As redemonstrated by Bomsztyk et al1 in this issue of Blood, top-down mass spectrometry (MS) of blood has been transformative for diagnosing and monitoring plasma cell disorders,2-7 and immunoglobulin light chain (AL) amyloidosis is no exception.3,8,9
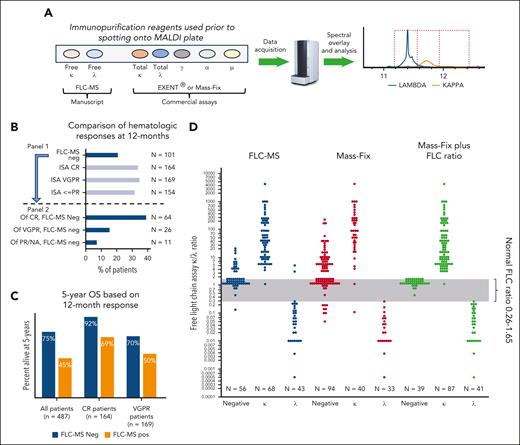
To date, every comparator study using a 5-bead MS-based assessment of hematologic response compared with standard immunofixation in either multiple myeloma or AL amyloidosis has favored the MS approach because of both improved sensitivity and specificity of the MS assays.5-9 Bomsztyk et al take this a step further in AL amyloidosis, which is a disease primarily of elevated free light chains (FLCs), by using an MS assay that detects FLCs (FLC-MS) rather than total light chains (those light chains bound to intact immunoglobulins plus FLCs [see figure panel A]).1 The authors studied 487 patients who were newly diagnosed with AL amyloidosis who were treated with bortezomib-based regimens with serial measures. All but 4 had their FLC peak identifiable by FLC-MS. Using FLC-MS, they demonstrated that fewer patients had as deep a response as estimated by standard hematologic measures (ie, the International Society of Amyloidosis amyloid response criteria, which are dependent on immunofixation and the nephelometric FLC assay) (see figure panel B, panel 1). Overall, only 21% of patients were FLC-MS negative at 12 months, and the likelihood of being FLC-MS negative increased with superior hematologic response status (see figure panel B, panel 2). Notably, 12 months after diagnosis, 32% of their patients had been classified as amyloid complete hematologic response, but only 39% of these had a negative FLC-MS, making only 13% of the entire cohort both amyloid complete response and FLC-MS negative.
(A) Representation of the MS assays, highlighting their differences. (B) Interaction of FLC-MS response at 12 months with standard amyloid hematologic response. N represents the total sample size from which the bar chart percent is calculated. Based on the study by Bomsztyk et al.1 (C) Interaction of FLC-MS response with standard amyloid hematologic response at 12 months, and 5-year overall survival among patients. Based on data from the study by Bomsztyk et al.1 (D) Relative performance of FLC-MS, Mass-Fix, and Mass-Fix plus nephelometric FLC ratio. Note the single Mass-Fix κ adjudicated as λ was actually a biclonal IgG κ plus free λ. Based on data from the study by Sepiashvili et al.10 CR, complete hematologic response; ISA, International Society of Amyloidosis; MALDI, matrix-assisted laser desorption/ionization; NA, not available; neg, negative; OS, overall survival; pos, positive; PR, partial response; VGPR, very good partial response.
(A) Representation of the MS assays, highlighting their differences. (B) Interaction of FLC-MS response at 12 months with standard amyloid hematologic response. N represents the total sample size from which the bar chart percent is calculated. Based on the study by Bomsztyk et al.1 (C) Interaction of FLC-MS response with standard amyloid hematologic response at 12 months, and 5-year overall survival among patients. Based on data from the study by Bomsztyk et al.1 (D) Relative performance of FLC-MS, Mass-Fix, and Mass-Fix plus nephelometric FLC ratio. Note the single Mass-Fix κ adjudicated as λ was actually a biclonal IgG κ plus free λ. Based on data from the study by Sepiashvili et al.10 CR, complete hematologic response; ISA, International Society of Amyloidosis; MALDI, matrix-assisted laser desorption/ionization; NA, not available; neg, negative; OS, overall survival; pos, positive; PR, partial response; VGPR, very good partial response.
FLC-MS negativity translated into a higher likelihood of organ response at 12 months, with 70% and 38% of organ response–eligible patients having a cardiac and renal response, respectively. Overall survival was also better among patients who had a negative (compared with a positive) FLC-MS in each of the following patient populations: (1) all, regardless of standard hematologic response; (2) complete hematologic responders; and (3) very good partial responders (see figure panel C). Patients achieving complete hematologic response but who remained FLC-MS positive had similar overall survival compared with patients in very good partial response.
These results are exciting, but there are several limitations to this study. First, although there are 2 commercially available top-down MS assays (Mass-Fix and EXENT), the FLC-MS, the assay used in this study, is not currently commercially available (see figure panel A), making this article academically interesting, but not yet applicable for general practice. It would have been useful had they contextualized the FLC-MS results relative to the EXENT assay. How do the existing MS assays perform relative to the FLC-MS assay? Investigators from the Mayo Clinic compared the FLC-MS with the Mass-Fix assay in the sera of 167 patients (see figure panel D).10 Concordance between Mass-Fix and FLC-MS assays was 74%, with the vast majority of the discrepancy due to the greater sensitivity of the FLC-MS. When the results of the FLC ratio derived from the FLC nephelometric assay were combined with the Mass-Fix result, concordance between these 2 commercially available assays and the FLC-MS assay was 88%, with the discordance relating predominantly to abnormal FLC ratios (typically favoring κ) in the setting of a negative FLC-MS. The ability of this combination rivaled the FLC-MS for detecting λ clones. Extrapolation to the present study is imperfect because the Mayo study was not done specifically in a population with AL amyloidosis. Bomsztyk et al reported on limited comparisons of the nephelometric FLC assay, immunofixation, and the FLC-MS. For example, they demonstrated that among those patients with AL in very good partial response who were also FLC-MS negative, of the discordant cases, approximately two-thirds were due to a positive immunofixation and approximately two-thirds to the difference between κ and λ FLC (dFLC) of >10 mg/L. Although one would expect that the FLC-MS would be more specific than the nephelometric FLC method, which largely relies on ratios—and the multivariate analyses in part bore out this prospect—the analyses would have been even more persuasive had hematologic complete response and good partial response been adjudicated using the commercially available MS assays rather than immunofixation, the latter of which has been well established to be less sensitive and less specific than the existing MS assays.2-8
The second major limitation of this study is no bone marrow data were provided such that the comparison between bone marrow minimal residual disease and FLC-MS could be made. Using the commercial assays that detect total light chains (rather than free light chains), there is a growing body of literative of how valuable the EXENT and the Mass-Fix assays can be in detecting bone marrow minimal residual disease among patients with AL amyloidosis8,9 as well as multiple myeloma.4-7
In short, this work provides exciting data on a yet to be approved assay. Monitoring hematologic response has come a long way in AL amyloidosis: from barely detectable monoclonal proteins by serum protein electrophoresis and immunofixation to nephelometric serum FLCs to total light chain–based MS assays and hopefully soon to FLC-MS assays. Until FLC-MS is commercially available, we will rely on the next best, which is total light chain–based MS and the nephelometric FLC assays of the blood (see figure panel D).
Conflict-of-interest disclosure: A.D. reports being on the advisory board and independent review committee of Janssen; being on the data monitoring safety committees of Oncopeptides and Sorrento; and receiving research funding from Alnylam, Pfizer, Takeda, and Bristol Myers Squibb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal