In the current issue of Blood, Mathioudaki et al1 use single-cell RNA sequencing to identify adhesion G-protein coupled receptor GPR56 as a marker of an antigen-experienced, antileukemic CD8+ T-cell subset that is associated with complete remission (CR) after allogeneic stem cell transplant (alloSCT) for acute myeloid leukemia (AML). By coupling in silico, high-throughput discovery technologies with immunophenotyping and in vitro cytotoxicity assays, the authors lay the groundwork for prospective investigation of GPR56 as a clinically predictive marker of post-SCT outcomes.
The graft-vs-leukemia (GVL) effect is widely recognized as the major determinant of alloSCT therapeutic efficacy for high-risk hematologic malignancies. Mechanistically, GVL is driven largely by donor T cells eliminating recipient leukemic cells. However, this antitumor alloreactivity must be highly specific and thus modulated by immunosuppressive agents to protect against graft-versus-host disease (GVHD). Post-SCT relapse is therefore chiefly attributed to failure of GVL, and identifying the responsible molecular pathways could illuminate novel therapeutic strategies to maximize GVL without increasing GVHD. Recent technological developments have enabled high-resolution inspection of the GVL effect, unveiling several complex cellular processes and new avenues for clinical exploration. Studies focused on malignant cells have documented loss of major histocompatibility complex expression, upregulation of immune-checkpoint ligands, secretion of inhibitory cytokines, and increased production of immunosuppressive enzymes as mechanisms for evading GVL.2 For immunotherapies that reinstate the GVL effect, T cell–derived determinants of outcome have been identified, such as expansion of precursor exhausted T cells after donor lymphocyte infusion and increased infiltration of cytotoxic CD8+ T cells after checkpoint blockade.3-5
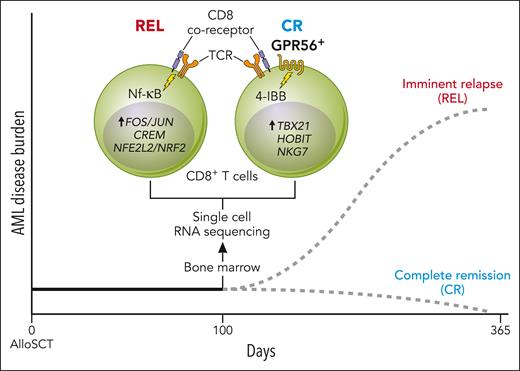
Here, the authors sought to understand the T cell–derived determinants of “endogenous” GVL in the early post-SCT period when the use of immunosuppressive GVHD prophylaxis is common. The authors generated single-cell transcriptomes on both marrow-infiltrating, CD34+ progenitors and CD3+ T cells collected 100 days after allo-SCT from patients with AML, to identify T-cell signatures associated with remaining in CR versus those found before imminent relapse (REL). Consistent with prior reports of an immunosuppressive phenotype and overall T-cell dysregulation in the REL state, the authors found enrichment of FOS/JUN, REL (NF-κB signaling pathway), CREM, and NFE2L2/NRF2 in the REL group.6,7 Likewise, differential expression analyses in CD8+ T cells revealed multiple genes in patients in CR linked to effector-memory function, including the TBX21 transcription factor regulon, targeting cytotoxic genes GZMB and KLRG1.8 Interestingly, among these differentially expressed genes was ADGRG1, encoding GPR56, a G-protein-coupled receptor and surface marker. GPR56 was previously associated with stemness and self-renewal phenotypes in AML as well as effector functions in T cells; however, the present study is the first to associate it with GVL (see figure).9,10
CD8+ T cells derived from patients who remained in CR after alloSCT for AML are characterized by a GPR56+, cytotoxic phenotype in contrast to those derived from patients who later relapsed with AML that exhibit GPR56−, immune-suppressive regulatory states. Professional illustration by Patrick Lane, ScEYEnce Studios.
CD8+ T cells derived from patients who remained in CR after alloSCT for AML are characterized by a GPR56+, cytotoxic phenotype in contrast to those derived from patients who later relapsed with AML that exhibit GPR56−, immune-suppressive regulatory states. Professional illustration by Patrick Lane, ScEYEnce Studios.
After correlating expression of GPR56 on the cell surface with increased CD8+ T-cell differentiation in the CR group, the authors investigated GPR56 as a potential marker of GVL activity in AML. Using a coculture model of the HL-60 AML cell line and CD33-directed chimeric antigen receptor (CAR) T cells expressing both CD28 and 4-1BB intracellular signaling domains, the authors showed upregulation of GPR56 upon CAR T-cell activation—which was not seen during in vitro CD3/CD28 costimulation assays that lack direct 4-1BB stimulation—implicating the 4-1BB signaling pathway in this process. Interferon-γ production was also stronger in CR patient-derived GPR56+ compared with GPR56-alloreactive T cells upon in vitro exposure to matched AML blasts. Together, these data associate GPR56 expression with antileukemic, cytotoxic capacity in simulated GVL contexts.
Mathioudaki et al have thus related GPR56 expression to CD8+ T-cell antileukemic cytotoxic activity and AML remission post-alloSCT. This study also demonstrates the potential clinical relevance of unbiased, high-throughput technologies such as single-cell transcriptomics. These data also raise an important question: does GPR56 expression on CD8+ T cells constitute a marker or mechanism of effective GVL activity? The present study provides substantive rationale for validation of the findings in larger clinical cohorts; further analyses of previously published molecular GVL studies; single-cell T-cell receptor sequencing for defining potential leukemia-specific, GPR56+ T-cell responses; and investigation of GPR56 downregulation as a possible method for immune evasion in AML relapse.4 Moreover, studies determining the effect of altered GPR56 expression on T-cell function can address potential mechanistic roles of GPR56 in T-cell recognition and cytotoxicity. Finally, the era of high-resolution, multimodal molecular measurements is in full force across translational science. Application of these capabilities to well-annotated clinical cohorts with longitudinal sampling offers the tantalizing prospect of additional regulatory networks whose therapeutic perturbation could transform post-SCT outcomes.
Conflict-of-interest disclosure: P.B. declares equity in Agenus, Amgen, Johnson & Johnson, Exelixis, and BioNTech and receives research support from Allogene Therapeutics. A.N.I. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal