 (21.4%) | 47 (11.7%) | .019 |
| 2 | 123 (25.2%) | 26 (23.2%) | 97 (25.8%) | .571 |
| 3 | 138 (28.3%) | 25 (22.3%) | 113 (20.1%) | .107 |
| 4 | 116 (23.8%) | 26 (23.2%) | 90 (24%) | .864 |
| Missing | 39 (8.0%) | 11 (9.8%) | 28 (7.5%) | .420 |
| Other organs | ||||
| Liver | 50 (10.2%) | 19 (17.0%) | 31 (8.3%) | .008 |
| Peripheral neuropathy | 29 (5.9%) | 6 (5.4%) | 23 (6.1%) | .761 |
| Autonomic neuropathy | 29 (5.9%) | 4 (3.6%) | 25 (6.7%) | .224 |
| Soft tissue | 80 (16.4%) | 14 (12.5%) | 66 (17.6%) | .201 |
| GI | 15 (3.1%) | 1 (0.8%) | 14 (0.4%) | .127 |
| Other | 4 (0.8%) | 0 | 4 (0.01%) | .272 |
| Number of organs involved | ||||
| 1 | 231 (47.3%) | 56 (50%) | 175 (46.7%) | .535 |
| 2 | 170 (34.8%) | 39 (34.8%) | 131 (34.9%) | .983 |
| 3 | 71 (14.5%) | 15 (13.4%) | 56 (14.9%) | .685 |
| 4 | 15 (3.1%) | 2 (1.7%) | 13 (3.5%) | .366 |
Significant P values are in bold type. GI, gastrointestinal; LV, left ventricular; LVEF, left ventricular ejection fraction; SPEP, serum protein electrophoresis.
Patients who were female and of a κ isotype were more likely to become FLC-MS negative at any point after therapy. Patients with a higher iFLC or dFLC at baseline were less likely to become FLC-MS negative, possibly reflecting a higher disease burden at baseline, although not statistically significant. There was no difference in disease severity based on Mayo staging between patients with FLC-MS positive and those with FLC-MC negative.
Concordance between FLC-MS and FLC by standard assays
A total of 483 patients (99.2%) had a monoclonal FLC peak identified using FLC-MS. Four patients had no clear monoclonal FLC isotype identified by FLC-MS, including 2 patients who had an atypical spectrum, 1 had no detectable clone in serum by FLC-MS and Freelite assay but identified as AL λ type on a renal biopsy by laser capture MS, and 1 patient had an insufficient volume for diagnostic testing. In patients who were assessable, FLC isotype detected by FLC-MS was 100% concordant with amyloid fibril type on tissue biopsy (4 patients as above were not assessable). Twenty-six patients (5.3%) had a dual κ and λ isotype identified by FLC-MS. The presence of N-linked glycosylation was identified in 73 patients (15%); 39 of 90 (37.8%) were κ, and 34 of 396 (8.6%) were λ.
Hematologic response and FLC-MS
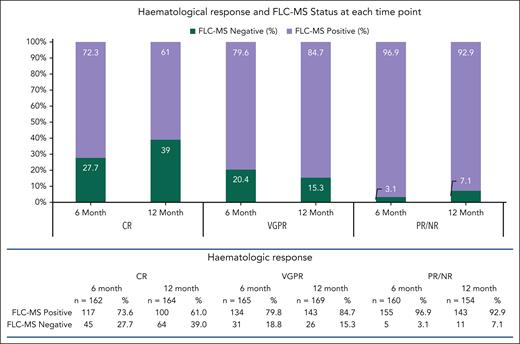
At the 6- and 12-month time points, by standard ISA criteria, a hematological CR was seen in 162 (33.2%) and 164 (33.7%) patients, a very good partial response (VGPR) in 165 (33.9%) and 169 (34.7%), and partial response (PR) or less in 160 (32.8%) and 154 (31.6%), respectively. By FLC-MS, 81 (16.6%) and 101 patients (20.7%) at 6 and 12 months were FLC-MS negative. Of those achieving a CR at 6 and 12 months, 45 (27.7%) and 64 patients (39%) were FLC-MS negative (Figure 1), respectively. At 12 months, only 87 of 164 patients (53.0%) were in a CR by Andromeda criteria, and of whom, 40 of 87 (46.0%) were FLC-MS negative by ISA criteria. At 6 and 12 months, FLC-MS negativity was noted in 31 (18.8%) and 26 (15.3%) for those in VGPR, respectively, and in 5 (3.1%) and 11 (7.1%) for those achieving PR or who were nonassessable by standard FLC.
Hematologic response at 6- and 12-month time points and FLC-MS status.
Of the patients in a VGPR and FLC-MS negative at 6- and 12-month time points, 2 (5.8%) and 2 (7.7%) had a dFLC >40 mg/L; 18 (52.9%) and 17 (65.4%) had dFLC >10 but <40 mg/L; 14 (41.2%) and 7 (26.9%) had dFLC <10 mg/L, respectively. At 6 and 12 months, 14 (41.2%) and 16 (61.5%) had the presence of an intact paraprotein detected by immunofixation, and 1 (2.94%) and 3 (11.5%) had a Bence Jones protein (BJP) detected, respectively. At 6 and 12 months, 16 (47.1%) and 9 patients (34.6%), respectively, had no evidence of BJP or paraprotein on IFE but were considered a VGPR due to an abnormal FLC ratio. Data for patients classed as PR by ISA criteria appear in the supplemental Material, available on the Blood website.
A total of 199 and 167 patients achieved a dFLC <10 mg/L at 6 and 12 months, respectively, of whom 50 (25.1%) and 48 (28.7%) were FLC-MS negative. Similarly, an iFLC <20 mg/L was seen in 154 and 101 patients at 6 and 12 months, with FLC-MS negativity in 45 (29.2%) and 38 (37.6%), respectively.
Organ response
A total of 290 patients (59.5%) had cardiac involvement at diagnosis, with 261 and 237 assessable for cardiac response at 6 and 12 months, respectively. Overall, at 6 and 12 months, 71 of 261 (27.2%) and 128 of 237 (54%) had a cardiac response, respectively. In patients reaching FLC-MS negativity, 15 of 39 (38%) and 31 of 44 (70%) had a cardiac response at 6 and 12 months, respectively (P = .08 vs P = .015 for FLC-MS positive). In accordance with the new graded cardiac response criteria,21 a cardiac CR in patients with FLC-MS negativity (vs positivity) at 12 months was achieved in 19.6% (vs 9.0%). By logistic regression, being FLC-MS negative (vs positive) at 12 months had a hazard ratio (HR) of 2.7302 (95% confidence interval, 1.2035-6.1932; P = .0162) for achieving a cardiac response.
For those in an Andromeda CR, 57 of 87 (65.5%) had cardiac involvement at diagnosis, and 40 (70.2%) had a cardiac response at 12 months. A cardiac response was seen in 17 of 23 (73.9%) who were FLC-MS negative vs 23 of 34 (67.6%) (P = .681) who were FLC-MS positive. Of those in an ISA CR but not Andromeda CR, a cardiac response was seen in 5 of 9 (55.5%) vs 12 of 30 (40%) (P = .409).
A total of 349 patients (71.1%) had renal involvement at diagnosis, with 303 and 313 patients assessable for a renal response at 6 and 12 months, respectively. Overall, at 6 and 12 months, 67 of 302 (22.2%) and 82 of 313 (26.2%) had a renal organ response, respectively. Of the patients reaching FLC-MS negativity, 15 of 58 (26%) and 25 of 66 (38%) had a renal response at 6 and 12 months, respectively (P = .45 and P = .015 compared with those with FLC-MS positivity).
Survival
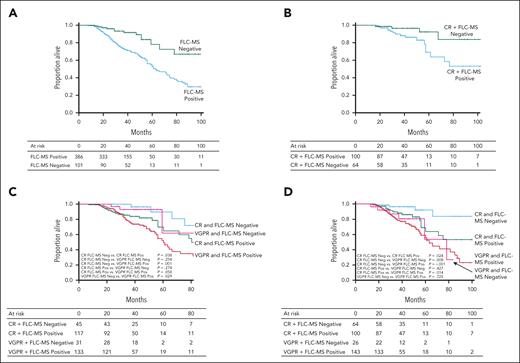
The median OS of the cohort was 72 months, and for patients achieving CR, VGPR, and PR, it was 110, 66, and 59 months, respectively. Median OS of patients who were FLC-MS negative vs positive (at both 6 and 12 months) was not reached (NR) vs 63 months (P ≤ .001), respectively (Figure 2A). At 3 and 5 years, 89% (vs 67%) and 73% patients (vs 45%) who were FLC-MS negative (vs positive) were alive.
Overall survival stratified by FLC-MS positivity or negativity: (A) OS from diagnosis based on FLC-MS status at 12-month landmark analysis; median OS, NR vs 63 months (P ≤ .001) in those with FLC-MS negative vs positive. (B) OS from diagnosis based on FLC-MS status at 12-month landmark analysis in patients who achieved a hematologic CR; median OS was NR vs 108 months (P = .009) in those with FLC-MS negative vs positive. (C) Six month analysis of OS from diagnosis by hematologic response combined with FLC-MS Status. CR – FLC MS negative, median OS NR, CR – FLC MS positive 80 months, VGPR – FLC MS negative 85 months, VGPR – FLC MS positive 60 months. (D) Twelve month analysis of OS from diagnosis by hematologic response combined with FLC-MS Status. CR + FLC-MS negative, median OS, NR; CR + FLC-MS positive, 108 months; VGPR, FLC-MS negative, 78 months; VGPR + FLC-MS positive, 63 months.
Overall survival stratified by FLC-MS positivity or negativity: (A) OS from diagnosis based on FLC-MS status at 12-month landmark analysis; median OS, NR vs 63 months (P ≤ .001) in those with FLC-MS negative vs positive. (B) OS from diagnosis based on FLC-MS status at 12-month landmark analysis in patients who achieved a hematologic CR; median OS was NR vs 108 months (P = .009) in those with FLC-MS negative vs positive. (C) Six month analysis of OS from diagnosis by hematologic response combined with FLC-MS Status. CR – FLC MS negative, median OS NR, CR – FLC MS positive 80 months, VGPR – FLC MS negative 85 months, VGPR – FLC MS positive 60 months. (D) Twelve month analysis of OS from diagnosis by hematologic response combined with FLC-MS Status. CR + FLC-MS negative, median OS, NR; CR + FLC-MS positive, 108 months; VGPR, FLC-MS negative, 78 months; VGPR + FLC-MS positive, 63 months.
The median OS in patients who were FLC-MS negative + ISA CR vs FLC-MS positive + ISA CR at 6 months was NR vs 80 months (P = .026); and at 12 months was NR vs 108 months (P = .024) (Figure 2B). In the Andromeda CR cohort, the median OS was better in those who did achieve FLC-MS negativity at 12 months (NR vs 110 months; P = .381), although not significant. In those who achieved an ISA CR (without Andromeda CR), a median OS was NR vs 56 months (FLC-MS negative vs positive; P = .116) (supplemental Figures 1 and 2)
For patients with VGPR, median OS at 6 months was 85 vs 60 months (P = .055) and at 12 months was 78 vs 63 months (P = .725) (Figure 2C-D). Patients who had dFLC <10 mg/L or iFLC <20 mg/L and were FLC-MS negative also had a significant improvement in survival compared with those who were FLC-MS positive (supplemental Figures 3 and 4).
The univariate analysis of the factors affecting survival is shown in Table 2. A multivariate model using cardiac disease stage and depth of response showed advanced cardiac disease stage and achieving FLC-MS negativity at 12 months were independently predictive of better outcomes. The HR incrementally increased with depth of response as graded by standard ISA criteria plus FLC-MS negativity/positivity (CR + FLC-MS negative–reference; CR-FLC-MS positive HR, 3.6; VGPR-FLC-MS negative HR, 5.08; VGPR-FLC-MS positive HR, 6.07; and PR or worse HR, 9.51) (Table 3).
Univariate analysis of factors affecting OS
| Univariate . | P . | HR (95% CI) . |
|---|---|---|
| Sex (female) | .067 | 0.726 (0.515-1.02) |
| Age | <.0001 | 1.033 (1.016-1.051) |
| iFLC∗ | .009 | 1.484 (1.111-1.981) |
| dFLC∗ | .029 | 1.321 (1.028-1.697) |
| NTProBNP∗ | <.0001 | 1.87 (1.447-2.416) |
| Creatinine∗ | <.0001 | 3.294 (1.852-5.858) |
| Bilirubin | .267 | 1.353 (0.793-2.309) |
| ALP | .861 | 0.948 (0.522-1.721) |
| Mayo 2004 staging with European modification | ||
| Stage 1 | Ref | |
| Stage 2 | .378 | 1.274 (0.744-2.18) |
| Stage 3a | <.0001 | 2.522 (1.529-4.161) |
| Stage 3b | .001 | 2.916 (1.533-5.545) |
| Mayo 2012 staging | ||
| Stage 1 | Ref | |
| Stage 2 | .194 | 1.592 (0.789-3.211) |
| Stage 3 | <.0001 | 3.646 (1.892-7.028) |
| Stage 4 | <.0001 | 3.654 (1.895-7.045) |
| Organ involvement | ||
| Cardiac | .011 | 1.575 (1.112-2.23) |
| Renal | .352 | 1.188 (0.826-1.708) |
| Liver | .753 | 0.925 ( 0.57-1.502) |
| Hematologic response at 12 mo | ||
| CR | Ref | |
| VGPR | .001 | 2.343 (1.409-3.895) |
| PR | <.0001 | 3.692 (2.196-6.208) |
| NR | <.0001 | 4.219 (1.932-9.213) |
| FLC-MS status, FLC-MS positive vs FLC-MS negative | <.0001 | 3.098 (1.752-5.478 |
| Combined hematologic response and FLC-MS status | ||
| 12-mo CR and FLC-MS negative | Ref | |
| 12-mo CR and FLC-MS positive | .019 | 3.37 (1.146-9.915) |
| 12-mo VGPR and FLC-MS negative | .015 | 4.801 (1.353-17.041) |
| 12-mo VGPR and FLC-MS positive | .002 | 6.054 (2.171-16.883) |
| 12-mo PR/NR | <.0001 | 10.15 (3.710-27.766) |
| Further response | ||
| 12-mo iFLC >20 mg/L vs <20 mg/L | <.0001 | 3.035 (1.845-4.992) |
| 12-mo dFLC >10 mg/L vs <10 mg/L | <.0001 | 2.108 (1.454-3.05) |
| Univariate . | P . | HR (95% CI) . |
|---|---|---|
| Sex (female) | .067 | 0.726 (0.515-1.02) |
| Age | <.0001 | 1.033 (1.016-1.051) |
| iFLC∗ | .009 | 1.484 (1.111-1.981) |
| dFLC∗ | .029 | 1.321 (1.028-1.697) |
| NTProBNP∗ | <.0001 | 1.87 (1.447-2.416) |
| Creatinine∗ | <.0001 | 3.294 (1.852-5.858) |
| Bilirubin | .267 | 1.353 (0.793-2.309) |
| ALP | .861 | 0.948 (0.522-1.721) |
| Mayo 2004 staging with European modification | ||
| Stage 1 | Ref | |
| Stage 2 | .378 | 1.274 (0.744-2.18) |
| Stage 3a | <.0001 | 2.522 (1.529-4.161) |
| Stage 3b | .001 | 2.916 (1.533-5.545) |
| Mayo 2012 staging | ||
| Stage 1 | Ref | |
| Stage 2 | .194 | 1.592 (0.789-3.211) |
| Stage 3 | <.0001 | 3.646 (1.892-7.028) |
| Stage 4 | <.0001 | 3.654 (1.895-7.045) |
| Organ involvement | ||
| Cardiac | .011 | 1.575 (1.112-2.23) |
| Renal | .352 | 1.188 (0.826-1.708) |
| Liver | .753 | 0.925 ( 0.57-1.502) |
| Hematologic response at 12 mo | ||
| CR | Ref | |
| VGPR | .001 | 2.343 (1.409-3.895) |
| PR | <.0001 | 3.692 (2.196-6.208) |
| NR | <.0001 | 4.219 (1.932-9.213) |
| FLC-MS status, FLC-MS positive vs FLC-MS negative | <.0001 | 3.098 (1.752-5.478 |
| Combined hematologic response and FLC-MS status | ||
| 12-mo CR and FLC-MS negative | Ref | |
| 12-mo CR and FLC-MS positive | .019 | 3.37 (1.146-9.915) |
| 12-mo VGPR and FLC-MS negative | .015 | 4.801 (1.353-17.041) |
| 12-mo VGPR and FLC-MS positive | .002 | 6.054 (2.171-16.883) |
| 12-mo PR/NR | <.0001 | 10.15 (3.710-27.766) |
| Further response | ||
| 12-mo iFLC >20 mg/L vs <20 mg/L | <.0001 | 3.035 (1.845-4.992) |
| 12-mo dFLC >10 mg/L vs <10 mg/L | <.0001 | 2.108 (1.454-3.05) |
iFLC, involved free light chain; ALP, alkaline phosphatase; 95% CI, confidence interval; mo, month; NTProBNP, N-terminal probrain natriuretic peptide; Ref, reference.
Multivariate analysis of factors affecting OS
| Multivariate (model 1) . | P . | HR (95% CI) . |
|---|---|---|
| Mayo 2004 staging with European modification | ||
| Mayo 2004 stage 1 | Ref | |
| Mayo 2004 stage 2 | .559 | 1.178 (0.68-2.038) |
| Mayo 2004 stage 3a | .001 | 2.302 (1.387-3.822) |
| Mayo 2004 stage 3b | .011 | 2.327 (1.217-4.449) |
| Combined hematologic response and FLC-MS status | ||
| 12mo CR and FLC-MS negative | Ref | |
| 12mo CR and FLC-MS positive | .021 | 3.576 (1.214-10.529) |
| 12mo VGPR and FLC-MS negative | .012 | 5.079 (1.421-18.155) |
| 12mo VGPR and FLC-MS positive | .001 | 6.068 (2.173-16.945) |
| 12mo PR/NR | <.0001 | 9.507 (3.469-26.058) |
| Multivariate (model 2) | ||
| Mayo 2012 staging | ||
| Mayo 2012 stage 1 | Ref | |
| Mayo 2012 stage 2 | .47 | 1.299 (0.639-2.639) |
| Mayo 2012 stage 3 | .02 | 2.223 (1.131-4.367) |
| Mayo 2012 stage 4 | .006 | 2.57 (1.31-5.044) |
| Creatinine∗ | .01 | 2.263 (1.213-4.219) |
| Combined hematologic response and FLC-MS status | ||
| 12-mo CR and FLC-MS negative | Ref | |
| 12-mo CR and FLC-MS positive | .097 | 2.517 (0.847-7.481) |
| 12-mo VGPR and FLC-MS negative | .125 | 2.823 (0.75-10.616) |
| 12-mo VGPR and FLC-MS positive | .005 | 4.476 (1.586-12.632) |
| 12-mo PR/NR | <.001 | 6.356 (2.303-17.538) |
| Multivariate (model 1) . | P . | HR (95% CI) . |
|---|---|---|
| Mayo 2004 staging with European modification | ||
| Mayo 2004 stage 1 | Ref | |
| Mayo 2004 stage 2 | .559 | 1.178 (0.68-2.038) |
| Mayo 2004 stage 3a | .001 | 2.302 (1.387-3.822) |
| Mayo 2004 stage 3b | .011 | 2.327 (1.217-4.449) |
| Combined hematologic response and FLC-MS status | ||
| 12mo CR and FLC-MS negative | Ref | |
| 12mo CR and FLC-MS positive | .021 | 3.576 (1.214-10.529) |
| 12mo VGPR and FLC-MS negative | .012 | 5.079 (1.421-18.155) |
| 12mo VGPR and FLC-MS positive | .001 | 6.068 (2.173-16.945) |
| 12mo PR/NR | <.0001 | 9.507 (3.469-26.058) |
| Multivariate (model 2) | ||
| Mayo 2012 staging | ||
| Mayo 2012 stage 1 | Ref | |
| Mayo 2012 stage 2 | .47 | 1.299 (0.639-2.639) |
| Mayo 2012 stage 3 | .02 | 2.223 (1.131-4.367) |
| Mayo 2012 stage 4 | .006 | 2.57 (1.31-5.044) |
| Creatinine∗ | .01 | 2.263 (1.213-4.219) |
| Combined hematologic response and FLC-MS status | ||
| 12-mo CR and FLC-MS negative | Ref | |
| 12-mo CR and FLC-MS positive | .097 | 2.517 (0.847-7.481) |
| 12-mo VGPR and FLC-MS negative | .125 | 2.823 (0.75-10.616) |
| 12-mo VGPR and FLC-MS positive | .005 | 4.476 (1.586-12.632) |
| 12-mo PR/NR | <.001 | 6.356 (2.303-17.538) |
Model 1 is based on Mayo staging 2004 with European modification, dFLC at baseline, creatinine, and the combined hematologic and FLC-MS response at 12 months. Creatinine and dFLC were found not to be significant on multivariate Cox regression analysis. Model 2 is based on the Mayo 2012 staging, creatinine, and the combined hematologic and FLC-MS response at 12 months.
eGFR, estimated glomerular filtration rate; Ref, reference.
Log10 transformation.
Discussion
To the best of our knowledge, this study reports the largest described cohort of patients with AL amyloidosis using FLC-MS to identify the presence of a monoclonal FLC at diagnosis and assess the impact of FLC-MS response at 6 and 12 months after treatment. The baseline light chain isotype identified by FLC-MS shows complete concordance with standard FLC assays and the amyloid fibril type on tissue biopsy. This study shows that FLC-MS negativity identifies a potential new category of deep response in AL amyloidosis that translates into significantly higher organ responses and marked survival benefit across all disease stages.
Patients who remained FLC-MS positive were more likely to have higher starting iFLC/dFLC and λ isotype at diagnosis. Because fewer patients with higher presenting FLC achieve FLC-MS negativity, it may be important to evaluate whether treatment regimens for such patients should be tailored differently with longer regimes, earlier consideration of transplantation, and maintenance therapy. All of these points need to be addressed in future studies.
The use of MS to detect the presence of a monoclonal protein has evolved over the last 5 years, with MS being considered a replacement for IFE for patients with multiple myeloma.6 We have previously reported FLC-MS in a small cohort of patients with AL amyloidosis using the technique described in this study with FLC immobilization with immunomagnetic beads and subsequent detection of the FLC by MALDI-TOF MS.8,11,14 It showed complete concordance with serum FLC by standard assay and amyloid fibril for light chain presence and isotype. In this cohort of 17 patients, 2 patients with normal FLC by standard assay after treatment but MRD positive on bone marrow using next generation flow cytometry demonstrated persistent FLC-MS peaks. The Mayo clinic group demonstrated in 33 patients with bone marrow MRD negativity (in hematologic CR) with AL amyloidosis that 12% had residual detectable FLC by LC-MS and MALDI-TOF MS, and it was associated with a poorer time to progression (at 50 months, 75% vs 13%; P = .003).14 Our study validates in a large cohort both the previous findings; the complete concordance of fibril light chain isotype with detected monoclonal FLC by FLC-MS and the persistence of abnormal FLC by FLC-MS in a significant proportion of patients with CR defined by standard ISA criteria. Here, only 20.7% of the total cohort became FLC-MS negative at 12 months, and 39% of patients in a hematologic CR by standard ISA criteria became FLC-MS negative.
Depth of response is directly linked to outcomes in AL amyloidosis especially for patients with advanced stage disease.22 Presently, organ response is governed by the reduction in FLC after therapy, and those who achieve at least a VGPR have better organ response.23-25 Furthermore, patients with AL amyloidosis who are bone marrow MRD negative by next generation flow cytometry have significantly better survival and organ responses.26,27 A European collaboration demonstrated that a renal response was seen in 92% vs 57%, and a cardiac response was demonstrated in 95% vs 71%, respectively, in those who were MRD negative vs positive.4 The use of FLC-MS to assess the depth of response or even as a surrogate of MRD is an attractive prospect because it can be performed on a serological sample without the need for an invasive procedure. The potential benefit of FLC-MS is that it can provide a further stratum of response without the limitations associated with the current Freelite assay, such as the impact of increased background polyclonal light chain levels, as seen in renal dysfunction.
To the best of our knowledge, this study is the first to report the impact of FLC-MS negativity on outcomes in AL amyloidosis. It was striking that the benefit of FLC-MS negativity translated to better survival even for patients in CR and those achieving a dFLC <10 mg/L (both currently considered as the “ideal” goals of treatment in AL amyloidosis). We note the current differences in the CR definition used by the ISA and that used in the Andromeda trial. Despite the increased stringency of the ISA criteria, less than half of the patients were FLC-MS at 12 months. Although not reaching statistical significance, the OS and organ response even in this cohort favoured those who were FLC-MS negative. Interestingly, the difference in outcome was more apparent in those who did not fulfil Andromeda criteria but were in an ISA CR in most cases due to increased polyclonal FLC in the context of renal dysfunction, in which 59 of 77 patients (76.6%) had an eGFR of <60 mL per minute per 1.73 m2. It is in this cohort, the utility of FLC-MS may be more apparent in determining potential ongoing amyloidotic disease progression.
At present, the FLC-MS assay is qualitative, but research is ongoing to determine whether FLC-MS can be used quantitatively. We hypothesize that in the context of an Andromeda CR, the level of the residual monoclonal FLC detected by FLC-MS may be lower than those detected in an ISA CR, in which the ratio is normal but the iFLC may be raised.
Although 15% of patients in standard VGPR were FLC-MS negative at 12 months, there was no statistical survival advantage compared with those who remained FLC-MS positive; however, due to the small numbers of patients who attained VGPR and who were FLC-MS negative, any conclusion is limited. However, it may suggest that a persistent intact M protein or very low level persistent amyloidotic FLC below threshold of FLC-MS methods of detection may be contributory. The presence of patients with FLC-MS negativity who only achieve VGPR appears puzzling, however, this can occur for a number of different reasons. Patients who do not have a normalized FLC ratio, a criterion for a CR, but a dFLC <40 mg/L are considered to be in VGPR. The lack of FLC ratio normalization is not solely due to an excess of amyloidotic light chain and can be attributed to changes in the polyclonal background. In contrast, an intact monoclonal protein in the serum is not detected by the FLC-MS assay and patients with essentially normal light chains but with a trace of intact monoclonal protein in the serum/urine would be classed as VGPR. A limitation of this study is that an additional MS assay for the intact M protein was not performed. Four patients for whom a Bence-Jones protein in the urine was identified on immunofixation but no abnormal FLC-MS spike detected on serology require further investigation. Unfortunately, due to the retrospective nature of the study, we are unable to repeat the urine tests to confirm the accuracy of IF positivity. At present, the FLC-MS has not been optimized to detect FLC in urine, although this a potential avenue for further research.
The FLC-MS assay is currently only available in a research capacity undertaken by the Binding Site/Thermo Fisher Scientific. Work is underway to validate the assay for it to become an additional component to the currently available EXENT assay. Critically, the EXENT assay measures individual immunoglobulins and "total" light chains, which include immunoglobulin-bound light chains and as well as "free" light chains. The FLC-MS assay uses FLC beads to specifically isolate the FLC (similar to the actual Freelite assay) and hence measure only the FLC. The total light chains measured by the EXENT technique are not sensitive enough to detect low level FLC below the polyclonal background and should not be used interchangeably with FLC-MS. The FLC-MS assay currently requires manual analysis of the spectra for each patient, but there is scope for this to be automated in a similar way to the EXENT and MASS-FIX assays. This process requires the development of a machine learning algorithm that would be refined with multiple generations of training data as has been performed for the EXENT assay. Over the last 4 years, protocols for the processing of the samples using automated liquid handlers to perform the immune precipitation and spot the samples onto MALDI target platelets have been developed and could easily be adapted for high throughput testing. MALDI-TOF MS is a high throughput MS technique taking <30 seconds for each patient’s sample to be analyzed. We appreciate a major limitation to our study is the lack of associated EXENT measurements, and a further study is underway looking at a combination of EXENT and FLC-MS assays.
We acknowledge other limitations of this study. This study only included patients with samples available at baseline, 6 months, and 12 months, thus by definition, a landmark analysis of 12-month survivors. The utility of FLC-MS assessment for early response and mortality remains unclear, as does its utility in prognosis in an unselected intent to treat population. However, the role of FLC-MS may lie in identifying long-term therapy needs that may correlate with long-term organ response rather than a parameter to affect early therapy changes. There were no parallel bone marrow samples to assess comparative bone marrow MRD assessment in patients with FLC-MS negativity. All patients in this cohort were treated with bortezomib-based regimens (commonly CyBorD) but none with upfront daratumumab. The results of the Andromeda study showed improved hematologic CR rates with daratumumab-CyBorD compared with CyBorD, and therefore, higher rates of FLC-MS negativity are likely as daratumumab-CyBorD becomes available as standard first-line treatment.18
In summary, this study, to our knowledge, is the first large cohort study of patients with AL amyloidosis using FLC detection by MS using a novel assay that detects monoclonal FLC based on the unique m/z value of the FLC and demonstrates that FLC-MS is a reliable method of detecting a monoclonal amyloidogenic FLC. Patients achieving FLC-MS negativity have significantly superior organ responses. Patients with no detectable residual monoclonal FLC by FLC-MS have significantly better OS, and FLC-MS negativity is an independent predictor of better survival in AL irrespective of the cardiac disease stage. FLC-MS is potentially a key serological MRD marker in AL amyloidosis. FLC-MS assessment should be validated in an independent cohort and, if findings confirmed, has the potential to be a new definition of response in AL amyloidosis and an end point of trials with novel agents in AL amyloidosis.
Acknowledgment
The authors thank all the clinical and laboratory staff at the National Amyloidosis Centre and The Binding Site.
Authorship
Contribution: J.B., S.R., and A.W. designed the study, performed research, analyzed the data, and wrote the manuscript; H.V.G., N.W., O.B., S.H., G.P., and J.G. designed the study, performed research, and wrote the manuscript; J.K., S.H., B.W., O.C., D.F., M.U.R., N.S., A.M.-N., L.V., C.W., M.F., P.N.H., J.D.G., and H.L. performed research and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: H.V.G. received research funding from the Binding Site. M.F. reports consulting income from Intellia, Novo-Nordisk, Pfizer, Eidos, Prothena, Akcea, Alnylam, Caleum, Alexion, Janssen, Ionis, and Astra-Zeneca. C.W. reports honoraria from Akcea, Alnylam, Novartis, and Pfizer. J.D.G has consulting income from Ionis, Eidos, Intellia, Alnylam, and Pfizer. G.P received an honorarium for advisory boards and educational support from Bristol Myers Squibb, Janssen, The Binding Site, Sanofi, Takeda, and GlaxoSmithKline. S.H., O.B., and N.W. are employees of The Binding Site. A.W. has consulting income from Alexia, Astra-Zeneca, Janssen, Attralus, and Prothena. The remaining authors declare no competing financial interests.
Correspondence: Ashutosh Wechalekar, National Amyloidosis Centre, University College London Medical School (Royal Free Campus), Rowland Hill St, London NW3 2PF, United Kingdom; email: a.wechalekar@ucl.ac.uk.
References
Author notes
Original data available upon reasonable request from the corresponding author, Ashutosh Wechalekar (a.wechalekar@ucl.ac.uk).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal