In the current issue of Blood, Seymour et al1 report the adverse event (AE) burden of continuous acalabrutinib versus ibrutinib treatment in the previously reported phase 3 randomized trial ELEVATE-RR for chronic lymphocytic leukemia (CLL).2 Approved covalent Bruton tyrosine kinase (BTK) inhibitors like ibrutinib and acalabrutinib have changed the treatment paradigm for CLL. This article emphasizes that the significant improvement in treatment options for CLL during the last decade raises important questions about the differences in risk-benefit for the different treatment options and for different subgroups of patients. These questions can be generalized and applied to treatment of hematological diseases as follows: (1) can we directly compare efficacy in terms of progression-free survival (PFS) between treatments of different lengths, (2) can we directly compare AEs by frequency and grade for treatments of different lengths, (3) can we assess efficacy as a primary outcome without taking discontinuation rates and safety profiles into account, and (4) can we combine measures such as the AE burden score applied by Seymour et al with efficacy assessment to weigh different good treatment options against each other?
When comparing indefinite treatment with different BTK inhibitors in relapsed or refractory CLL, similar efficacy of ibrutinib and acalabrutinib has been demonstrated,2 although superior PFS was demonstrated for zanubrutinib compared with ibrutinib.3 Diving into these studies, higher discontinuation rates due to toxicity were seen with ibrutinib compared with both acalabrutinib and zanubrutinib. These higher discontinuation rates may, at least in part, explain the difference in PFS. For both studies, however, precautions about bias leading to discontinuance of the different BTK inhibitors in an open label trial should be kept in mind. The importance of discontinuation rates given the indefinite length of treatment with BTK inhibitors is further emphasized by real-world data, which demonstrated a median of less than 3 years to discontinuation when not participating in a clinical trials,4 less than half the length reported in randomized clinical trials.5
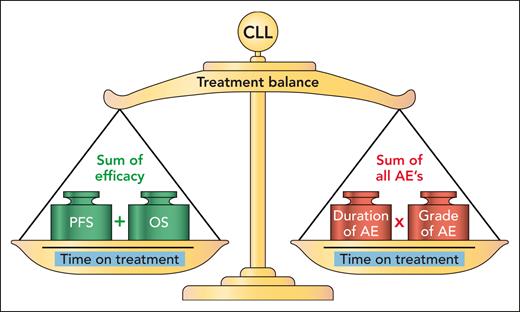
One way of assessing the impact of AEs for continuous therapy drugs is to report time to event of AEs, as implemented in the previously mentioned trials and reported for real-world data.2-4 Together with discontinuation rates due to AEs, progression, and other reasons, this can provide a clearer assessment of the burden of AEs in each of the different treatment options. Seymour et al add a combined assessment of AE burden by summarizing all AEs according to grade and duration while taking time on treatment into account (see figure, right side). This seems to give a fair comparison of 2 different continuous treatments. However, it does not consider the limitation of different lengths of treatments. By contrast, the CLL14 trial addressed this issue by prolonging the standard treatment with chlorambucil to the same duration as the experimental venetoclax-based arm.6 However, this remains infeasible for comparison with the indefinite length of treatment with BTK inhibitors or for more intensive chemotherapy as exemplified by the Alliance trial comparing 6 cycles of bendamustine-rituximab versus indefinite treatment with ibrutinib-based regimens.7 Another example of this dilemma is the GAIA/CLL13 trial, which compared 3 different experimental arms with treatment duration between 12 and 36 months with a chemoimmunotherapy arm of 6 cycles.8 In the GAIA/CLL13 trial, the most intensive experimental arm combining venetoclax, obinutuzumab, and ibrutinib for frontline treatment of CLL led to the longest PFS, although the PFS was not statistically different from the arm with venetoclax and obinutuzumab. However, the toxicity of the triplet arm combining venetoclax, obinutuzumab, and ibrutinib resulted in higher burden of AEs, in particular due to infections and cardiac events. These clinical trials emphasize the need to combine AE burden scores as presented here by Seymour et al with assessment of efficacy, as here proposed by the CLL treatment balance (see figure). This approach considers the treatment duration (whether planned duration or shortened due to progression, toxicity, or other reasons) both when counting the efficacy and when assessing the burden of AEs. Still, this approach needs adjusted weights for impact of PFS, overall survival, and different types of AEs.
Seymour et al applied assessment of AE burden including the sum of duration and grade of all AEs in relation to time on treatment. The proposed CLL treatment balance also considers efficacy in terms of PFS and overall survival (OS) with time on treatment included on both sides of the balance. Professional illustration by Patrick Lane, ScEYEnce Studios.
Seymour et al applied assessment of AE burden including the sum of duration and grade of all AEs in relation to time on treatment. The proposed CLL treatment balance also considers efficacy in terms of PFS and overall survival (OS) with time on treatment included on both sides of the balance. Professional illustration by Patrick Lane, ScEYEnce Studios.
Even without considering the financial implications, the ongoing toxicity of indefinite BTK inhibitor treatment2,3 and higher discontinuation rates of BTK inhibitors outside clinical trials4 emphasize that time-limited therapy is needed as the main treatment option in CLL. The reporting of exposure-adjusted incidence rates of AEs and AE burden by Seymour et al should thus be used as a step toward identifying optimal combinations for time-limited treatment. Such trials should aim at optimizing the CLL treatment balance (see figure) for individual subgroups of patients with CLL. Outside of clinical trials, data-driven medicine is necessary to identify the optimal balance for subgroups of patients with CLL by individualized risk prediction. This approach is exemplified by the CLL Treatment Infection Model (CLL-TIM) algorithm, a data-driven algorithm based on >4000 patients with CLL, identifying patients at high risk of serious infections or in need of CLL treatment by pattern recognition within routine health data.9 CLL-TIM is currently implemented in 1 electronic health record system and used for patient selection within the PreVent-ACaLL trial (clinicaltrials.gov: NCT03868722). However, such data-driven approaches should also be applied to data from clinical trials. This could be combined with minimal residual disease (MRD)-guided treatment duration and treatment intensity to further decrease AE burden while preserving treatment efficacy, as exemplified by the MRD-guided VISION/HO141 trial with venetoclax and ibrutinib for relapsed CLL.10 To facilitate implementation of a CLL treatment balance (see figure) assessment for CLL trials in particular, and hematology trials in general, it is recommended that the Food and Drug Administration, the European Medicines Agency, and other health authorities request mandatory sharing of clinical trial data for data-driven analyses while preserving data privacy.
Conflict-of-interest disclosure: C.U.N. received research funding and/or consultancy fees from AbbVie, Janssen, AstraZeneca, Beigene, Genmab, CSL Behring, Lilly, Takeda, and Octapharma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal