Key Points
Acalabrutinib has a favorable benefit-risk profile, including lower incidence of cardiovascular-related toxicities, vs ibrutinib.
AE burden score allowed further comparison of the safety of acalabrutinib vs ibrutinib accounting for AE duration, recurrence, and grade.
Abstract
ELEVATE-RR demonstrated noninferior progression-free survival and lower incidence of key adverse events (AEs) with acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia. We further characterize AEs of acalabrutinib and ibrutinib via post hoc analysis. Overall and exposure-adjusted incidence rate was assessed for common Bruton tyrosine kinase inhibitor–associated AEs and for selected events of clinical interest (ECIs). AE burden scores based on previously published methodology were calculated for AEs overall and selected ECIs. Safety analyses included 529 patients (acalabrutinib, n = 266; ibrutinib, n = 263). Among common AEs, incidences of any-grade diarrhea, arthralgia, urinary tract infection, back pain, muscle spasms, and dyspepsia were higher with ibrutinib, with 1.5- to 4.1-fold higher exposure-adjusted incidence rates. Incidences of headache and cough were higher with acalabrutinib, with 1.6- and 1.2-fold higher exposure-adjusted incidence rate, respectively. Among ECIs, incidences of any-grade atrial fibrillation/flutter, hypertension, and bleeding were higher with ibrutinib, as were exposure-adjusted incidence rates (2.0-, 2.8-, and 1.6-fold, respectively); incidences of cardiac events overall (the Medical Dictionary for Regulatory Activities system organ class) and infections were similar between arms. Rate of discontinuation because of AEs was lower for acalabrutinib (hazard ratio, 0.62; 95% confidence interval, 0.41-0.93). AE burden score was higher for ibrutinib vs acalabrutinib overall and for the ECIs atrial fibrillation/flutter, hypertension, and bleeding. A limitation of this analysis is its open-label study design, which may influence the reporting of more subjective AEs. Overall, event-based analyses and AE burden scores demonstrated higher AE burden overall and specifically for atrial fibrillation, hypertension, and hemorrhage with ibrutinib vs acalabrutinib. This trial was registered at www.clinicaltrials.gov as #NCT02477696.
Introduction
Bruton tyrosine kinase inhibitors (BTKis) are effective targeted therapies for chronic lymphocytic leukemia (CLL).1 Ibrutinib, the first covalent BTKi approved for CLL/small lymphocytic lymphoma, is highly effective but associated with adverse events (AEs), particularly cardiovascular toxicities, that can lead to treatment discontinuation.1-5 In addition to its targeted binding of BTK, a variety of receptor and nonreceptor tyrosine kinases are covalently inhibited by ibrutinib,6 likely contributing to AEs.1,7-10
Acalabrutinib is a more selective, covalent BTKi approved for CLL/small lymphocytic lymphoma with decreased off-target activity vs ibrutinib as demonstrated by in vitro studies.1,7,11 In the phase 3 head-to-head ELEVATE-RR trial (NCT02477696), after a median study follow-up of 40.9 months, progression-free survival with acalabrutinib was noninferior to ibrutinib (median progression-free survival of 38.4 months for both acalabrutinib and ibrutinib as assessed by an independent review committee) in patients with previously treated CLL with del(17p) or del(11q).12 Any-grade atrial fibrillation/flutter was significantly less frequent (9.4% vs 16.0%), with a longer median time to event (28.8 vs 16.0 months) with acalabrutinib vs ibrutinib, and fewer patients discontinued treatment because of AEs with acalabrutinib (14.7%) vs ibrutinib (21.3%).12
The ELEVATE-RR trial provides an opportunity to further characterize the safety profiles of acalabrutinib and ibrutinib, including through the use of the AE burden score, which is a novel statistical methodology that combines AE duration, recurrence, and grade weighting into a single score.13 Herein, we report data from post hoc analyses of ELEVATE-RR to compare the safety profiles of acalabrutinib and ibrutinib using additional methods, including event-based comparisons and AE burden score analyses.
Methods
Study design and treatment
ELEVATE-RR14 was a phase 3, randomized, international, multicenter, open-label, noninferiority trial comparing the efficacy and safety of acalabrutinib and ibrutinib in adults with previously treated CLL. Patients who were eligible had previously received ≥1 prior therapy and required treatment per the International Workshop on Chronic Lymphocytic Leukemia 2008 criteria,15 had an Eastern Cooperative Oncology Group performance status score of ≤2, and had del(17)(p13.1) and/or del(11)(q22.3) by interphase fluorescence in situ hybridization. Key exclusion criteria were presence of significant cardiovascular disease, receipt of concomitant warfarin or vitamin K antagonist equivalent, and prior treatment with a BTK or B-cell lymphoma 2 inhibitor. History of atrial fibrillation/flutter was not an exclusion criterion.
Patients were randomized to receive oral acalabrutinib 100 mg twice daily or oral ibrutinib 420 mg once daily until disease progression or unacceptable toxicity; randomization was stratified by del(17p) status (yes or no), Eastern Cooperative Oncology Group performance status score (2 vs ≤1), and number of prior therapies (1-3 vs ≥4). The protocol was approved by an institutional review board and an independent ethics committee before the study. All patients provided written informed consent. The study was conducted in accordance with the protocol, local regulations, and the principles of the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practices.
Safety assessments
These post hoc analyses further characterize the safety profiles of acalabrutinib and ibrutinib in ELEVATE-RR, including incidence and exposure-adjusted incidence rate (events per 100 person-months) of common BTKi-associated AEs (events of any grade occurring in ≥10% in either arm) and events of clinical interest (ECIs; as defined by Medical Dictionary for Regulatory Activities [MedDRA] version 23.0), including overall cardiac events (MedDRA system organ class), atrial fibrillation/flutter, hypertension, bleeding, and infections. AEs selected for assessment in this analysis were typically more common (occurring in >10% of patients) and based on those described previously by Lipsky and Lamanna as BTKi treatment–related AEs necessitating specialized management16; the included ECIs were also defined by regulatory bodies and included in the product label safety information of both products.
ECIs of atrial fibrillation/flutter, hypertension, and bleeding were further characterized by time to first onset, cumulative incidence, incidence within specific patient subgroups (age [<65 years, ≥65 years], number of prior lines of therapy [1-3, ≥4], and a history of a given event [yes or no]), and AE management (dose reductions, treatment discontinuations, and relevant concomitant medications). Among patients with atrial fibrillation/flutter or hypertension, concomitant medications for these events (ie, angiotensin-converting enzyme inhibitors, β-blockers, calcium channel blockers, and all other antihypertensives) were also examined in patients with or without prior history of event. Possible association with other subsequent AEs was assessed, including incidence of hypertension, hemorrhage, or major hemorrhage subsequent to atrial fibrillation/flutter events, and of atrial fibrillation/flutter, hemorrhage, or major hemorrhage subsequent to hypertension events. A subsequent AE was an event that began after the start date of the ECI, regardless of the end date of either event. Cumulative incidence of atrial fibrillation/flutter and hypertension over time was evaluated in patient subgroups with or without a history of these events; prevalence of any-grade atrial fibrillation/flutter, hypertension, and hemorrhage was analyzed by yearly interval up to year 5.
The ECI of “infections” was further characterized by time to onset, incidence within specific patient subgroups (age [<65 years, ≥65 years], number of prior lines of therapy [1-3, ≥4]), infections leading to treatment discontinuation and dose reduction, and type of infection (upper respiratory tract infections, urinary tract infections, pneumonia, sepsis, or opportunistic infections). Serious infections and infections resulting in death were also reported.
A supplemental analysis assessed incidences of any-grade cardiac arrhythmias, any-grade hypertension, and grade ≥3 infection by age, sex, number of prior therapies, concomitant medication use, comorbidities, lymphocyte counts, and hemoglobin levels at baseline.
AE burden was further assessed using a calculated AE burden score that considers grade, duration, and recurrence of the AE based on methodology adapted from Ruppert et al.13 An AE burden score was assessed for AEs overall; the ECIs of atrial fibrillation/flutter, hypertension, bleeding, and infections; and the typically more common (occurring in >10% of patients) BTKi-associated symptomatic AEs of fatigue, headache, diarrhea, and musculoskeletal events. Overall, AE burden score was analyzed by time interval and polypharmacy status (polypharmacy defined as receipt of >3 concomitant medications at baseline) as a surrogate for comorbidities.17
Patient-reported outcome (PRO) assessments
PRO assessments with potential relevance to safety outcomes included changes from baseline in the European Organization for Research and Treatment of Cancer Quality of Life Questionnaires-Core 30 (EORTC QLQ-C30) Global Health Status (GHS) and the EuroQoL Five-Dimension 3 Level (EQ-5D-3L) visual analog score (VAS), both of which assess patient-reported health-related quality of life/health status. Both were completed in the first week of treatment (first visit after randomization), at week 12, every 4 weeks thereafter until week 24, and then every 12 weeks thereafter until treatment discontinuation.18,19
Statistical analysis
Cumulative incidence of selected ECIs and time to treatment discontinuation overall (for any reason) and because of AEs were analyzed using the Kaplan-Meier method and Cox proportional hazards model. Time to event onset was analyzed descriptively. For analysis of AE prevalence by yearly interval, multiple onsets of the same AE within a specific yearly interval were counted once, and the same AE term continuing across several yearly intervals was counted in each interval.
Grade 1 to 4 AEs were weighted per their Common Terminology Criteria for Adverse Events (version 4.03) severity grades; grade 5 AEs were weighted as 10 to emphasize the impact of the fatal outcome.20 Patients with no AE had a score of 0. Separate scores were calculated for grade 1 to 4 AEs and grade 1 to 5 AEs. For AEs that were ongoing either at the time of death, end of study, or data cutoff date, the end date was imputed as the earliest of either death date, end of study date, or data cutoff date. One patient had a missing AE start date, which was imputed as the treatment start date. For the analysis of AE burden score by time interval, the end of the AE was defined as the earliest of the end of the AE, end of follow-up year, or end of treatment-emergent period, for each yearly interval; patients had to be exposed to treatment within the specific year of follow-up to be included in the analysis for that year. AE burden scores were analyzed using a 2-sided Wilcoxon rank-sum test.
PROs were analyzed in the intent-to-treat population. Changes from baseline in EORTC QLQ-C30 GHS and EQ-5D-3L VAS were analyzed using least squares means and mixed model repeated measures. Meaningful improvements in EORTC QLQ-C30 GHS and EQ-5D-3L VAS were defined as a change in score greater than +8 and a change in score of +7 or greater, respectively.
Results
Patients
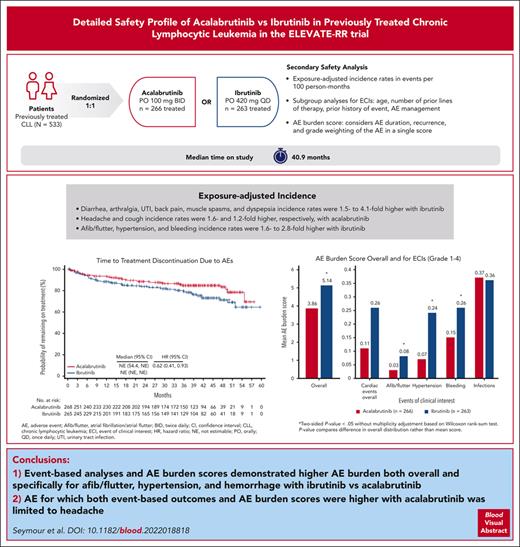
A total of 533 patients were randomized (acalabrutinib, n = 268; ibrutinib, n = 265); overall, the median patient age was 66 years; 45% had del(17p), 64% had del(11q), and 51% of patients had del(17p) and/or TP53 mutation (supplemental Table 1, available on the Blood website). The median number of previous therapies was 2 (range, 1-12). At data cutoff (15 September 2020), the median time on study was 40.9 months (range, 0-59.1 months); 46% of patients treated with acalabrutinib vs 41% of those treated with ibrutinib remained on treatment.12 Reasons for treatment discontinuation (acalabrutinib vs ibrutinib) were disease progression (31% [n = 82] vs 26% [n = 68]), AEs (15% [n = 40] vs 22% [n = 59]), consent withdrawn (3% [n = 7] vs 3% [n = 7]), death (2% [n = 5] vs 2% [n = 6]), investigator decision (2% [n = 5] vs 2% [n = 5]), and other (1% [n = 3] vs 3% [n = 9]).12 Median time to treatment discontinuation was 44.0 months for acalabrutinib vs 37.8 months for ibrutinib (hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.65-1.03; Figure 1A). Median time to treatment discontinuation because of AEs was not reached in either arm; the HR favored acalabrutinib (HR, 0.62; 95% CI, 0.41-0.93; Figure 1B). Median (range) treatment exposure was 38.3 months (0.3-55.9) for acalabrutinib and 35.5 months (0.2-57.7) for ibrutinib. In total, 529 patients (acalabrutinib, n = 266; ibrutinib, n = 263) were analyzed for safety.
Time to treatment discontinuation. Kaplan-Meier plot for time to treatment discontinuation overall (A) and because of AEs (B).
Time to treatment discontinuation. Kaplan-Meier plot for time to treatment discontinuation overall (A) and because of AEs (B).
Safety
Among the most common any-grade AEs, incidences of diarrhea, arthralgia, urinary tract infection, back pain, muscle spasms, and dyspepsia were higher with ibrutinib (P < .05; Table 1); exposure-adjusted incidence rates were 1.5- to 4.1-fold higher with ibrutinib for these AEs. Incidences of headache and cough were higher with acalabrutinib (P < .05); exposure-adjusted incidence rates of these events were 1.6- and 1.2-fold higher compared with ibrutinib.
Most common AEs
| Common AEs by SOC and PT . | Incidence, % . | Exposure-adjusted incidence rate∗ . | ||||||
|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |||||
| Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | |
| Blood and lymphatic system disorders | ||||||||
| Anemia | 22 | 19 | 12 | 13 | 1.2 | 1.2 | 0.6 | 0.6 |
| Neutropenia | 21 | 25 | 20 | 23 | 1.7 | 1.9 | 1.4 | 1.5 |
| Thrombocytopenia | 15 | 13 | 10 | 7 | 0.8 | 0.7 | 0.5 | 0.3 |
| Gastrointestinal disorders | ||||||||
| Diarrhea | 35 | 46† | 1 | 5† | 1.9 | 2.8 | <0.1 | 0.2 |
| Nausea | 18 | 19 | 0 | <1 | 0.9 | 0.8 | 0 | <0.1 |
| Constipation | 12 | 14 | 0 | 1 | 0.5 | 0.6 | 0 | <0.1 |
| Vomiting | 11 | 14 | <1 | 1 | 0.4 | 0.5 | <0.1 | <0.1 |
| Dyspepsia | 4 | 12† | 0 | 0 | 0.1 | 0.5 | 0 | 0 |
| General disorders, administration site conditions, and injury | ||||||||
| Pyrexia | 23 | 19 | 3 | 1 | 1.1 | 1.0 | 0.1 | <0.1 |
| Fatigue | 20 | 17 | 3† | 0 | 0.9 | 0.9 | 0.1 | 0 |
| Peripheral edema | 10 | 14 | 0 | <1 | 0.5 | 0.6 | 0 | <0.1 |
| Musculoskeletal and connective tissue disorders | ||||||||
| Arthralgia | 16 | 23† | 0 | 1 | 0.6 | 1.3 | 0 | <0.1 |
| Myalgia | 9 | 10 | 1 | <1 | 0.4 | 0.5 | <0.1 | <0.1 |
| Back pain | 8 | 13† | 0 | 1 | 0.3 | 0.5 | 0 | <0.1 |
| Muscle spasms | 6 | 13† | 0 | 1 | 0.2 | 0.7 | 0 | <0.1 |
| Nervous system disorders | ||||||||
| Headache | 35† | 20 | 2† | 0 | 1.8 | 1.1 | <0.1 | 0 |
| Dizziness | 11 | 10 | 0 | 0 | 0.5 | 0.5 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||||||
| Cough | 29† | 21 | 1 | <1 | 1.3 | 1.1 | <0.1 | <0.1 |
| Dyspnea | 14 | 9 | 2 | <1 | 0.5 | 0.4 | 0.1 | <0.1 |
| Common AEs by SOC and PT . | Incidence, % . | Exposure-adjusted incidence rate∗ . | ||||||
|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |||||
| Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | |
| Blood and lymphatic system disorders | ||||||||
| Anemia | 22 | 19 | 12 | 13 | 1.2 | 1.2 | 0.6 | 0.6 |
| Neutropenia | 21 | 25 | 20 | 23 | 1.7 | 1.9 | 1.4 | 1.5 |
| Thrombocytopenia | 15 | 13 | 10 | 7 | 0.8 | 0.7 | 0.5 | 0.3 |
| Gastrointestinal disorders | ||||||||
| Diarrhea | 35 | 46† | 1 | 5† | 1.9 | 2.8 | <0.1 | 0.2 |
| Nausea | 18 | 19 | 0 | <1 | 0.9 | 0.8 | 0 | <0.1 |
| Constipation | 12 | 14 | 0 | 1 | 0.5 | 0.6 | 0 | <0.1 |
| Vomiting | 11 | 14 | <1 | 1 | 0.4 | 0.5 | <0.1 | <0.1 |
| Dyspepsia | 4 | 12† | 0 | 0 | 0.1 | 0.5 | 0 | 0 |
| General disorders, administration site conditions, and injury | ||||||||
| Pyrexia | 23 | 19 | 3 | 1 | 1.1 | 1.0 | 0.1 | <0.1 |
| Fatigue | 20 | 17 | 3† | 0 | 0.9 | 0.9 | 0.1 | 0 |
| Peripheral edema | 10 | 14 | 0 | <1 | 0.5 | 0.6 | 0 | <0.1 |
| Musculoskeletal and connective tissue disorders | ||||||||
| Arthralgia | 16 | 23† | 0 | 1 | 0.6 | 1.3 | 0 | <0.1 |
| Myalgia | 9 | 10 | 1 | <1 | 0.4 | 0.5 | <0.1 | <0.1 |
| Back pain | 8 | 13† | 0 | 1 | 0.3 | 0.5 | 0 | <0.1 |
| Muscle spasms | 6 | 13† | 0 | 1 | 0.2 | 0.7 | 0 | <0.1 |
| Nervous system disorders | ||||||||
| Headache | 35† | 20 | 2† | 0 | 1.8 | 1.1 | <0.1 | 0 |
| Dizziness | 11 | 10 | 0 | 0 | 0.5 | 0.5 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||||||
| Cough | 29† | 21 | 1 | <1 | 1.3 | 1.1 | <0.1 | <0.1 |
| Dyspnea | 14 | 9 | 2 | <1 | 0.5 | 0.4 | 0.1 | <0.1 |
Common AEs were those occurring in ≥10% of patients in either treatment arm that were not already captured as an ECI.
Acala, acalabrutinib; Ibru, ibrutinib; PT, preferred term; SOC, system organ class.
Reported as events per 100 person-months.
2-sided P value < .05 without multiplicity adjustment, for comparison of incidence based on the Barnard exact test (indicated in bold).
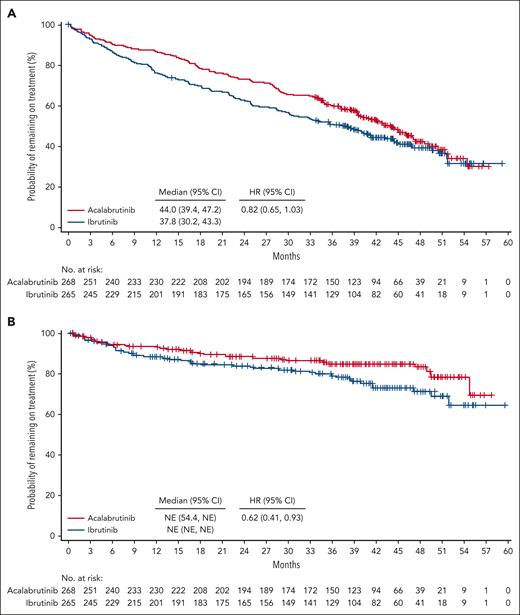
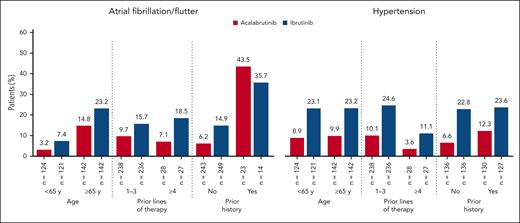
Among cardiovascular ECIs, incidences of any-grade atrial fibrillation/flutter, hypertension, and bleeding were higher with ibrutinib (P < .05); exposure-adjusted incidence rates were 2.0-, 2.8-, and 1.6-fold higher, respectively (Table 2). Ventricular arrhythmias were rare overall (ibrutinib, 3 patients; acalabrutinib, 0 patients). Sudden cardiac death was reported in 1 patient treated with ibrutinib vs 0 patients treated with acalabrutinib. Median time to onset of atrial fibrillation/flutter was nearly twice as long with acalabrutinib vs ibrutinib, whereas the median time to onset of hypertension was similar between treatment arms (supplemental Table 2). Prevalence of atrial fibrillation/flutter and hypertension was numerically lower for acalabrutinib vs ibrutinib in each yearly time interval, with absolute differences of 3.2% and 10.5%, respectively, seen in year 5 (supplemental Figure 1); cumulative incidences of atrial fibrillation/flutter and hypertension were also lower for acalabrutinib vs ibrutinib at each time point (P < .05), with absolute differences of 7.5% and 18.2%, respectively, seen at 36 months (supplemental Figure 2). Treatment discontinuation because of atrial fibrillation/flutter occurred only in the ibrutinib arm (n = 7). Among all patients, concomitant medication use for both atrial fibrillation/flutter and hypertension was less common in the acalabrutinib arm (supplemental Table 2). The post–atrial fibrillation/flutter incidences of hemorrhage and hypertension and the posthypertension incidence of atrial fibrillation/flutter were generally similar in both treatment arms, whereas the posthypertension incidence of hemorrhage (28.0% vs 37.7%) was higher with ibrutinib (supplemental Table 2). Atrial fibrillation/flutter and hypertension occurred consistently less frequently with acalabrutinib across most patient subgroups (Figure 2). Cox proportional-hazards analysis of new-onset atrial fibrillation/flutter and new-onset hypertension showed relative rate reductions of 63% and 77%, respectively, favoring acalabrutinib (Figure 3). Among patients with histories of atrial fibrillation/flutter or hypertension, Cox proportional-hazards analysis found no evidence of a difference in on-study atrial fibrillation/flutter between treatment arms; a relative rate reduction of 54% favoring acalabrutinib was seen for on-study hypertension (Figure 3).
Selected ECIs
| ECIs . | Incidence, % . | Exposure-adjusted incidence rate∗ . | AE burden score, mean (SD) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Grades 1-4 . | Grades 1-5 . | |||||||
| Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | |
| Cardiac events overall† | 24 | 30 | 9 | 10 | 1.2 | 1.9 | 0.4 | 0.5 | 0.11 (0.355) | 0.26 (1.059) | 0.11 (0.354) | 0.26 (1.053) |
| Afib/flutter | 9 | 16‡ | 5 | 4 | 0.4 | 0.7 | 0.2 | 0.1 | 0.03 (0.187) | 0.08§(0.316) | 0.03 (0.187) | 0.08§(0.316) |
| HTN‖ | 9 | 23‡ | 4 | 9‡ | 0.4 | 1.2 | 0.1 | 0.4 | 0.07 (0.336) | 0.24§(0.682) | 0.07 (0.336) | 0.24§(0.682) |
| Bleeding events¶ | 38 | 51‡ | 4 | 5 | 2.4 | 3.8 | 0.1 | 0.2 | 0.15 (0.377) | 0.26§(0.568) | 0.18 (0.667) | 0.26§(0.568) |
| Major bleeding events# | 5∗∗ | 5†† | 4 | 5 | 0.2 | 0.2 | 0.1 | 0.2 | 0.02 (0.143) | 0.01 (0.153) | 0.05 (0.576) | 0.01 (0.153) |
| Infections‡‡ | 78 | 81 | 31 | 30 | 8.9 | 10.4 | 1.6 | 2.0 | 0.37 (1.056) | 0.36 (0.797) | 0.46 (1.513) | 0.41 (0.904) |
| ECIs . | Incidence, % . | Exposure-adjusted incidence rate∗ . | AE burden score, mean (SD) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Grades 1-4 . | Grades 1-5 . | |||||||
| Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | Acala n = 266 . | Ibru n = 263 . | |
| Cardiac events overall† | 24 | 30 | 9 | 10 | 1.2 | 1.9 | 0.4 | 0.5 | 0.11 (0.355) | 0.26 (1.059) | 0.11 (0.354) | 0.26 (1.053) |
| Afib/flutter | 9 | 16‡ | 5 | 4 | 0.4 | 0.7 | 0.2 | 0.1 | 0.03 (0.187) | 0.08§(0.316) | 0.03 (0.187) | 0.08§(0.316) |
| HTN‖ | 9 | 23‡ | 4 | 9‡ | 0.4 | 1.2 | 0.1 | 0.4 | 0.07 (0.336) | 0.24§(0.682) | 0.07 (0.336) | 0.24§(0.682) |
| Bleeding events¶ | 38 | 51‡ | 4 | 5 | 2.4 | 3.8 | 0.1 | 0.2 | 0.15 (0.377) | 0.26§(0.568) | 0.18 (0.667) | 0.26§(0.568) |
| Major bleeding events# | 5∗∗ | 5†† | 4 | 5 | 0.2 | 0.2 | 0.1 | 0.2 | 0.02 (0.143) | 0.01 (0.153) | 0.05 (0.576) | 0.01 (0.153) |
| Infections‡‡ | 78 | 81 | 31 | 30 | 8.9 | 10.4 | 1.6 | 2.0 | 0.37 (1.056) | 0.36 (0.797) | 0.46 (1.513) | 0.41 (0.904) |
Acala, acalabrutinib; Afib/flutter, atrial fibrillation/flutter; CNS, central nervous system; HTN, hypertension; Ibru, ibrutinib.
Reported as events per 100 person-months.
Based on MedDRA cardiac system organ class terms.
2-sided P value < .05 without multiplicity adjustment, for comparison of incidence based on the Barnard exact test (indicated in bold).
2-sided P value < .05 without multiplicity adjustment based on Wilcoxon rank-sum test (indicated in bold). P value compares difference in overall distribution rather than mean score.
Includes HTN, blood pressure increase, and blood pressure systolic increase.
Bleeding events occurring in ≥10% of patients in either treatment arm include contusion and epistaxis.
Any hemorrhagic event that was serious, grade ≥3, or a CNS hemorrhage (any grade).
Of 12 patients with major bleeding events in the acalabrutinib arm, CNS-related hemorrhage events were reported in 4 patients (1 with subdural hematoma [grade 3], 2 with intracranial hemorrhage [grade 3 and grade 5], and 1 with cerebral hemorrhage [grade 2]).
Of 14 patients with major bleeding events in the ibrutinib arm, CNS-related hemorrhage events were reported in 1 patient who had both grade 1 subdural hematoma and grade 3 intracranial hemorrhage.
Infections occurring in ≥10% of patients in either treatment arm include upper respiratory tract infection, pneumonia, bronchitis, nasopharyngitis, and urinary tract infection.
Incidence of atrial fibrillation/flutter and hypertension in patient subgroups.
Incidence of atrial fibrillation/flutter and hypertension in patient subgroups.
Cumulative incidence of atrial fibrillation/flutter and hypertension by prior history. Cumulative incidence of atrial fibrillation/flutter (A) and hypertension (B) in patients with and without prior history.
Cumulative incidence of atrial fibrillation/flutter and hypertension by prior history. Cumulative incidence of atrial fibrillation/flutter (A) and hypertension (B) in patients with and without prior history.
Characteristics of any-grade bleeding events are shown in supplemental Table 3. Median time to event onset was similar for acalabrutinib and ibrutinib (supplemental Table 3). Cumulative incidence was lower for acalabrutinib vs ibrutinib at each time point, with an absolute difference of 14.6% seen at 36 months (supplemental Figure 3). Any-grade bleeding prevalence was consistently lower in each yearly time interval with acalabrutinib (supplemental Figure 4). Overall bleeding events were less frequent with acalabrutinib vs ibrutinib regardless of age and in patients with 1 to 3 prior lines of therapy (supplemental Table 3). Dose modifications because of bleeding events occurred in very few patients in both arms.
Infection rates (any grade and grade ≥3; Table 2) and cumulative incidences (supplemental Figure 5) were similar between treatment arms, with a decrease in any-grade infection prevalence by yearly interval seen with both treatments (supplemental Figure 6). Total rates of serious infections (28.9% vs 29.7%) and infections resulting in death (4.9% vs 6.5%) were also similar for acalabrutinib vs ibrutinib, respectively. Fungal opportunistic infections occurred in 3.8% of patients treated with acalabrutinib (Pneumocystis jirovecii pneumonia [n = 5]; bronchopulmonary aspergillosis [n = 3]; and Aspergillus infection, cerebral aspergillosis, and cryptococcosis [n = 1 patient each]) and 1.9% of patients treated with ibrutinib (esophageal candidiasis [n = 2]; and coccidioidomycosis, cerebral aspergillosis, and bronchopulmonary aspergillosis [n = 1 patient each]). Other characteristics of any-grade infections were generally similar between treatment arms (supplemental Table 4).
In the supplemental analysis, baseline characteristics were balanced between treatment arms; when incidence of any-grade cardiac arrhythmias, any-grade hypertension, and grade ≥3 infection was analyzed per these baseline characteristics, the most notable difference between the treatment arms was seen for any-grade hypertension, for which the incidence was considerably higher for ibrutinib across most of the baseline characteristics assessed (supplemental Table 5). In addition, increasing age was associated with higher rates of arrhythmias and infections (supplemental Table 5).
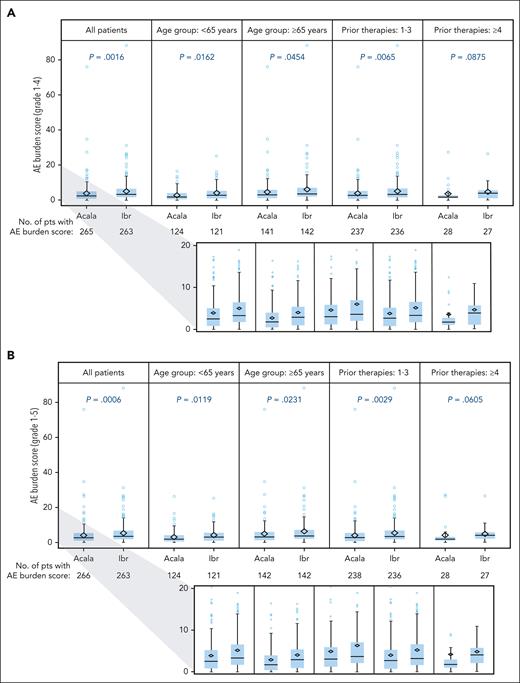
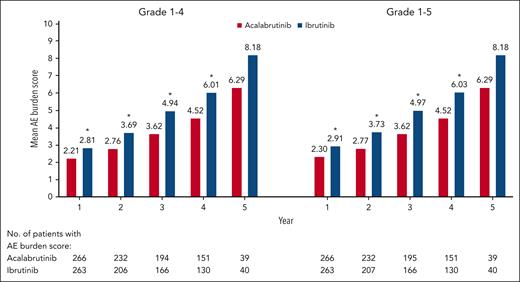
The mean (standard deviation [SD]) overall AE burden score considering all AEs together was higher for ibrutinib vs acalabrutinib for both the grade 1 to 4 analysis (5.14 [7.281] vs 3.86 [6.087]; P = .0016) and the grade 1 to 5 analysis (5.28 [7.294] vs 4.00 [6.272]; P = .0006) (Figure 4). It was higher with ibrutinib in certain patient subgroups, including those aged <65 years, those aged ≥65 years, and those with 1 to 3 prior therapies, in both the grade 1 to 4 (P ≤ .0454) and grade 1 to 5 (P ≤ .0231) analyses; it was also numerically higher with ibrutinib among patients with ≥4 prior therapies but the difference was not significant (Figure 4). When analyzed by yearly interval, the overall AE burden score was higher with ibrutinib during each of the first 4 years for both the grade 1 to 4 and grade 1 to 5 analyses (P < .05); it was numerically higher with ibrutinib during year 5 but these differences were not significant (Figure 5). Among patients with polypharmacy at baseline (acalabrutinib, n = 132; ibrutinib, n = 144), mean (SD) overall AE burden scores were higher with ibrutinib vs acalabrutinib, both with (6.74 [9.079] vs 5.11 [7.997]; P = .0006) and without (6.54 [9.078] vs 4.94 [7.920]; P = .0016) the inclusion of grade 5 events. Similar findings were also seen in those without polypharmacy at baseline (acalabrutinib, n = 134; ibrutinib, n = 119), with mean (SD) overall AE burden scores of 3.51 (3.534) vs 2.89 (3.595) for ibrutinib vs acalabrutinib, respectively, with grade 5 events included (P = .0006), and of 3.45 (3.545) vs 2.80 (3.089), respectively, without grade 5 events included (P = .0016).
Summary of AE burden score by treatment arm and subgroup. AE burden score by treatment arm and subgroup for grade 1 to 4 (A) and grade 1 to 5 (B) AEs. P values based on a 2-sided Wilcoxon rank-sum test without adjustment for multiplicity. Lower figures in each panel show a magnification of the 0 to 20 portion of the scale. Acala, acalabrutinib; Ibr, ibrutinib; pts, patients.
Summary of AE burden score by treatment arm and subgroup. AE burden score by treatment arm and subgroup for grade 1 to 4 (A) and grade 1 to 5 (B) AEs. P values based on a 2-sided Wilcoxon rank-sum test without adjustment for multiplicity. Lower figures in each panel show a magnification of the 0 to 20 portion of the scale. Acala, acalabrutinib; Ibr, ibrutinib; pts, patients.
Overall AE burden score by time interval. ∗P < .05 based on a 2-sided Wilcoxon rank-sum test without adjustment for multiplicity.
Overall AE burden score by time interval. ∗P < .05 based on a 2-sided Wilcoxon rank-sum test without adjustment for multiplicity.
Among ECIs, the AE burden scores for atrial fibrillation/flutter, hypertension, and hemorrhage were each higher with ibrutinib than acalabrutinib (P < .05); however, no evidence of a difference was observed between treatments for major hemorrhage (Table 2). The AE burden score for cardiac events overall was numerically but not statistically higher for ibrutinib vs acalabrutinib, even when including grade 5 events. No evidence of a difference in AE burden score was seen between treatment arms for infections. For the common BTKi-associated symptomatic AEs, the AE burden scores for diarrhea and headache were higher with acalabrutinib (P < .05); the AE burden score for musculoskeletal events was higher with ibrutinib (P = .0229). No difference in AE burden score was observed between the treatment arms for fatigue (supplemental Table 6).
PROs
An increase (improvement) in EORTC QLQ-C30 GHS was observed at week 12 (first–time point assessment) and stabilized thereafter in both treatment arms with no statistical differences seen between treatments; the improvements were not considered clinically meaningful (ie, >+8; supplemental Table 7). An increase (improvement) in EQ-5D-3L VAS score was observed at week 12 that increased until week 180, with similar findings reported in both treatment arms; these improvements also were not considered clinically meaningful (ie, ≥+7; supplemental Table 7).
Discussion
The primary analysis of the phase 3 ELEVATE-RR trial demonstrated noninferior efficacy and a lower rate of key AEs with acalabrutinib vs ibrutinib in previously treated CLL.12 However, incidence of AEs as an isolated measure can be insufficient in defining the toxicity profile of prolonged treatments, because it does not consider the course of AEs, their onset, progression, or cumulative effects nor account for drug exposure.21 This secondary analysis of the ELEVATE-RR trial further defines the tolerability profile of acalabrutinib and ibrutinib based on in-depth event-based analyses and AE burden scores accounting for event duration, recurrence, and severity grade, which also support a better overall tolerability profile for acalabrutinib vs ibrutinib. Acalabrutinib demonstrated lower overall and exposure-adjusted incidence rates and lower AE burden scores for atrial fibrillation/flutter, hypertension, and bleeding, compared with ibrutinib. Moreover, cumulative incidences of hypertension and atrial fibrillation/flutter were lower with acalabrutinib in patients without a history of these events; cumulative incidence of hypertension was lower with acalabrutinib in patients with a history of events. In patients with a prior history of atrial fibrillation, cumulative incidence was similar for acalabrutinib vs ibrutinib. The incidence, exposure-adjusted incidence rate, and AE burden score for infections were generally similar between treatment arms.
Since the approval of ibrutinib in 2014, BTKis have reshaped the treatment of CLL. However, long-term data have demonstrated safety concerns with ibrutinib, potentially because of its broader kinase activity.16 For example, at concentrations of <10 nM, ibrutinib was shown to inhibit 8 of 9 kinases that have a conserved cysteine residue similar to BTK (BMX, ITK, TEC, TXK, EGFR, ERBB2, ERBB4, and BLK), whereas the more selective BTKi acalabrutinib does not inhibit these kinases at similar concentrations.7,22 In the primary analysis of ELEVATE-RR, at ∼41 months of follow-up, the occurrence of AEs leading to treatment discontinuation was lower with acalabrutinib (15%) vs ibrutinib (21%).12 A novel finding in this secondary analysis is that the rate of treatment discontinuation because of AEs was lower with acalabrutinib vs ibrutinib (HR, 0.62; 95% CI, 0.41-0.93) based on Kaplan-Meier analysis. Because BTKis as monotherapy are administered continuously until disease progression, minimizing tolerability issues is key to maximizing long-term treatment benefits.
Atrial fibrillation is a well-characterized safety concern with ibrutinib.16 The mechanism underlying ibrutinib-induced arrhythmias, particularly atrial fibrillation, is not yet completely understood; however, preclinical evidence in 2 rodent models has separately implicated off-target inhibition of PI3K-Akt signaling (reduced PI3K(p110α) expression and reduced Akt activation) and of C-terminal Src kinase, respectively.23,24 Ibrutinib was also shown to inhibit kinases downstream of C-terminal Src kinase (lymphocyte cell kinase and Src) in peripheral blood samples of patients with CLL, whereas acalabrutinib showed minimal inhibition of these kinases.25 In the primary analysis of ELEVATE-RR, atrial fibrillation/flutter ECI incidence, a secondary end point of the trial, was significantly lower with acalabrutinib vs ibrutinib (9.4% vs 16.0%).12 By comparison, the rate of any-grade atrial fibrillation as a preferred term (excluding atrial flutter) was 11% with ibrutinib in the RESONATE trial at a similar median follow-up (44 months).3 In this post hoc analysis, the acalabrutinib arm had a longer median time to atrial fibrillation/flutter onset (28.8 vs 16.0 months) and a lower number of treatment discontinuations because of atrial fibrillation/flutter (0 vs 7) vs ibrutinib, the latter of which could have been impacted by the nonblinded study design and presumptions regarding increased atrial fibrillation incidence with ibrutinib. The cumulative incidence of atrial fibrillation/flutter over time was also lower with acalabrutinib in patients without a history of atrial fibrillation at baseline; no evidence of a difference was observed in those with a history of atrial fibrillation/flutter at baseline. Of note, the acalabrutinib arm had more patients with a history of atrial fibrillation/flutter at baseline compared with ibrutinib (n = 23 vs n = 14, respectively), although numbers of patients overall were small, limiting the ability to detect differences in this patient subgroup.
Overall cardiac event incidence based on the MedDRA system organ class was numerically lower in the acalabrutinib arm (24%) vs the ibrutinib arm (30%), but the difference was not statistically significant. Although incidence of hypertension was significantly higher with ibrutinib (23%) than with acalabrutinib (9%), hypertension does not fall under the MedDRA system organ class of cardiac events; therefore, it does not contribute to differences in the overall rate of cardiac events between treatment arms.
Hypertension has also been identified as a potential safety concern with ibrutinib.16 Possible mechanisms proposed for BTKi-induced hypertension include inhibition of vascular endothelial growth factor, inhibition of the PI3K pathway, and downregulation of endothelial nitric oxide production.5 In the primary analysis of ELEVATE-RR, both any-grade and grade ≥3 hypertension were significantly higher with ibrutinib vs acalabrutinib.12 In the RESONATE trial after a similar follow-up of 44 months, grade ≥3 hypertension was reported in 8% of patients receiving ibrutinib, which is consistent with the rate of 9% reported for ibrutinib in this secondary analysis.3 In this analysis, hypertension was also less frequent in patients treated with acalabrutinib across assessment time points and among all subgroups of age and number of prior lines of therapy, and in those without any history. The ibrutinib arm also had a numerically higher proportion of patients receiving concomitant medications for hypertension, and a higher proportion of patients with hemorrhage subsequent to hypertension, which could suggest greater clinical impact of these AEs in patients receiving ibrutinib. Longer-term data will be important to define the overall risk with these agents.
Although the mechanism is not completely understood, on-target BTK inhibition contributes to bleeding risk.16 The increased bleeding risk reported with ibrutinib may be exacerbated by additional inhibition of other Src kinases and Tec kinases that acalabrutinib does not inhibit, resulting in platelet dysfunction.26 In the primary analysis of ELEVATE-RR, bleeding events occurred less frequently in patients treated with acalabrutinib vs those treated with ibrutinib (38.0% vs 51.3%); however, major bleeding events were similar.12 In this post hoc analysis, any-grade bleeding events also occurred less frequently in the acalabrutinib arm when analyzed by age group and among those with 1 to 3 prior lines of therapy. Fewer patients received antithrombotics after onset of atrial fibrillation in the acalabrutinib arm vs the ibrutinib arm, which could also have contributed to less bleeding; however, numbers overall were low (5.3% vs 9.1%).
The AE burden score methodology used in this analysis was adapted from the methodology developed by Ruppert et al in their post hoc analysis of the Alliance trial of ibrutinib vs bendamustine + rituximab in older patients with previously untreated CLL.13 This tool could provide a standardized framework for understanding global AE burden that includes the impact of the recurrence and duration of these AEs, which are not captured by crude per-patient incidence rates. In the Alliance analysis, grade 5 AEs were not included in the score calculation, whereas this analysis includes scoring with and without grade 5 events. In the Alliance analysis, which compared 2 therapies with different mechanisms and durations of exposure, ibrutinib was shown to have a lower overall AE burden score than bendamustine + rituximab13; our analysis of the head-to-head ELEVATE-RR study, comparing 2 therapies in the same therapeutic class with similar exposures, demonstrated a lower AE burden score with acalabrutinib vs ibrutinib overall (considering all AEs) and for the ECIs of atrial fibrillation/flutter, hypertension, and hemorrhage. The overall AE burden score was also lower for acalabrutinib vs ibrutinib over time. Polypharmacy has been significantly associated with a high comorbidity burden, which did not affect outcomes with ibrutinib in a previous retrospective analysis.17 The presence or absence of polypharmacy status at baseline did not affect the lower overall AE burden score seen with acalabrutinib vs ibrutinib in our analysis. The increase in AE burden score observed in both treatment arms in patients with polypharmacy may provide further evidence for use of polypharmacy as a surrogate for comorbidities.
Limitations of this analysis include the use of an open-label study design, allowing investigators and patients to be aware of the assigned treatment. This could influence the reporting of more subjective AEs (eg, headache); however, other more objective AEs (eg, atrial fibrillation and hypertension) are measurable and less influenced by bias. The study was not powered to detect differences between treatment arms among most of the safety outcomes in this analysis except for atrial fibrillation and grade ≥3 infections, and post hoc statistical testing was not controlled for multiplicity. In the AE burden score analysis, scores were not differently weighted for AEs that resulted in treatment discontinuation, which may result in an underestimation of the true burden in these cases. A limitation imposed by study design in calculating the AE burden should also be acknowledged, whereby the duration of any event was dependent on the timing of clinical evaluations based on the clinical trial protocol; therefore, persistence between visits cannot be confirmed. This could have affected the precision of the duration component of the AE burden score, although it would not be expected to affect 1 treatment arm more than the other. Improvements in patient-reported health-related quality of life/health status were observed in both treatment arms, which were not considered clinically meaningful and were not statistically different between treatments. A limitation of the PRO findings is that the first postbaseline PRO assessment was not conducted until week 12, thus any treatment differences within the first 12 weeks, for example because of early toxicity, would not have been captured. Some of the AEs for which treatment differences were observed (eg, hypertension) are generally asymptomatic and would not influence the PRO outcomes. Overall, the PRO tools used in ELEVATE-RR do not specifically assess quality-of-life outcomes related to AEs. However, a quality-adjusted time without symptoms or toxicity survival analysis previously compared acalabrutinib with ibrutinib using data from the ELEVATE-RR trial, which further corroborated the better tolerability profile of acalabrutinib compared with that of ibrutinib from a quality-of-life perspective.27
Overall, in this post hoc analysis of the head-to-head ELEVATE-RR trial of acalabrutinib vs ibrutinib in patients with previously treated CLL, a greater BTKi-related toxicity burden was seen with ibrutinib relative to acalabrutinib. In particular, the incidence and exposure-adjusted incidence rate of atrial fibrillation/flutter, hypertension, and bleeding were lower with acalabrutinib vs ibrutinib. Cumulative incidences of hypertension and atrial fibrillation/flutter were also lower with acalabrutinib in patients without any history of these events. AE burden scores, which were lower with acalabrutinib vs ibrutinib overall, and for atrial fibrillation/flutter, hypertension, and hemorrhage, further support the superior tolerability of acalabrutinib vs ibrutinib. This secondary analysis of the ELEVATE-RR trial further characterized the differential safety profiles of acalabrutinib and ibrutinib in patients with previously treated CLL, which may help inform treatment selection in this patient population.
Acknowledgments
The authors thank the investigators and coordinators at each of the clinical sites, the patients who participated in this trial and their families, and Sophia Sohoni, who contributed to the conduct of the study.
The study was funded by AstraZeneca. Medical writing assistance, funded by AstraZeneca, was provided by Robert J. Schoen, of Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors.
Authorship
Contribution: J.F.S., J.C.B., R.R.F., S.O., and J.A.W. designed the study; J.C.B., P.G., A.P.K., A.C.-K., R.R.F., S.O., T.M., A.M., S.S., W.J., and J.A.W. were study investigators; J.F.S., J.C.B., P.G., A.P.K., R.R.F., S.O., T.M., S.S., W.J., and J.A.W. recruited patients or provided study materials; A.C.-K., R.R.F., S.O., S.S., M.d.B., and W.J. collected and assemble the data; J.F.S., R.R.F., K.H., E.J., and M.d.B. analyzed the data; J.F.S., J.C.B., P.G., A.P.K., R.R.F., S.O., J.R.B., A.M., S.S., N.B., P.M., K.H., E.J., M.d.B., and J.A.W. interpreted the data; and all authors critically reviewed and revised this manuscript and approved the manuscript for submission.
Conflict-of-interest disclosure: J.F.S. serves on the advisory board of AbbVie, AstraZeneca, Celgene, Genentech, Genor Bio, Gilead, Janssen, MorphoSys, Roche, and Sunesis; is a speaker for AbbVie, Celgene, and Roche; reports research funding from AbbVie, Celgene, Janssen, and Roche; provides expert testimony to Celgene, Roche, and TG Therapeutics; and reports consultancy for TG Therapeutics. J.C.B. serves as a consultant for Syndax, Trillium, AstraZeneca, Novartis, Newave, and Kronos; and serves as the scientific advisory board chair of, and is a major stockholder with, Vincerx Pharma. T.M. declares honoraria from AstraZeneca, AbbVie, Janssen, Alexion, Gilead, Roche, and Novartis. P.G. serves as a consultant for AbbVie, AstraZeneca, BeiGene, BMS, Janssen, Lilly/Loxo, MSD, and Roche; and reports research funding from AbbVie, AstraZeneca, BMS, and Janssen. A.P.K. serves as a consultant for AbbVie, AstraZeneca, BeiGene, BMS, Janssen, and Roche/Genentech; and reports research funding from AbbVie, AstraZeneca, BMS, Janssen, and Roche/Genentech. A.C.-K. reports advisory board honoraria, research support, board of director, and trial participation with Ascentage, BeiGene, Cellectar, Starton, Janssen, and Pharmacyclics. R.R.F. serves as a consultant for AbbVie, AstraZeneca, BeiGene, Genentech, Incyte, Janssen, Loxo, MEI Pharma, Pharmacyclics, Sanofi, TG Therapeutics, and X4 Pharmaceuticals. S.O. serves as a consultant for AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, AstraZeneca, Autolus, BMS, Celgene, DynaMed, Eli Lilly and Company, Gilead, GlaxoSmithKline, Janssen Oncology, Johnson & Johnson, Juno Therapeutics, MEI Pharma Inc, Merck, and NOVA Research; and declares research funding from Acerta, Alliance, BeiGene, Caribou Biosciences, Gilead, Kite, Loxo Oncology, Mustang, Nurix, Pfizer, Pharmacyclics, Regeneron, Sunesis, and TG Therapeutics. J.R.B. serves as a consultant for AbbVie, Acerta/AstraZeneca, BeiGene, Bristol Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Grifols Worldwide Operations, Hutchmed, iOnctura, Janssen, MEI Pharma, Pfizer, and Pharmacyclics; and declares research funding from BeiGene, Gilead, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, and TG Therapeutics. A.M. serves as a consultant/advisor for AbbVie, Acerta, Adaptive, AstraZeneca, BeiGene, DTRM Biopharma, Genentech, Curio, Dava, Octopharma, Janssen, Johnson & Johnson, Loxo, Nurix, Genmab, BMS, Pharmacyclics, Sunesis, and TG Therapeutics; reports research funding from AbbVie, Octopharma, Acerta, Adaptive, BeiGene, DTRM Biopharma, Genentech, Genmab, Nurix, Janssen, Johnson & Johnson, Loxo, Pharmacyclics, Sunesis, and TG Therapeutics; and reports other relationship(s) with TG Therapeutics (DSMB). S.S. reports receiving advisory board honoraria, research support, travel support, and speaker fees from, and trial participation with AbbVie, AstraZeneca, BeiGene, BMS, Gilead, GSK, Hoffman-La Roche, Janssen, Novartis, Pharmacyclics, and Sunesis. N.B., P.M., K.H., E.J., and M.d.B. reports employment and stock ownership with AstraZeneca. W.J. received research funding from AstraZeneca, Janssen, BeiGene, and Lilly. J.A.W. received research funding from AbbVie, Janssen, Karyopharm, MorphoSys, Pharmacyclics, and Schrodinger; and serves as a consultant for AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Loxo, Newave, Pharmacyclics, and Schrodinger.
Correspondence: John F. Seymour, Haematology Department, Peter MacCallum Cancer Centre, 305 Grattan St, Melbourne, VIC 3000, Australia; e-mail: john.seymour@petermac.org.
References
Author notes
Presented in part at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 to 14 December 2021; the 2021 International Workshop on Chronic Lymphocytic Leukemia, virtual, 17 to 20 September 2021; and the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 to 13 December 2022.
Data underlying the findings described in this manuscript may be obtained in accordance with the data sharing policy of AstraZeneca described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal