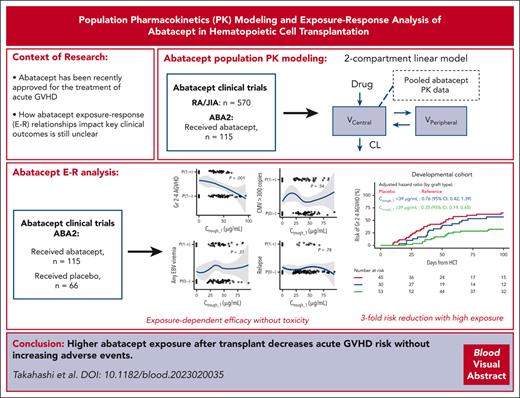
Key Points
We demonstrate a favorable exposure-response relationship between the Ctrough_1 of abatacept and a lower risk of gr 2or 4 aGVHD.
No association was found between abatacept Ctrough_1 and key safety outcomes, including relapse, cytomegalovirus, or Epstein-Barr virus viremia.
Abstract
In the ABA2 study, the T-cell costimulation blockade agent, abatacept, was safe and effective in preventing acute graft-versus-host disease (aGVHD) after unrelated-donor hematopoietic cell transplant (HCT), leading to US Food and Drug Administration approval. Here, we performed a determination of abatacept pharmacokinetics (PK), which enabled an examination of how abatacept exposure-response relationships affected clinical outcomes. We performed a population PK analysis of IV abatacept using nonlinear mixed-effect modeling and assessed the association between abatacept exposure and key transplant outcomes. We tested the association between the trough after dose 1 (Ctrough_1) and grade (GR) 2 or 4 aGVHD (GR2-4 aGVHD) through day +100. An optimal Ctrough_1 threshold was identified via recursive partitioning and classification tree analysis. This demonstrated that abatacept PK was characterized by a 2-compartment model with first-order elimination. The ABA2 dosing regimen was based on previous work targeting a steady-state abatacept trough of 10 μg/mL. However, a higher Ctrough_1 (≥39 μg/mL, attained in ∼60% of patients on ABA2) was associated with a favorable GR2-4 aGVHD risk (hazard ratio, 0.35; 95% confidence interval, 0.19-0.65; P < .001), with a Ctrough_1 <39 μg/mL associated with GR2-4 aGVHD risk indistinguishable from placebo (P = .37). Importantly, no significant association was found between Ctrough_1 and key safety indicators, including relapse, and cytomegalovirus or Epstein-Barr virus viremia. These data demonstrate that a higher abatacept Ctrough_1 (≥39 μg/mL) was associated with a favorable GR2-4 aGVHD risk, without any observed exposure-toxicity relationships. This trial was registered at www.clinicaltrials.gov as #NCT01743131.
Introduction
Hematopoietic cell transplant (HCT) from both HLA-matched and HLA-mismatched unrelated donors (URDs) represents a life-saving treatment for patients with hematologic malignancies. However, graft-versus-host disease (GVHD), relapse, and infection remain major causes of transplant failure.1 Recently, a phase 2 clinical trial (ABA2, NCT01743131),2 along with real-world evidence from the Center for International Blood and Marrow Transplant Research,3 led to the US Food and Drug Administration (FDA) approval of abatacept for acute GVHD (aGVHD) prevention,4 representing the first such FDA approval for GVHD prevention in the history of the field. Abatacept is a fusion protein composed of the antigen-binding domain of CTLA-4 combined with a humanized Fc portion of immunoglobulin G4, designed to prolong its in vivo half-life. Abatacept inhibits T-cell costimulation through binding to the CD80 and CD86 receptors on antigen presenting cells (APCs), thereby blocking key costimulation signals that occur through the binding of CD28 on T cells and CD80/86 on APCs.5 IV abatacept was initially approved by the FDA for patients with rheumatoid arthritis (RA) in 20056 and polyarticular juvenile idiopathic arthritis (JIA) in 2008.7 In the ABA2 study, abatacept demonstrated efficacy in preventing aGVHD in patients after URD-HCT.2 Importantly, the ABA2 study revealed an acceptable safety profile with no increase in relapse or viral reactivation, resulting in a favorable risk-benefit profile.
The ABA2 trial used the dose of abatacept (10 mg/kg) that was identified for adults and children aged >5 years with RA and JIA.8,9 However, ABA2 incorporated a unique dosing schedule, with 4 doses of 10 mg/kg given on days −1, +5, +14, and +28,10 that was partly based on that used with belatacept for kidney transplants.11 This dosing schedule includes a compression of the first 3 doses of abatacept, compared with that in the RA/JIA dosing (giving 3 doses rather than 2 in the first 2 weeks), to achieve higher initial trough levels and increase early abatacept exposure. One of the accepted tenets of abatacept dosing, which is based on repeated long-term dosing (every 4 weeks) and pharmacokinetic-pharmacodynamic (PK-PD) analysis in RA/JIA, is to target a steady-state trough concentration (Ctrough) >10 μg/mL.12,13 However, high Ctrough interpatient variability was observed in adult patients with RA receiving IV abatacept.14 Notably, with the compressed initial doses of abatacept at 10 mg/kg per dose, the 10-patient ABA1 pilot HCT study demonstrated a much higher mean Ctrough (45.6 ± 2.5 μg/mL),10 which raised a question of the optimal dosing of abatacept required for aGVHD prevention, in order to achieve desirable efficacy and safety. To address this question, we performed a PK-PD analysis with samples collected during the 185-patient ABA2 trial. This detailed PK analysis enabled an exposure-response (E-R) study in a cohort that received a uniform dose and schedule of abatacept during URD HCT. Such an E-R study leverages detailed PK analysis for patients receiving abatacept, and identifies associations of abatacept exposure with key efficacy (GVHD) and safety (viral reactivation and relapse) end points. This represents a critical analysis, given the potentially divergent PK profiles of patients with RA/JIA and those undergoing HCT, and the possibility to optimize abatacept dosing for HCT to minimize potential toxicities and maximize efficacy. Our results provide evidence for a positive E-R relationship for abatacept and the prevention of aGVHD and, importantly, do not identify negative exposure-toxicity relationships with relapse or viral reactivation.
Methods
Schema
The present study analyzed PK data from the ABA2 trial and correlated these with clinical outcomes. ABA2 is a previously reported multicenter phase 2 study of abatacept as GVHD prophylaxis in combination with standard methotrexate and calcineurin inhibitor in URD HCT (NCT01743131; patients enrolled from 1 March 2013 to 30 November 2016). Written informed consent was obtained from all patients on the ABA2 trial. Results of the primary analysis and the study protocol were published previously.2,15 The study protocol was approved by the institutional review board of all participating centers. ABA2 used a randomized double-blind, placebo-controlled design in patients receiving 8/8 HLA-matched (ie, HLA A, B, C, and DRB1) URD-HCT, and a single-arm open-label design in patients receiving 7/8 HLA-matched grafts. The ABA2 trial enrolled 185 patients, with abatacept given to 73 patients in the 8/8 study arm and 43 patients in the 7/8 arm, whereas 69 patients in the 8/8 arm received placebo. Among the 116 HCT patients who received abatacept, 1 patient without PK data was excluded from the current analysis (therefore, 184 of 185 patients are reported here). Abatacept was given IV at 10 mg/kg per dose based on actual body weight, with a maximum dose of 1000 mg, which was administered on days −1, +5, +14, and +28.
Abatacept PK sampling and population PK analysis
We performed population PK modeling, which leveraged pooled abatacept PK data from 4872 plasma abatacept concentrations derived from 685 patients enrolled in 8 clinical trials, including ABA2 (both 8/8 and 7/8 cohorts, n = 115 patients), as well as 6 trials for adults with RA (n = 386 patients), and 1 trial for children with JIA (n = 184 patients) from the Bristol Myers Squibb clinical development program for intravenous abatacept (supplemental Tables 1 and 2, available on the Blood website). The PK sampling schedule for ABA2 was on days −1 (predose), +5 (predose), +14 (predose), +21, +28 (predose), +35, +42, +63, +100, +180, +270, and +365, and 10 minutes after the end of the hour-long drug infusion on days −1 and +28. To determine abatacept exposure, plasma abatacept concentrations were quantified using a validated enzyme-linked immunosorbent assay method with a lower limit of quantification of 1.0 μg/mL. We leveraged the data from RA and JIA trials in addition to the ABA2 study to delineate the characteristics of abatacept PK in patients receiving HCT in comparison with those receiving RA/JIA. In addition, the wide range of abatacept dosing (0.2-10.0 mg/kg per dose) and concentration-time data in the pooled data enabled the development of highly robust population PK models (supplemental Table 2).
Population PK modeling
Details of the population PK modeling are found in supplemental Methods.
E-R analysis for the ABA2 study
The E-R analysis to assess HCT-related outcomes included 184 patients enrolled on the ABA2 study (69 patients received placebo and 115 patients received abatacept), which were subsequently divided into a developmental cohort of 128 patients and a validation cohort of 56 patients for some analyses. Because E-R analysis and validation are most robust when modeling higher-frequency events, we chose Grade 2 to 4 (GR2-4) aGVHD until day +100 (with an incidence of 48.9% in the combined ABA2 data set; Table 2) as the primary outcome for this analysis. Exploratory outcomes included day +100 GR3-4 aGVHD, day +100 steroid-refractory aGVHD, engraftment of neutrophil and platelets, and day +180 GR3-4 aGVHD-free survival (aGFS) as well as these day +365 outcomes: overall survival, nonrelapse mortality, all chronic GVHD (cGVHD), moderate-to-severe cGVHD, relapse, severe GVHD–free survival (survival without relapse, GR3-4 aGVHD, or moderate-to-severe cGVHD; ie, GRFS), cytomegalovirus (CMV) infection (viremia >300 copies per mL or invasive disease), and Epstein-Barr virus (EBV) reactivation (of any viral load). There were too few posttransplant lymphoproliferative disease events (4 cases across all patients in the ABA2 trial) to effectively include them in the model. Relapse was treated as a competing risk for GVHD outcomes and as a censoring event in engraftment and CMV/EBV infection outcomes. Death was treated as a competing risk for all outcomes, except when death was an event of interest (ie, overall survival, nonrelapse mortality, aGFS, and GVHD-relapse–free survival). No censoring for other reasons (eg, lost to follow-up) was observed during the outcome follow-up periods.
Patient characteristics
| Characteristics . | Total (N = 184) . | Developmental cohort (n = 128) . | Validation cohort (n = 56) . | P value . |
|---|---|---|---|---|
| HLA match: 7/8 vs 8/8, n (%) | 42 (23) vs 142 (77) | 30 (23) vs 98 (77) | 12 (21) vs 44 (79) | .76 |
| Abatacept vs placebo, n (%) | 115 (63) vs 69 (38) | 83 (65) vs 45 (35) | 32 (57) vs 24 (43) | .32 |
| Age in y, median (range) | 44 (7-76) | 45 (7-76) | 40 (8-76) | >.99 |
| Age ≥21 y, n (%) | 134 (72) | 93 (73) | 41 (73) | .94 |
| Race, White, n (%) | 153 (83) | 109 (85) | 44 (79) | .27 |
| PS ≥ 90%, n (%) | 135 (73) | 96 (75) | 39 (70) | .45 |
| Disease, n (%) | .15 | |||
| AML | 67 (36) | 42 (33) | 25 (45) | |
| ALL | 56 (30) | 40 (31) | 16 (29) | |
| MDS | 37 (20) | 25 (20) | 12 (21) | |
| Other | 24 (13) | 21 (16) | 3 (5) | |
| CIBMTR disease stage, n (%) | .27 | |||
| Early | 110 (60) | 82 (64) | 28 (50) | |
| Intermediate | 43 (23) | 26 (20) | 17 (30) | |
| Advanced | 30 (16) | 19 (15) | 11 (20) | |
| Other | 1 (1) | 1 (1) | 0 | |
| Conditioning regimen, n (%) | .26 | |||
| Busulfan/cyclophosphamide | 61 (33) | 41 (32) | 20 (36) | |
| TBI/cyclophosphamide | 57 (31) | 43 (34) | 14 (25) | |
| Fludarabine/melphalan | 49 (27) | 30 (23) | 19 (34) | |
| Busulfan/fludarabine | 17 (9) | 14 (11) | 3 (5) | |
| Conditioning: MAC vs RIC, n (%) | 136 (74) vs 48 (26) | 99 (77) vs 29 (23) | 37 (66) vs 19 (34) | .11 |
| Patient CMV seropositive, n (%) | 79 (43) | 57 (45) | 22 (39) | .68 |
| Graft: BM vs PBSC, n (%) | 79 (43) vs 105 (57) | 54 (42) vs 74 (58) | 25 (45) vs 31 (55) | .76 |
| Characteristics . | Total (N = 184) . | Developmental cohort (n = 128) . | Validation cohort (n = 56) . | P value . |
|---|---|---|---|---|
| HLA match: 7/8 vs 8/8, n (%) | 42 (23) vs 142 (77) | 30 (23) vs 98 (77) | 12 (21) vs 44 (79) | .76 |
| Abatacept vs placebo, n (%) | 115 (63) vs 69 (38) | 83 (65) vs 45 (35) | 32 (57) vs 24 (43) | .32 |
| Age in y, median (range) | 44 (7-76) | 45 (7-76) | 40 (8-76) | >.99 |
| Age ≥21 y, n (%) | 134 (72) | 93 (73) | 41 (73) | .94 |
| Race, White, n (%) | 153 (83) | 109 (85) | 44 (79) | .27 |
| PS ≥ 90%, n (%) | 135 (73) | 96 (75) | 39 (70) | .45 |
| Disease, n (%) | .15 | |||
| AML | 67 (36) | 42 (33) | 25 (45) | |
| ALL | 56 (30) | 40 (31) | 16 (29) | |
| MDS | 37 (20) | 25 (20) | 12 (21) | |
| Other | 24 (13) | 21 (16) | 3 (5) | |
| CIBMTR disease stage, n (%) | .27 | |||
| Early | 110 (60) | 82 (64) | 28 (50) | |
| Intermediate | 43 (23) | 26 (20) | 17 (30) | |
| Advanced | 30 (16) | 19 (15) | 11 (20) | |
| Other | 1 (1) | 1 (1) | 0 | |
| Conditioning regimen, n (%) | .26 | |||
| Busulfan/cyclophosphamide | 61 (33) | 41 (32) | 20 (36) | |
| TBI/cyclophosphamide | 57 (31) | 43 (34) | 14 (25) | |
| Fludarabine/melphalan | 49 (27) | 30 (23) | 19 (34) | |
| Busulfan/fludarabine | 17 (9) | 14 (11) | 3 (5) | |
| Conditioning: MAC vs RIC, n (%) | 136 (74) vs 48 (26) | 99 (77) vs 29 (23) | 37 (66) vs 19 (34) | .11 |
| Patient CMV seropositive, n (%) | 79 (43) | 57 (45) | 22 (39) | .68 |
| Graft: BM vs PBSC, n (%) | 79 (43) vs 105 (57) | 54 (42) vs 74 (58) | 25 (45) vs 31 (55) | .76 |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BM, bone marrow, CIBMTR, the Center for International Blood and Marrow Transplant Research; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cell; PS, performance status; RIC, reduced intensity conditioning; TBI, total body irradiation.
Summary of clinical outcomes
| Outcome . | Total (N = 184) . | Developmental cohort (n = 128) . | Validation cohort (n = 56) . | P value . |
|---|---|---|---|---|
| Cumulative incidence, % (95% CI) | ||||
| aGVHD, day +100 | ||||
| GR2-4 | 48.9 (41.5-55.9) | 49.2 (40.3-57.6) | 48.2 (34.5-60.6) | .87 |
| GR3-4 | 8.7 (5.2-13.3) | 8.6 (4.5-14.3) | 8.9 (3.2-18.2) | .93 |
| Steroid refractory | 9.2 (5.6-14.0) | 11.7 (6.9-18.0) | 3.6 (0.7-11.0) | .08 |
| cGVHD, d +365 | ||||
| Mild to severe | 49.5 (42.0-56.5) | 52.3 (43.3-60.6) | 42.9 (29.6-55.4) | .27 |
| Moderate to severe | 41.3 (34.1-48.3) | 44.5 (35.7-52.9) | 33.9 (21.8-46.4) | .22 |
| aGFS, d +180 | 9.8 (6.0-14.6) | 9.4 (5.1-15.2) | 10.7 (4.3-20.5) | .08 |
| GRFS, d +365 | 14.1 (9.5-19.6) | 14.8 (9.3-21.6) | 12.5 (5.4-22.7) | .70 |
| Overall survival, d +365 | 17.9 (12.8-23.8) | 20.3 (13.8-27.7) | 12.5 (5.4-22.6) | .20 |
| Nonrelapse mortality, d +365 | 10.3 (6.5-15.2) | 11.7 (6.9-18.0) | 7.1 (2.3-15.9) | .34 |
| Relapse, d +365 | 15.9 (10.9-21.7) | 16.4 (10.4-23.5) | 14.6 (6.8-25.3) | .79 |
| CMV infection | ||||
| >300 copies per mL | 39.5 (32.1-46.7) | 43.0 (33.9-51.7) | 31.2 (19.4-43.8) | .29 |
| Invasive disease | 6.9 (3.7-11.3) | 8.2 (4.2-14.0) | 3.8 (0.7-11.5) | .27 |
| Any EBV viremia | 45.5 (35.7-54.7) | 44.0 (32.2-55.3) | 48.0 (31.6-62.6) | .30 |
| Day, median (95% CI) | ||||
| Neutrophil engraftment | 17.0 (11.6-29.9) | 17.0 (11.2-32.1) | 19.0 (12.0-28.6) | .24 |
| Platelets engraftment | 20.0 (11.0-59.7) | 19.5 (2.8-55.4) | 22.0 (14.0-79.8) | .07 |
| Outcome . | Total (N = 184) . | Developmental cohort (n = 128) . | Validation cohort (n = 56) . | P value . |
|---|---|---|---|---|
| Cumulative incidence, % (95% CI) | ||||
| aGVHD, day +100 | ||||
| GR2-4 | 48.9 (41.5-55.9) | 49.2 (40.3-57.6) | 48.2 (34.5-60.6) | .87 |
| GR3-4 | 8.7 (5.2-13.3) | 8.6 (4.5-14.3) | 8.9 (3.2-18.2) | .93 |
| Steroid refractory | 9.2 (5.6-14.0) | 11.7 (6.9-18.0) | 3.6 (0.7-11.0) | .08 |
| cGVHD, d +365 | ||||
| Mild to severe | 49.5 (42.0-56.5) | 52.3 (43.3-60.6) | 42.9 (29.6-55.4) | .27 |
| Moderate to severe | 41.3 (34.1-48.3) | 44.5 (35.7-52.9) | 33.9 (21.8-46.4) | .22 |
| aGFS, d +180 | 9.8 (6.0-14.6) | 9.4 (5.1-15.2) | 10.7 (4.3-20.5) | .08 |
| GRFS, d +365 | 14.1 (9.5-19.6) | 14.8 (9.3-21.6) | 12.5 (5.4-22.7) | .70 |
| Overall survival, d +365 | 17.9 (12.8-23.8) | 20.3 (13.8-27.7) | 12.5 (5.4-22.6) | .20 |
| Nonrelapse mortality, d +365 | 10.3 (6.5-15.2) | 11.7 (6.9-18.0) | 7.1 (2.3-15.9) | .34 |
| Relapse, d +365 | 15.9 (10.9-21.7) | 16.4 (10.4-23.5) | 14.6 (6.8-25.3) | .79 |
| CMV infection | ||||
| >300 copies per mL | 39.5 (32.1-46.7) | 43.0 (33.9-51.7) | 31.2 (19.4-43.8) | .29 |
| Invasive disease | 6.9 (3.7-11.3) | 8.2 (4.2-14.0) | 3.8 (0.7-11.5) | .27 |
| Any EBV viremia | 45.5 (35.7-54.7) | 44.0 (32.2-55.3) | 48.0 (31.6-62.6) | .30 |
| Day, median (95% CI) | ||||
| Neutrophil engraftment | 17.0 (11.6-29.9) | 17.0 (11.2-32.1) | 19.0 (12.0-28.6) | .24 |
| Platelets engraftment | 20.0 (11.0-59.7) | 19.5 (2.8-55.4) | 22.0 (14.0-79.8) | .07 |
GRFS, GVHD-relapse-free survival (ie, survival without relapse, aGVHD GR3-4, or moderate-to-severe cGVHD).
E-R model development
Details of the E-R model development and its validation are found in supplemental Methods.
Multivariable analysis and cumulative incidence plots
Using the validated Ctrough_1 risk group–outcome pair, multivariable analysis was performed to identify the final E-R model by adjusting for significant clinical covariates. This process was conducted for binary Ctrough_1-based risk groups (placebo and Ctrough_1 low vs Ctrough_1 high) in accordance with the aforementioned target Ctrough_1 identification process (described in supplemental Methods). We also performed this multivariate analysis for 3 risk groups (placebo vs Ctrough_1 low vs Ctrough_1 high) to evaluate the difference between placebo and the <39 μg/mL Ctrough_1 group. Finally, cumulative incidence function plots were created to visualize the effects of Ctrough_1-based risk groups.
Analysis of the impact of AUC0-28 and AUC0-infinity on cGVHD
Because cGVHD may be affected by longer exposure variables, we calculated both the area under the curve (AUC) from day 0 to +28 (AUC0-28) and the AUC from day 0 to infinity (AUC0-infinity) and performed a smooth regression analysis of these values vs the incidence of cGVHD.
Analysis of the interactions between immune reconstitution and abatacept exposure
The ABA2 study collected detailed information regarding the post-HCT reconstitution of T cells, B cells, and monocytes.2 This enabled an analysis of the effects of abatacept exposure on the reconstitution of immune cells after transplant via logistic regression and smooth regression analyses. We also assessed the impact of early (day −1 and +5) B-cell and monocyte counts (as representative APCs) on abatacept PK.
Results
Population PK modeling
Abatacept PK was characterized using an IV 2-compartment model with first-order elimination (supplemental Tables 3 and 4), which was consistent with the PK characteristics of abatacept in previous reports.12-14 The final population PK model from the developmental cohort included body weight and donor-recipient HLA matching (ie, HLA 7/8 vs 8/8; supplemental Figure 1) as both statistically and clinically significant covariates (supplemental Figure 2). Tests for model diagnostics and parameter uncertainty for PK modeling (including graphical goodness of fit,16 the sampling-importance-resampling method,17 and the prediction-corrected visual predictive check test18; supplemental Methods) indicated an acceptable model across the different disease cohorts (ie, HLA 7/8 vs HLA 8/8 vs RA/JIA; supplemental Table 4; supplemental Figures 3-5).
E-R analysis for ABA2
Characteristics of the ABA2 participants (both placebo and abatacept cohorts combined) in the developmental and validation cohorts are shown in Table 1. Clinical outcomes of interest (combined for patients receiving abatacept those receiving placebo) are summarized in Table 2. No statistical differences in patient characteristics and clinical outcomes were observed between the developmental and the validation cohorts.
Positive E-R relationship between abatacept Ctrough_1 and GR2-4 aGVHD without an E-R relationship for relapse or CMV or EBV reactivation
The median Ctrough_1 was 42.9 μg/mL (range, 19.9-94.7 μg/mL; interquartile range, 31.7-54.1) among patients in the developmental cohort who received abatacept (supplemental Figure 6). Of note, although all patients in both the 7/8 and 8/8 cohorts reached the planned target trough of 10 μg/mL, the median Ctrough_1 was different for the HLA 7/8 cohort (56.0 μg/mL) vs the HLA 8/8 cohort (38.9 μg/mL) (P < .01). We interrogated the predictability of Ctrough_1 as a continuous variable at specific thresholds for each of the clinical outcomes listed in Table 2, using a recursive partitioning algorithm. From this analysis, along with a bootstrap aggregating approach,19 Ctrough_1 was identified as the best classifier for the GR2-4 aGVHD outcome for the developmental cohort (Figure 1), with a Ctrough_1 threshold ≥39 μg/mL (95% confidence interval (CI), 24.1-50.9) significantly associated with a lower risk of GR2-4 aGVHD (P < .001 using Fine-Gray testing). Local polynomial regression plots visually corroborated these results (Figure 2). Thus, the Ctrough_1 threshold of 39 μg/mL was carried forward for further analysis and discussion. Of note, logistic regression analysis also identified significant E-R relationships between Ctrough_1 and several key secondary outcomes, including GR3-4 aGVHD, steroid-refractory aGVHD, and aGFS (supplemental Figure 7). However, an optimal Ctrough_1 threshold was not identifiable via recursive partitioning with acceptable crossvalidated errors for these secondary outcomes, because of small sample sizes. Importantly, there were no relationships identified between Ctrough_1 and 3 of the major complications of transplant: relapse, CMV reactivation/infection, and EBV reactivation (Figure 3).
Classification tree analysis with bootstrap for aGVHD GR2-4 at day +100. Classification tree analysis of the developmental cohort is shown (n = 128) (the validation cohort [n = 56] is not included). This method uses recursive partitioning based on the Gini purity index and enables the identification of variables that best predict GR2-4 aGVHD. Each box represents a node (second row in a box: the percentage of GR2-4 aGVHD within the node; third row in a box: sample size of the node and fraction of the initial node size). The initial tree was pruned per the crossvalidated error to avoid overfitting. The final model predicts the risk of aGVHD GR2-4 at day +100 by Ctrough_1 ≥39 vs <39 μg/mL (31% vs 62%). Ctrough_1 was identified as the most predictive classifier in 73.6% of the 1000 bootstrap data sets. Of these, the median Ctrough_1 threshold value was 39.0 μg/mL (95% CI, 24.1-50.9). BuCy, busulfan/cyclophosphamide; BuFlu, busulfan/fludarabine; CyTBI, cyclophosphamide/total body irradiation; FluMel, fludarabine/melphalan.
Classification tree analysis with bootstrap for aGVHD GR2-4 at day +100. Classification tree analysis of the developmental cohort is shown (n = 128) (the validation cohort [n = 56] is not included). This method uses recursive partitioning based on the Gini purity index and enables the identification of variables that best predict GR2-4 aGVHD. Each box represents a node (second row in a box: the percentage of GR2-4 aGVHD within the node; third row in a box: sample size of the node and fraction of the initial node size). The initial tree was pruned per the crossvalidated error to avoid overfitting. The final model predicts the risk of aGVHD GR2-4 at day +100 by Ctrough_1 ≥39 vs <39 μg/mL (31% vs 62%). Ctrough_1 was identified as the most predictive classifier in 73.6% of the 1000 bootstrap data sets. Of these, the median Ctrough_1 threshold value was 39.0 μg/mL (95% CI, 24.1-50.9). BuCy, busulfan/cyclophosphamide; BuFlu, busulfan/fludarabine; CyTBI, cyclophosphamide/total body irradiation; FluMel, fludarabine/melphalan.
E-R relationships, using local polynomial regression (developmental cohort). Shown in the blue line is the local polynomial regression (ie, Loess smooth regression) for E-R relationships for 3 exposure variables: (A) Ctrough_1; (B) Cmax_1; and (C) AUC1. The shaded area represents the 95% CI. Ctrough_1 showed the lowest P value (logistic regression). Each dot represents a single patient and associated Ctrough_1 and the probability of the event (0 [−], event absent; 1 [+], event present). AUC1, AUC of abatacept concentration-time; Cmax_1, maximum abatacept concentration after the first dose.
E-R relationships, using local polynomial regression (developmental cohort). Shown in the blue line is the local polynomial regression (ie, Loess smooth regression) for E-R relationships for 3 exposure variables: (A) Ctrough_1; (B) Cmax_1; and (C) AUC1. The shaded area represents the 95% CI. Ctrough_1 showed the lowest P value (logistic regression). Each dot represents a single patient and associated Ctrough_1 and the probability of the event (0 [−], event absent; 1 [+], event present). AUC1, AUC of abatacept concentration-time; Cmax_1, maximum abatacept concentration after the first dose.
Exposure-outcome relationships based on the Ctrough_1 of key secondary outcomes in the developmental cohort. Shown are graphical assessments of E-R relationships, using scatter plots with local polynomial regression lines (ie, Loess smooth regression; blue line). Each dot represents a single patient and associated Ctrough_1 and the probability of the event (0 [−], event absent; 1 [+], event present). This analysis demonstrates no associations between Ctrough_1 and any of the tested outcomes. The shaded area represents 95% CI for the typical value. CMV- and EBV-related outcomes are assessed as approximation because the censoring event (ie, relapse) is treated as absence of the outcome of interest. All of the other outcomes do not have censoring events. P values are calculated using logistic regression.
Exposure-outcome relationships based on the Ctrough_1 of key secondary outcomes in the developmental cohort. Shown are graphical assessments of E-R relationships, using scatter plots with local polynomial regression lines (ie, Loess smooth regression; blue line). Each dot represents a single patient and associated Ctrough_1 and the probability of the event (0 [−], event absent; 1 [+], event present). This analysis demonstrates no associations between Ctrough_1 and any of the tested outcomes. The shaded area represents 95% CI for the typical value. CMV- and EBV-related outcomes are assessed as approximation because the censoring event (ie, relapse) is treated as absence of the outcome of interest. All of the other outcomes do not have censoring events. P values are calculated using logistic regression.
Validation of the E-R model
In the validation cohort, the median Ctrough_1 among those who received abatacept was 46.2 μg/mL (range, 26.0-83.1; supplemental Figure 6). A significant association between Ctrough_1 ≥39 μg/mL and GR2-4 aGVHD was also observed in this cohort (P = .03).
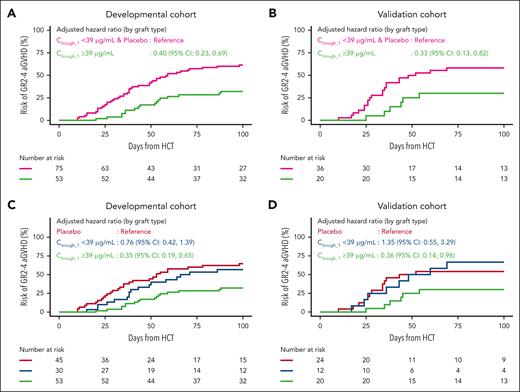
A predictor Ctrough_1 ≥39 μg/mL identifies patients who have a lower cumulative incidence of GR2-4 aGVHD
In both the developmental and validation cohorts, multivariable analysis demonstrated that a Ctrough_1 ≥39 μg/mL was independently associated with a lower risk of developing GR2-4 aGVHD at day +100, even after adjusting for graft type (Figure 4), which was the only significant clinical covariate identified through univariate analysis. As such, patients with a Ctrough_1 ≥39 μg/mL demonstrated a lower cumulative incidence of GR2-4 aGVHD, with a hazard ratio (HR) of 0.40 (95% CI, 0.23-0.69) for the developmental cohort and 0.33 (95% CI, 0.13-0.82) for the validation cohort; Figure 4A-B). Of note, this analysis did not reveal a significant difference in the cumulative incidence and HR of GR2-4 aGVHD between patients receiving placebo and those who were treated with abatacept but achieved a Ctrough_1 <39 μg/mL (HR, 0.76; 95% CI, 0.42-1.39 for the developmental cohort; and HR, 1.35; 95% CI, 0.55-3.29 for the validation cohort; Figure 4C-D). In addition, the cumulative incidence of GR2-4 aGVHD when Ctrough_1 was divided into quartiles and tertiles corroborated the decreasing risk of aGVHD with increasing Ctrough_1 (supplemental Figure 8).
Cumulative incidence of GR2-4 aGVHD divided by Ctrough_1. (A) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo and those with Ctrough_1 <39 μg/mL (pink) vs those with a Ctrough_1 ≥39 μg/mL (green) (developmental cohort). (B) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo and those with Ctrough_1 <39 μg/mL (pink) vs those with a Ctrough_1 ≥39 μg/mL (green) (validation cohort). (C) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo (red), those with Ctrough_1 <39 μg/mL (blue), and those with a Ctrough_1 ≥39 μg/mL (green) (developmental cohort). (D) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo (red), those with Ctrough_1 <39 μg/mL (blue), and those with a Ctrough_1 ≥39 μg/mL (green) (validation cohort). The number of patients at risk are listed below the graph. Cumulative incidence plots represent unadjusted estimates, whereas the HR estimates are adjusted for the graft type.
Cumulative incidence of GR2-4 aGVHD divided by Ctrough_1. (A) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo and those with Ctrough_1 <39 μg/mL (pink) vs those with a Ctrough_1 ≥39 μg/mL (green) (developmental cohort). (B) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo and those with Ctrough_1 <39 μg/mL (pink) vs those with a Ctrough_1 ≥39 μg/mL (green) (validation cohort). (C) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo (red), those with Ctrough_1 <39 μg/mL (blue), and those with a Ctrough_1 ≥39 μg/mL (green) (developmental cohort). (D) Cumulative incidence of GR2-4 aGVHD for patients receiving placebo (red), those with Ctrough_1 <39 μg/mL (blue), and those with a Ctrough_1 ≥39 μg/mL (green) (validation cohort). The number of patients at risk are listed below the graph. Cumulative incidence plots represent unadjusted estimates, whereas the HR estimates are adjusted for the graft type.
Sensitivity analysis for the impact of 7/8 vs 8/8 HLA abatacept exposure on clinical outcomes
Because we observed different PK for patients in the 7/8 vs 8/8 cohorts (supplemental Table 4; supplemental Figure 1) in a post hoc analysis, we investigated the E-R relationships for each of these cohorts separately. Of note, because of the smaller sample sizes (n = 42 for the HLA 7/8 cohort, and n = 142 for the HLA 8/8 cohort), we did not divide these further into developmental and validation cohorts. As shown in supplemental Figure 9, this analysis demonstrated that Ctrough_1 remained significantly associated with GR2-4 aGVHD for each cohort and also confirmed a lack of exposure-toxicity associations in the 2 cohorts. Moreover, recursive partitioning was able to confirm Ctrough_1 ≥39 μg/mL as associated with a lower risk of GR2-4 aGVHD for the HLA 8/8 cohort (HR, 0.33; 95% CI, 0.17-0.64; supplemental Figure 10). A threshold of Ctrough_1 could not be identified for the HLA 7/8 cohort, likely because of the small sample size.
Sensitivity analysis for the association of cGVHD with longer exposure variables
Although this study was mostly focused on aGVHD, given the importance of cGVHD as a transplant outcome and the potential importance of longer exposure measurements for this end point, we analyzed the AUC from day 0 to +28 (AUC0-28) and the AUC from day 0 to infinity (AUC0-infinity) and performed a smooth regression analysis of these values vs those of cGVHD. As shown in supplemental Figure 11, we found no association between these longer exposure measures and cGVHD.
Abatacept Ctrough_1 and immune reconstitution
The collection of detailed hematologic and immune reconstitution data on ABA22 enabled an analysis of the impact of abatacept exposure on hematopoietic reconstitution as well as an interrogation for any relationship between individual patient antigen presenting cell counts and abatacept PK. As depicted in supplemental Figure 12, we measured the relationship of Ctrough_1 with the following reconstitution variables: time to neutrophil engraftment, time to platelet engraftment, day +100 total T-cell (CD3+) count, day +100 CD4+ T-cell count, day +100 CD8+ T-cell count, and day +100 regulatory T-cell count, which demonstrated that none were affected by the abatacept Ctrough_1. Notably, the day +100 total B-cell counts were directly related to the Ctrough_1, with higher day +100 B cells positively correlating with higher Ctrough_1 values. We speculate that this may be because of the increased control of GVHD with higher abatacept Ctrough_1 and the potential protective effect of GVHD control on the more slow-to-reconstitute B-cell compartment after HCT.20 Additionally, we examined whether B-cell and monocyte counts (both of which express CD80/86) on days −1 or +5 (the time points corresponding to Ctrough_1) affected abatacept PK. As shown in supplemental Figure 13, we found no association between day −1 or +5 B-cell or monocyte counts and abatacept PK parameters.
Impact of a dose maximum for abatacept on the Ctrough_1
The identification of an optimal Ctrough_1 ≥39 μg/mL suggested that patients with a body weight >100 kg might be at a risk of not achieving this exposure, given the practice of capping the abatacept dose at 1000 mg that was used in the ABA2 trial. This risk is underscored by the fact that in addition to the current analysis of the HCT cohort, all previous abatacept population PK analyses12-14 identified body weight as a key determinant of exposure (ie, the greater the weight, the higher the clearance and volume of distributions; supplemental Table 4). Of note, 17 of 115 (15%) patients in the ABA2 trial who received abatacept had a body weight ≥100 kg (range, 100-143 kg), with 8 of 17 (47%) of these patients having a body weight of between 100 and 110 kg. Although no difference in the risk of GR2-4 aGVHD was observed based on a body weight >100 kg (P = .67), we considered that, especially with capping the abatacept dose at 1000 kg, patients with a body weight significantly >100 kg might be at an increased risk of not attaining an optimal Ctrough_1. To investigate this, we performed a simulation of the Ctrough_1 over a range of body weights and investigated the role of capping the dose at 1000 mg (Figure 5). As shown in the figure, patients with a body weight of >100 kg demonstrated a progressively downward trend in the proportion who would attain the target Ctrough_1, suggesting the possibility that capping the abatacept dose is not optimal for patient undergoing HCT.
Exposure simulation for Ctrough_1. Shown is the simulation of Ctrough_1 for patients in the 7/8 URD HCT cohort (left) and the 8/8 URD HCT cohort (right). The top graphs depict simulations that include a maximum dose of 1000 mg. The bottom graphs depict simulations that have no maximum dose. The black line represents the 50th percentile and the gray range represents the 90th percentile interval (bottom: 5th, top: 95th) of 1000 simulated Ctrough_1 values. The range above the red dashed lines represents the optimal target for GR 2-4 aGVHD (Ctrough_1 ≥39 μg/mL).
Exposure simulation for Ctrough_1. Shown is the simulation of Ctrough_1 for patients in the 7/8 URD HCT cohort (left) and the 8/8 URD HCT cohort (right). The top graphs depict simulations that include a maximum dose of 1000 mg. The bottom graphs depict simulations that have no maximum dose. The black line represents the 50th percentile and the gray range represents the 90th percentile interval (bottom: 5th, top: 95th) of 1000 simulated Ctrough_1 values. The range above the red dashed lines represents the optimal target for GR 2-4 aGVHD (Ctrough_1 ≥39 μg/mL).
Discussion
Here, we report an E-R analysis of abatacept for aGVHD prevention, which helped identify 2 clinically and biologically important findings. Firstly, this analysis demonstrated an E-R association between a higher abatacept Ctrough_1 (≥39 μg/mL) and a lower risk of GR2-4 aGVHD. This finding is distinct from the E-R analysis in RA/JIA populations, in which maximum abatacept efficacy was reported with steady-state Ctrough_1 ≥10 μg/mL.12-14 The ABA2 dosing design resulted in all patients achieving the RA/JIA target of 10 μg/mL. However, although ∼60% of patients receiving abatacept attained a Ctrough_1 ≥39 μg/mL, the other 40% demonstrated lower Ctrough_1 values (19.9-38.9 μg/mL) and in these patients, although in a relatively small sample size, the risk of GR2-4 aGVHD was comparable with that of the placebo group. This result underscores the central role that early T-cell activation plays in the initiation of aGVHD,21 and the biologic impact of abatacept on the control of this disease.
The second finding resulted from the assessment of the exposure-toxicity profile of abatacept during URD HCT, with the PK-PD analysis being notable for the lack of any exposure-toxicity relationship with key HCT adverse events. In particular, there was a lack of association between relapse and abatacept exposure, and no exposure-toxicity relationship for CMV reactivation/end organ disease and EBV reactivation. Although this result needs to be interpreted cautiously, given that the trial was not specifically powered to assess these outcomes, and, with respect to relapse, the fact that few trial participants had advanced disease, and no correlation was found between abatacept exposure and this critical outcome, these results suggest a favorable PK-PD profile of this new agent for aGVHD prevention.
Our population PK model was largely consistent with the previously reported model structure in the RA/JIA population12-14; however, 1 notable difference was the decreased clearance in patients receiving HCT as compared with those with RA/JIA. This disparity may be explained by the difference in the number of abatacept target cells in HCT vs RA/JIA. Target-mediated drug disposition (ie, the higher the target amount, the faster the clearance) is a well-established tenet of elimination kinetics in therapeutic proteins.22 The targets of abatacept, CD80 and CD86, are expressed on APCs, which are likely expanded and activated in patients with RA/JIA because of chronic inflammation.23 In contrast, patients receiving HCT undergo cytotoxic chemo-irradiation therapy before the administration of abatacept, and at early time points after transplantation patients receiving HCT have not yet fully reconstituted donor APCs.24 Both of these factors would lead to decreased abatacept target concentrations and thus a lower degree of target-mediated drug clearance. It is notable that within the ABA2 study, we did not find an association between B-cell and monocyte counts immediately after the start of abatacept therapy and individual abatacept PK parameters. This observation suggests that although lower APC levels may have increased abatacept levels on the whole in patients receiving HCT compared with patients with RA/JIA, to the extent that we could query this relationship within ABA2, we could not detect a relationship between individual patient hematologic/immune reconstitution and abatacept PK.
The discovery of an HCT-specific E-R relationship is also congruent with previous in vitro studies with abatacept, which have demonstrated that, at a molecular level, the binding capacity of abatacept to CD80 and CD86 increases in a concentration-dependent manner.25 This may be particularly relevant for CD86, with previous work demonstrating a sigmoidal function to describe CD86 receptor occupancy by abatacept, with 50% occupancy at 26.9 μg/mL and approximate occupancy <5% at 10 μg/mL, 80% at 40 μg/mL, and 90% at 50 μg/mL.26 Our analysis of abatacept PK-PD in HCT implies that the biologic effects of abatacept may be more pronounced at concentrations ≥39 μg/mL compared with ≥10 μg/mL.
Of the secondary analyses interrogated, the finding that there was a significant association between abatacept Ctrough_1 and a decreased risk of steroid-refractory aGVHD has implications for the efficacy of abatacept, as well as for its ability to decrease HCT-associated complications. In terms of efficacy, this observation suggests that abatacept has biologic activity against the severe as well as milder manifestations of aGVHD. In terms of mitigating HCT-associated complications, there are also important implications of this relationship: given that patients with steroid-refractory aGVHD are treated with a panoply of secondary agents, many of which have significant immunosuppression-related and systemic toxicities,27 increased abatacept Ctrough_1 would be expected to decrease the need for patient treatment with these immunosuppressive therapies, and their associated risks. Because details of the secondary agents used were not part of the planned ABA2 analysis, a detailed examination of the impact of Ctrough_1 on exposure to other immunosuppressive drugs remains an important area for future studies.
One of the unexpected findings of this PK-PD analysis was that patients who received transplants from 8/8 HLA-matched donors demonstrated lower abatacept exposure (supplemental Figure 1) compared with those who received transplants from 7/8 HLA-matched donors. This difference was because of lower abatacept clearance and lower volume of distribution in the latter group. Although these 2 groups had selected differences in baseline characteristics (including lower median age and fewer underrepresented minorities in the 8/8 cohort), none of these baseline factors were found to be significantly associated with abatacept PK in this study. However, the present study was not specifically powered to detect these differences, and future analyses, especially with regard to differences in abatacept clearance in underrepresented minorities, are clearly warranted. The difference in abatacept clearance is also unlikely to be due to differences in the conduct of transplantation between the 7/8 and 8/8 cohorts, or differences in supportive care. These patients were enrolled contemporaneously, such that transplant standards were the same for both, and the majority of patients in both cohorts received intensive conditioning regimens, which is the major driver for requirements of supportive care. Indeed, the individual conditioning regimens used were not found to influence abatacept PK. Moreover, abatacept, as a large-molecule drug, is expected to have minimal drug-drug interactions because it is not a substrate for common drug transporters or metabolizing enzymes.22 Although the reasons for the HLA disparity–based PK differences are currently unclear, this PK-PD analysis suggests a novel explanation for the fact that the 7/8 abatacept cohort demonstrated outcomes that were at least as good, and sometimes superior, to the 8/8 abatacept cohort in the ABA2 study.2,15
This study does have certain limitations. Firstly, the sample size for the HCT cohort was relatively small, suggesting that further validation in a larger cohort would be advantageous, and may enable the detection of an optimal Ctrough_1 threshold for additional exploratory outcomes, including those with high clinical significance, such as GR3-4 aGVHD, steroid-refractory aGVHD, and aGFS. Each of these outcomes did demonstrate a positive E-R relationship by logistic regression (supplemental Figure 7) but, likely based on sample size, an optimal Ctrough_1 threshold could not be identified. Secondly, it should be noted that unlike previous studies with chronic abatacept dosing in RA/JIA, the 4-dose ABA2 dosing regimen was not designed to reach a steady-state. Thus, this E-R model based on Ctrough_1 may not be directly comparable with the steady-state Ctrough in the RA/JIA regimen. This study also did not evaluate the impact of other concomitant medications, including calcineurin inhibitor exposures, on abatacept exposure or aGVHD. Although the protocol-specified targets for calcineurin inhibitor exposures likely mitigated this impact, future studies would be needed to rigorously evaluate any relationship between other medications and abatacept E-R.
One of the more provocative implications of this analysis is that it may be advantageous to escalate doses 2, 3, and/or 4 in patients for whom the abatacept Ctrough_1 is <39 μg/mL, with the assumption that by increasing total abatacept exposure in this manner, the higher risk of GR2-4 aGVHD in patients with Ctrough_1 <39 μg/mL could be mitigated. It is also possible that delivering a higher loading-dose of abatacept on day −1 could achieve more efficient target saturation, thereby increasing the likelihood of reaching the Ctrough_1 target. Although these personalized- or loading-dose strategies would be feasible, it is important to note that, because of the fact that a uniform dosing regimen was used in ABA2, it is not possible to model the impact of these modifications on either efficacy or safety events with the current data set. Thus, although dose optimization of abatacept could have a salutary effect on transplant outcome, further clinical trials would be necessary to determine how best to deploy each of these strategies during abatacept prophylaxis, especially to accurately model both efficacy and toxicity if PK-based dose escalation was initiated.
One immediately actionable recommendation that has emerged from this E-R analysis is that capping of abatacept at 1000 mg is likely not necessary, and may potentially be disadvantageous in the HCT cohort. Our population PK model, along with previous models derived from RA/JIA,12-14 describe a continuous negative association between body weight and abatacept exposure. As such, the probability of attaining the target Ctrough_1 would be improved in patients with a body weight >100 kg if dose capping at 1000 mg was removed. This may be especially important given emerging data for an increased risk of GVHD in the setting of obesity.28-30 Moreover, a potential negative effect of this dose capping was suggested in a previous study in RA cohorts, which used weight-tiered fixed IV abatacept dosing with the same maximum dose of 1000 mg (ie, 500 mg for those with a body weight <60 kg, 750 mg for body weights of 60-100 kg, and 1000 mg for body weight ≥100 kg). This study also reported an association between a body weight ≥100 kg and a poor joint symptom response (odds ratio, 0.61; 95% CI, 0.44-0.84).12
In conclusion, we have developed and validated a Ctrough_1 threshold model to determine E-R relationships of abatacept in URD HCT. Our results identify an E-R relationship for efficacy in the ABA2 study, without identifying any exposure-toxicity relationships. This analysis provides further support for the positive attributes of abatacept for preventing aGVHD, and creates a foundation for future PK-based dosing modifications to optimize CD28:CD80/86 costimulation blockade for GVHD prophylaxis.
Acknowledgments
The authors thank the patients and families who participated in the ABA2 clinical trial. The authors acknowledge the scientists at Bristol Myers Squibb who made previous PK and study data available for this analysis, and for input and helpful discussions on data analysis and PK modeling. The authors also thank James Kaminski for early discussions on statistical analysis.
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) grants R01-HL095791 and P01- HL158505 (L.S.K.). B.R.B. is supported by NIH National Institute of Allergy and Infectious Diseases (NIAID) grant R37 AI 34495 and NIH NHLBI grant R01 HL56067. This work was also partially funded by Bristol Myers Squibb. M.A.P. is supported by NIH NIAID grant1U01AI126612-01A1, NIH National Cancer Institute grant P30CA040214, and NIH NHLBI grant 2UG1HL069254.
Authorship
Contribution: A.L., J.T.H., B.B., K.B., and Y.S. generated data; T.T., M.A.-K., M.J., and L.S.K. analyzed the data; and T.T., M.A.-K., M.J., B.B., K.B., Y.S., A.Y., D.S.N., S.W.C., J.D., C.D., R.G., M.G., A.C.H., D.J., N.L., N.F., M.A.P., S.S., A.P., K.R.S., G.A.Y., B.R.B., J.T.H., B.W., A.L., M.Q., and L.S.K. wrote and edited the manuscript.
Conflict-of-interest disclosure: B.R.B. receives research funding from Carisma Therapeutics; is a consultant at Magenta Therapeutics; and is a founder of Tmunity. L.S.K. is on the scientific advisory board for HiFiBio and Mammoth Biosciences; reports research funding from Magenta Therapeutics, Bluebird Bio, Bristol Myers Squibb, Novartis, Gilead, EMD-Serono, and Regeneron Pharmaceuticals; reports consulting fees from Vertex Pharmaceuticals; reports grants and personal fees from Bristol Myers Squibb during the conduct of the study; and reports grants and personal fees from Bristol Myers Squibb outside the submitted work; her financial interests with Bristol Myers Squibb are managed under an agreement with Harvard Medical School; she has a patent “Method to prevent relapse after transplant,” which is pending, and a patent “Method to prevent GVHD after transplant” with royalties paid. The remaining authors declare no competing financial interests.
Correspondence: Leslie S. Kean, Stem Cell Transplantation Program, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, 1 Blackfan Circle, Karp Research Bldg 08215, Boston, MA 02115; e-mail: leslie.kean@childrens.harvard.edu.
References
Author notes
Data are available on request from the corresponding author, Leslie S. Kean (leslie.kean@childrens.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Classification tree analysis with bootstrap for aGVHD GR2-4 at day +100. Classification tree analysis of the developmental cohort is shown (n = 128) (the validation cohort [n = 56] is not included). This method uses recursive partitioning based on the Gini purity index and enables the identification of variables that best predict GR2-4 aGVHD. Each box represents a node (second row in a box: the percentage of GR2-4 aGVHD within the node; third row in a box: sample size of the node and fraction of the initial node size). The initial tree was pruned per the crossvalidated error to avoid overfitting. The final model predicts the risk of aGVHD GR2-4 at day +100 by Ctrough_1 ≥39 vs <39 μg/mL (31% vs 62%). Ctrough_1 was identified as the most predictive classifier in 73.6% of the 1000 bootstrap data sets. Of these, the median Ctrough_1 threshold value was 39.0 μg/mL (95% CI, 24.1-50.9). BuCy, busulfan/cyclophosphamide; BuFlu, busulfan/fludarabine; CyTBI, cyclophosphamide/total body irradiation; FluMel, fludarabine/melphalan.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/8/10.1182_blood.2023020035/2/m_blood_bld-2023-020035-gr1.jpeg?Expires=1769392819&Signature=T8dL2Io7xowFWP1PxV-ZClISRMglUi81kh3C7bgz9hFfUhJQTaDfL7~EJiwgih3WI3wuOZ8iN-MfjAZzH5ZHJQnqoWzUZX~bse7rpbCXv~H82-D2TVIVLgj882NWXt6ER7hT5JBdaEGl-smxTyUzGZ0PFAiHFxphGcxYTuoqJ9K5xNlsMWaPvLfsQ0yrmjDXZEBZQA6Pmkb51gzkntdduaTa-l-3IHLjop~6kfcywRtpJynHiBNN2Qoi7TR6Y3xcZ20sJM2yqze3C-pW2b~6LDAkmEww1rToV3hgV0BOWybGMM574KKD9hx5rUnDdYTNjvMJMeFdFEY8XJO5H6q3Lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![E-R relationships, using local polynomial regression (developmental cohort). Shown in the blue line is the local polynomial regression (ie, Loess smooth regression) for E-R relationships for 3 exposure variables: (A) Ctrough_1; (B) Cmax_1; and (C) AUC1. The shaded area represents the 95% CI. Ctrough_1 showed the lowest P value (logistic regression). Each dot represents a single patient and associated Ctrough_1 and the probability of the event (0 [−], event absent; 1 [+], event present). AUC1, AUC of abatacept concentration-time; Cmax_1, maximum abatacept concentration after the first dose.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/8/10.1182_blood.2023020035/2/m_blood_bld-2023-020035-gr2.jpeg?Expires=1769392819&Signature=MwWteFtGVqq~g62BNlzM80XAKJfcZrC5K~P49kcHCgEFW22RRZxIrvadOBFIP4aCZ9lt2lE5CXbUXTjONM-0zufFr1tBKIH0CoIQfgtWzIgXDf26Ge4PQdBSpDKGa2meSlG6tqTsMH53GKpWStARHxo90xn44BhnkJWo1XJlr87Nuh828MQbq2RROJVSUjPfzUsgWG83sOWwJ1roqkNCsfRqDsn~MAN7HNRiSlH1cOybzPddsvOlqBCJnTC0lh0BApjM5GXx8PmLaYwezkPa4Ox5-TwUFY9MT4aP9bR5lzNWvKXk4T0lU46Oq2ltsVdF1mfJRVbzdmu0wkn3o3T3Kg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Exposure-outcome relationships based on the Ctrough_1 of key secondary outcomes in the developmental cohort. Shown are graphical assessments of E-R relationships, using scatter plots with local polynomial regression lines (ie, Loess smooth regression; blue line). Each dot represents a single patient and associated Ctrough_1 and the probability of the event (0 [−], event absent; 1 [+], event present). This analysis demonstrates no associations between Ctrough_1 and any of the tested outcomes. The shaded area represents 95% CI for the typical value. CMV- and EBV-related outcomes are assessed as approximation because the censoring event (ie, relapse) is treated as absence of the outcome of interest. All of the other outcomes do not have censoring events. P values are calculated using logistic regression.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/8/10.1182_blood.2023020035/2/m_blood_bld-2023-020035-gr3.jpeg?Expires=1769392819&Signature=ZDKHwhSGRCTeQuDKr~ftwNQO~kXJK3XVc29ghjwkGOp-7jt9n4nNknjKYr9u-850ZacURG~O3hMzfxIDjENtFOLdlfByvhshfVQmKvSuQIamYaJyJ5zqDepQiP3ou7WTkA-MxPZclP46k9TjF7pudZGMJ2rzP4nKeJ3-9OWMbeGCYHje4Cf7R7GBvvONg8T4VXa-bhIU9kKm5fSocNJxYq7vMBuvihwkXvJq82IHtqqRVkv~lsdqgthlwukZFWaitAn8rUWwTDq3wfAeabTYfBZ1mIHZ8AyP3tmbLFkxj1RqsUKKHLSKMruWeDdtBC9V7FldwoT~0Nyfup8eOVPtrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal