Key Points
oIL-2Rβ Tregs can be selectively expanded in vivo with oIL-2 administered after allo-HSCT.
Smaller numbers of oIL-2Rβ Tregs–oIL-2 are needed to reduce clinical scores, improve GVHD survival, and maintain GVT responses.
Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative option for patients with hematological disorders and bone marrow (BM) failure syndromes. Graft-versus-host disease (GVHD) remains a leading cause of morbidity posttransplant. Regulatory T cell (Treg) therapies are efficacious in ameliorating GVHD but limited by variable suppressive capacities and the need for a high therapeutic dose. Here, we sought to expand Treg in vivo by expressing an orthogonal interleukin 2 receptor β (oIL-2Rβ) that would selectively interact with oIL-2 cytokine and not wild-type (WT) IL-2. To test whether the orthogonal system would preferentially drive donor Treg expansion, we used a murine major histocompatibility complex–disparate GVHD model of lethally irradiated BALB/c mice given T cell–depleted BM from C57BL/6 (B6) mice alone or together with B6Foxp3+GFP+ Treg or oIL-2Rβ–transduced Treg at low cell numbers that typically do not control GVHD with WT Treg. On day 2, B6 activated T cells (Tcons) were injected to induce GVHD. Recipients were treated with phosphate-buffered saline (PBS) or oIL-2 daily for 14 days, then 3 times weekly for an additional 14 days. Mice treated with oIL-2Rβ Treg and oIL-2 compared with those treated with PBS had enhanced GVHD survival, in vivo selective expansion of Tregs, and greater suppression of Tcon expansion in secondary lymphoid organs and intestines. Importantly, oIL-2Rβ Treg maintained graft-versus-tumor (GVT) responses in 2 distinct tumor models (A20 and MLL-AF9). These data demonstrate a novel approach to enhance the efficacy of Treg therapy in allo-HSCT using an oIL-2/oIL-2Rβ system that allows for selective in vivo expansion of Treg leading to GVHD protection and GVT maintenance.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative therapy for patients with hematological malignancies. However, efficacy is limited by graft-versus-host disease (GVHD), the second leading cause of mortality following tumor relapse.1,2 Acute GVHD (aGVHD) is a highly inflammatory process mediated by T cells in the donor graft that recognize allo-antigens on host cells and cause targeted tissue damage.3 The development of novel GVHD preventive strategies is warranted because current prophylaxis protocols still leave more than half of patients who receive allo-HSCT at risk of developing GVHD.3,4
Regulatory T cells (Tregs) are a key immunoregulatory cell type known to prevent autoimmunity, promote tolerance, and maintain immune homeostasis.5 Polyclonal Treg transfer has been shown to suppress aGVHD in preclinical and early clinical trials.6-12 The variable Treg suppressive capacity and high cell dose required for therapeutic effect hinder use in the clinic. To date, there is no pharmacological approach to selectively expand Tregs. As genetic engineering approaches become more readily accessible, there is an interest to modify Tregs to enhance function, stability, persistence, and homing to sites of inflammation13 because such approaches would reduce the Treg dose needed and increase the efficacy of clinically translating Treg therapies. Treatment with low-dose interleukin 2 (IL-2) in chronic GVHD or autoimmune disease can result in a significant reduction in inflammation that allows disease improvement. The limitation of this approach is based on the fact that IL-2 can also target other immune cells, such as activated T cells (Tcons) and natural killer cells, leading to toxicity.14-17 IL-2/anti–IL-2 antibody complexes that preferentially bind CD25 have been used to expand Tregs.18,19 Such complexes have shown protective effects in a chronic GVHD model but were highly deleterious when used in an aGVHD model because of concurrent stimulation of alloreactive T cells and Tregs.20 Thus, more specific IL-2 methods are needed to preferentially stimulate Tregs and avoid stimulation of GVHD pathogenic T cells to effectively reduce incidence of aGVHD.
The orthogonal IL-2 receptor β (oIL-2Rβ) and cytokine pair were developed as a strategy to selectively expand cell populations of interest.21 The oIL-2 cytokine preferentially binds to the oIL-2Rβ and not the endogenous IL-2Rβ counterpart.21 The selectivity of the orthogonal system prevents undesired stimulation of non-Treg IL-2Rβ–expressing immune cells which has been a key limitation of other IL-2–based strategies.22-24 We hypothesized that engineering Tregs using the oIL-2/IL-2Rβ pair would preferentially boost Treg numbers in vivo such that the onset and severity of aGVHD could be significantly decreased following in vivo oIL-2 administration.
Methods
Mice
BALB/cJ (H-2kd), C57BL/6J (H-2kb), and B6.SJL-Ptprca/BoyJ (H-2kb, CD45.1+) mice were purchased from the Jackson Laboratory. To facilitate enrichment and obtain highly purified Foxp3-expressing Tregs, we used as sources of Tregs the C57BL/6 Foxp3GFP-DTR mice (B6-Foxp3GFP:H2kb, CD45.2+) that were provided by Alexander Rudensky (Memorial Sloan Kettering Cancer Center, New York, NY), C57BL/6-albino background, Foxp3.luciferase-DTR mice (B6-Foxp3GFP/Luc:H2b, CD45.1+), in which expression of green fluorescent protein/luciferase is controlled by the Foxp3 promoter, were provided by Gunther Hammerling (Heidelberg, Germany), and B6 Foxp3-GFP knockin mice were kindly provided by Vijay Kuchroo (Harvard University, Boston, MA) and bred in our animal colony. Animal protocols were approved by the Institutional Animal Care and Use Committees of Stanford University and the University of Minnesota.
HCST protocol for GVHD and GVT experiments
Recipient mice (BALB/c) were lethally irradiated with 8.8 Gy total body irradiation (cesium-137 source) split over 2 doses administered 4 hours apart. Mice received grafts containing bone marrow (BM) alone (5 × 106) that were depleted of CD4+ and CD8+ T cells (Tcons) (MicroBeads; Miltenyi) or with transduced/sorted-fresh Tregs (fTregs) coinjected via tail vein (1 × 106 or 0.2 × 106). Two days later, mice received an IV injection of Tcons (1 × 106) that were isolated from a single-cell suspension obtained from the spleens of donor mice by using a T Cell–Negative Isolation Kit (Stemcell). Animals that underwent transplantation were housed with water containing sulfamethoxazole and trimethoprim, monitored daily, and weight and GVHD scores were assessed weekly. Mice were treated daily with 25 000 IU of oIL-2 or phosphate-buffered saline (PBS) intraperitoneally for 14 days after irradiation and then 3 times a week for an additional 14 days. Survival curves were analyzed using the Kaplan-Meier method and log-rank test. All the animal experiments were performed with sex-matched mice between 8 and 12 weeks of age. For graft-versus-tumor (GVT) experiments, 2 × 105 A20-Luciferase+YFP+ B-cell lymphoma cell line (A20luc-yfp) or 1 × 104 MLL-AF9 leukemia cells (from BALB/c background), received from Sophie Paczesny’s laboratory (Medical University of South Carolina), were administered IV at the time of BM transplantation and recipients were examined by bioluminescent imaging (BLI). Mice were monitored daily and euthanized if moribund or when they developed lower limb paralysis.
All experiments in this manuscript used 5:1 Tcons:Tregs except for survival studies in which 1:1 was also used (supplemental Table 1; available on the Blood website).
Treg transduction
Retroviral supernatant was placed on 24-well plates coated with RetroNectin (Takara). Supernatant was removed after 2 hours of centrifugation at 2000g at 32°C. Preactivated Tregs were recovered and inoculated into the virus-loaded plates together with refreshed human IL-2 (1000 IU/mL) and CD3/CD28 beads (1:3 cell to beads ratio) and incubated for 72 hours at 37°C. Human IL-2 was replenished on day 2. Nontransduced (NT) Tregs were cultured on a virus-unloaded plate under the same conditions. Transduction efficiency was determined by truncated human epidermal growth factor receptor–positive (tEGFR+) cells by flow cytometry.
Treg suppression assay
Tregs were isolated from lymph nodes (LNs) and spleen from B6 GFP mice and sorted to >98% Foxp3+ purity. Tregs were transduced as described above. CD25-depleted T cells were isolated from CD45.1 mice and labeled with CellTrace Violet to be used as responder cells in 96-well round bottom plates. Responder cells were stimulated with anti-CD3/CD28 beads at responder to bead ratio of 2:1 in the presence of no IL-2, wild-type (WT) IL-2 (1000 IU/mL), or oIL-2 (100 000 IU/mL) and cultured with or without NT, tEGFR, or oIL-2Rβ Tregs at a 1:12 Treg:Tcon effector to target ratio. Cells were harvested on day 4, and proliferation was determined by CellTrace Violet dilution compared with Tcon alone group.
BLI studies
After intraperitoneal injection of D-Luciferin Firefly (Biosynth), the bioluminescent signal was acquired with AMI Imager and images were analyzed with Aura software (Spectral Instruments Imaging, Tucson, AZ). In some experiments, mice were euthanized after BLI (ex vivo BLI), organs were aligned, and the image captured.
Statistics
Data are presented as mean ± standard deviation. Statistical analyses were performed using Student unpaired t test with Bonferroni correction for multiple comparisons or a log-rank (Mantel-Cox) test in survival studies, using GraphPad Prism version 9 software. P < .05 was considered statistically significant.
Results
Selective expansion of oIL-2Rβ–transduced Treg in vitro
Tregs from C57BL/6 Foxp3-GFP reporter mice were retrovirally transduced with a construct expressing the oIL-2Rβ followed by a T2A self-cleaving peptide to coexpress a nonfunctional tEGFR. Control-transduced Tregs were retrovirally transduced with a construct only encoding tEGFR, and transduction efficiency was measured via tEGFR expression. tEGFR and oIL-2Rβ Tregs averaged 80% tEGFR expression with >98% CD25+Foxp3+ (Figure 1A-B). To understand how tEGFR Tregs (without oIL-2Rβ) compared with oIL-2Rβ Tregs at the transcriptomic level before oIL-2 stimulation, we performed RNA-seq. We found tEGFR Tregs and oIL-2Rβ Tregs shared similar transcriptomic profiles with upregulated expression of genes associated with activation and suppression (ie, Ifngr1, Gzmb, and Ctla2a) and downregulated genes associated with a more naïve state (ie, Tcf7, Ccr6, and Cd83) compared with fTregs (Figure 1C). Phenotypic analysis following transduction supported RNA-seq findings and demonstrated comparable levels of expression of CTLA-4, CD25, inducible costimulator (ICOS), and GITR between tEGFR and oIL-2Rβ Tregs, which were higher than fTregs (Figure 1D; supplemental Figure 1). We found a significantly higher expression of Lag3 and lower expression of Ki67 in oIL-2Rβ Tregs compared with tEGFR Tregs (Figure 1D; supplemental Figure 1).
oIL-2 selectively expands oIL-2Rβ transduced Tregs in vitro and enhances Treg suppressive function. (A) Representative flow cytometry plot displaying CD25+Foxp3+ Treg expression (left) and frequency (right) in NT, tEGFR, and oIL-2Rβ Tregs (right). (B) Representative histogram plots of tEGFR expression (left) and frequency (right) in NT, tEGFR Tregs, and oIL-2Rβ Tregs. (C) Heatmap and hierarchical clustering of the differential gene expression from fTregs, tEGFR Tregs, and oIL-2Rβ Tregs. Genes related with activation and Treg suppression are highlighted. Expression for each gene is scaled across single rows. (D) Heatmap displaying mean fluorescence intensity (MFI) of Treg markers CTLA-4, CD25, ICOS, Lag3, and Ki67 in Tregs freshly harvested (day 0 [D0]) or tEGFR Tregs and oIL-2Rβ Tregs on day 7 after harvest. (E) Absolute number of Tregs after 4-day stimulation with WT (1000 IU/mL) or oIL-2 (100 000 IU/mL). Data are representative of 4 independent experiments. (F) Percent suppression of CD4+ and CD8+ T cells after coculture with Tregs at 1:12 Treg:Tcon ratio. (G) Representative histograms show dilution of proliferation dye on CD4+ T cells after coculture with Tregs at 1:12 Treg:Tcon ratio. Data are representative of 3 independent experiments. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. Error bars indicate the standard deviation (SD) of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CTV, CellTrace Violet; ns, no significance.
oIL-2 selectively expands oIL-2Rβ transduced Tregs in vitro and enhances Treg suppressive function. (A) Representative flow cytometry plot displaying CD25+Foxp3+ Treg expression (left) and frequency (right) in NT, tEGFR, and oIL-2Rβ Tregs (right). (B) Representative histogram plots of tEGFR expression (left) and frequency (right) in NT, tEGFR Tregs, and oIL-2Rβ Tregs. (C) Heatmap and hierarchical clustering of the differential gene expression from fTregs, tEGFR Tregs, and oIL-2Rβ Tregs. Genes related with activation and Treg suppression are highlighted. Expression for each gene is scaled across single rows. (D) Heatmap displaying mean fluorescence intensity (MFI) of Treg markers CTLA-4, CD25, ICOS, Lag3, and Ki67 in Tregs freshly harvested (day 0 [D0]) or tEGFR Tregs and oIL-2Rβ Tregs on day 7 after harvest. (E) Absolute number of Tregs after 4-day stimulation with WT (1000 IU/mL) or oIL-2 (100 000 IU/mL). Data are representative of 4 independent experiments. (F) Percent suppression of CD4+ and CD8+ T cells after coculture with Tregs at 1:12 Treg:Tcon ratio. (G) Representative histograms show dilution of proliferation dye on CD4+ T cells after coculture with Tregs at 1:12 Treg:Tcon ratio. Data are representative of 3 independent experiments. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. Error bars indicate the standard deviation (SD) of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CTV, CellTrace Violet; ns, no significance.
To assess whether oIL-2 could be used to selectively expand oIL-2Rβ Treg in vitro, we stimulated NT Tregs, tEGFR Tregs, and oIL-2Rβ Tregs with either WT or oIL-2 at the indicated concentrations for 4 days. We found that WT IL-2 comparably stimulated NT, tEGFR, and oIL-2Rβ Tregs, leading to a sixfold expansion (Figure 1E). Whereas oIL-2 failed to expand NT and tEGFR Tregs, oIL-2 significantly expanded oIL-2Rβ Tregs comparably to WT IL-2–stimulated Treg groups (Figure 1E). Next, we used suboptimal Treg doses in an in vitro Treg suppression assay. We found that NT, tEGFR, and oIL-2Rβ Tregs comparably suppressed CD4+ and CD8+ T-cell proliferation in the absence of IL-2 by ∼30% and 20%, respectively, at a Treg:T-cell ratio of 1:12 (Figure 1F-G). Adding WT IL-2 decreased suppressive function in all Treg groups to ∼10% in both CD4+ and CD8+ T cells at the same Treg:T-cell ratio used in the experiment without IL-2 (Figure 1F-G). The addition of oIL-2 resulted in tEGFR and NT Tregs having comparable suppression of CD4+ and CD8+ T-cell proliferation, ∼30% and 20%, respectively, and comparable suppression in the absence of IL-2. In contrast, oIL-2Rβ Tregs stimulated with oIL-2 demonstrated a significant increase in suppression of CD4+ and CD8+ T cells of 42% and 35%, respectively, compared with NT and tEGFR Tregs (Figure 1F-G). Overall, we demonstrate that mouse oIL-2Rβ Tregs stimulated with oIL-2 will selectively expand and gain suppressor function when cultured under suboptimal Treg conditions in vitro.
oIL-2Rβ Treg and oIL-2 system permits the administration of lower numbers of Treg to suppress aGVHD lethality
To assess the capacity in vivo of oIL-2Rβ Treg to improve aGVHD survival and ameliorate disease severity, we used a major histocompatibility complex mismatch murine model (Figure 2A). Compared with mice that only received Tcon (aGVHD), mice transplanted with 1 × 106 of oIL-2Rβ Treg or fTregs at a Tcon:Treg ratio of 1:1 displayed a significant survival benefit with the highest survival advantage observed in the cohort of mice receiving oIL-2Rβ Tregs and oIL-2 compared with oIL-2Rβ and PBS (Figure 2B). The oIL-2Rβ Treg group treated with oIL-2 had significantly improved survival when compared with the group that received fTreg transplantation (reference group, P = .03) but not when compared with the PBS group (P = .07). Importantly, when a suboptimal dose of oIL-2Rβ Tregs (2 × 105, Tcon:Treg ratio of 5:1) was infused, the only group that was able to significantly suppress lethal aGVHD was mice treated with oIL-2Rβ Tregs and oIL-2 (Figure 2D). In addition, mice treated with oIL-2Rβ Tregs and injected with oIL-2 had improved aGVHD clinical scores compared with those injected with PBS (Figure 2C,E).
In vivo injection of oIL-2 and oIL-2Rβ Tregs protect from lethal GVHD. (A) Schema of aGVHD mouse model. Survival curve (B) and aGVHD clinical scores (C) of mice treated with 1 × 106 of fTregs or oIL-2Rβ Tregs and injected with PBS or oIL-2 at a Tcon:Treg ratio of 1:1. Survival curves (D) and aGVHD clinical scores (E) of mice treated with oIL-2Rβ Tregs and injected with oIL-2 or PBS at a Tcon:Treg ratio of 5:1. Data are pooled from 3 independent experiments with a total of 10 to 15 mice per group. Error bars indicate standard error of the mean. P values are indicated when significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Survival curves were plotted using the Kaplan-Meier method and compared using a log-rank test. HSCT used BM transplantation depleted in T cells. Total body irradiation (TBI) was done without BM transplant. FACS, fluorescence-activated cell sorted; IP, immunoprecipitation; TCD, T cell depleted.
In vivo injection of oIL-2 and oIL-2Rβ Tregs protect from lethal GVHD. (A) Schema of aGVHD mouse model. Survival curve (B) and aGVHD clinical scores (C) of mice treated with 1 × 106 of fTregs or oIL-2Rβ Tregs and injected with PBS or oIL-2 at a Tcon:Treg ratio of 1:1. Survival curves (D) and aGVHD clinical scores (E) of mice treated with oIL-2Rβ Tregs and injected with oIL-2 or PBS at a Tcon:Treg ratio of 5:1. Data are pooled from 3 independent experiments with a total of 10 to 15 mice per group. Error bars indicate standard error of the mean. P values are indicated when significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Survival curves were plotted using the Kaplan-Meier method and compared using a log-rank test. HSCT used BM transplantation depleted in T cells. Total body irradiation (TBI) was done without BM transplant. FACS, fluorescence-activated cell sorted; IP, immunoprecipitation; TCD, T cell depleted.
oIL-2 administration drives in vivo expansion and activation of oIL-2Rβ Treg during aGVHD suppression
BLI revealed that administration of oIL-2 induced expansion of the donor luciferase transgenic (luc) oIL-2Rβ Treg (Tregluc+) population in the early-phase posttransplant (Figure 3A) with significant differences at day 7 when compared with the administration of PBS (Figure 3B). Similar statistical differences were observed between these groups with the 1:1 ratio of oIL-2Rβ Tregs:Tcons (1 × 106:1 × 106) and 1:5 ratio (0.2 × 106:1 × 106) (Figure 3B). To assess oIL-2Rβ Tregsluc+ localization throughout the body, recipients were euthanized at different time points after transplantation for ex vivo BLI analysis. A similar pattern of Treg distribution was observed between animals administered oIL-2Rβ Tregsluc+ and injected with oIL-2 or PBS. We found an accumulation of oIL-2Rβ Tregs as early as day 2 posttransplant in the secondary lymphoid organs (SLOs) including peripheral LNs (pLNs), mesenteric LNs (mLNs), and spleen, and in the gastrointestinal tract (GIT) (Figure 3C). The expansion of oIL-2Rβ Tregsluc+ in the spleen and mLNs was higher in the groups treated with oIL-2 than in those treated with PBS (Figure 3D), with significant differences observed when compared with PBS at day 7 (spleen P = .02, mLN P = .007). Flow cytometric analysis of peripheral blood (PB), pLNs, mLNs, and spleen indicated a significant increase of oIL-2Rβ Tregluc+ engraftment in the animals treated with oIL-2 compared with control animals treated with PBS (Figure 3E).
Administration of oIL-2 expands oIL-2Rβ Tregs early after allo-HSCT. Expansion of donor oIL-2Rβ Tregluc+ cells in BALB/c recipients. After 8.8 Gy TBI, BALB/c mice received TCD BM + CD45.1+ Foxp3GFP+luc+ Tregs (1 × 106 or 0.2 × 106) that were transduced with oIL-2Rβ. To induce aGVHD, the mice were infused with 1 × 106 of C57BL/6 T cells on day 2. (A-B) Whole-body photons derived from Tregluc+ cells expanding in BALB/c recipients at days 7 and 14 after allo-HSCT. (A) The animals that received oIL-2 protein (n = 10) (top) and those that received PBS (n = 10) (bottom) are shown. (B) Quantification of whole-body photons using the 1:1 (top) and 5:1 (bottom) Tcon:Treg ratio. (C-D) Ex vivo BLI pictures of donor oIL-2Rβ Tregluc+ in the spleen, pLNs, mLNs, and GIT imaging. (C) The animals that received oIL-2 protein (top) and those injected with PBS at different time points after allo-HSCT (bottom) are shown. (D) Mean ± SD of total flux (photons per second [p/s]) obtained from each region of interest. Photons derived from Tregluc+ cells in the spleen (left) and mLNs (right) at days 2, 3, 5, and 7 after allo-HSCT. (E) PB, spleen, pLNs, and mLNs were recovered and analyzed by flow cytometry on day 7 after allo-HSCT. Pooled data from 3 independent experiments including 4 to 5 mice per group per each experiment. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Color bar represents signal intensity code over body surface area.
Administration of oIL-2 expands oIL-2Rβ Tregs early after allo-HSCT. Expansion of donor oIL-2Rβ Tregluc+ cells in BALB/c recipients. After 8.8 Gy TBI, BALB/c mice received TCD BM + CD45.1+ Foxp3GFP+luc+ Tregs (1 × 106 or 0.2 × 106) that were transduced with oIL-2Rβ. To induce aGVHD, the mice were infused with 1 × 106 of C57BL/6 T cells on day 2. (A-B) Whole-body photons derived from Tregluc+ cells expanding in BALB/c recipients at days 7 and 14 after allo-HSCT. (A) The animals that received oIL-2 protein (n = 10) (top) and those that received PBS (n = 10) (bottom) are shown. (B) Quantification of whole-body photons using the 1:1 (top) and 5:1 (bottom) Tcon:Treg ratio. (C-D) Ex vivo BLI pictures of donor oIL-2Rβ Tregluc+ in the spleen, pLNs, mLNs, and GIT imaging. (C) The animals that received oIL-2 protein (top) and those injected with PBS at different time points after allo-HSCT (bottom) are shown. (D) Mean ± SD of total flux (photons per second [p/s]) obtained from each region of interest. Photons derived from Tregluc+ cells in the spleen (left) and mLNs (right) at days 2, 3, 5, and 7 after allo-HSCT. (E) PB, spleen, pLNs, and mLNs were recovered and analyzed by flow cytometry on day 7 after allo-HSCT. Pooled data from 3 independent experiments including 4 to 5 mice per group per each experiment. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Color bar represents signal intensity code over body surface area.
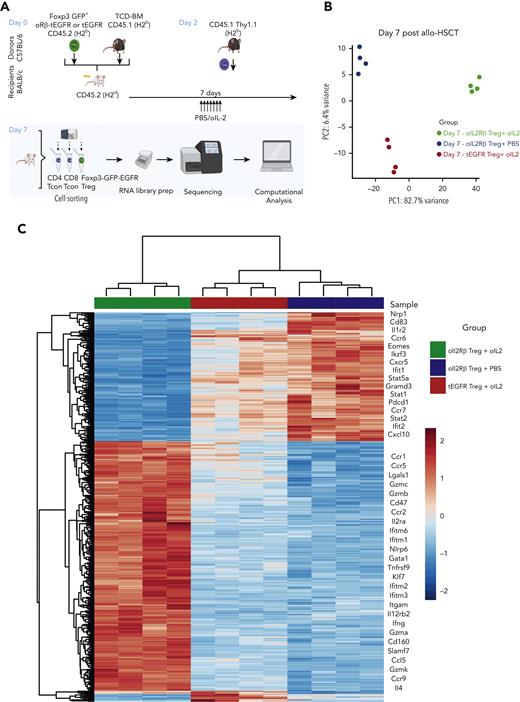
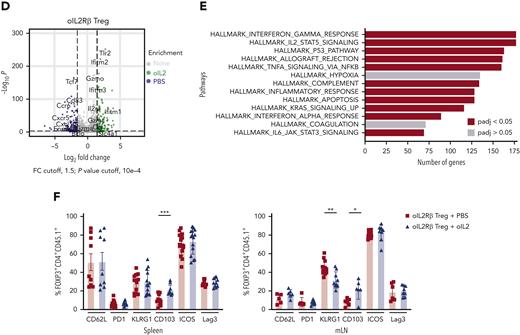
To understand how oIL-2Rβ Tregs stimulated by oIL-2 administration shapes the transcriptomic profile of Tregs in the aGVHD setting, we performed RNA-seq as we have previously described.27 At day 7 after allo-HSCT, we reisolated donor-derived oIL-2Rβ or tEGFR Tregs (CD45.2+ H2b+ GFP+ tEGFR+) and CD4+ and CD8+ T cells (CD45.1+Thy1.1+) (Figure 4A). Principal component analysis (PCA) of the top 1000 most differentially expressed genes (DEGs) across all samples revealed that 82.7% of variance was explained by PC1, which segregated oIL-2Rβ Tregs injected with oIL-2 from the group injected with PBS and tEGFR Tregs injected with oIL-2 (Figure 4B). Comparing oIL-2Rβ Tregs with tEGFR Tregs stimulated with oIL-2, we found 197 DEGs (Figure 4C; supplemental Figure 2A). Examination of the 314 DEGs (P = .001, ±1.5 log2 fold change) revealed 197 upregulated and 117 downregulated genes in the oIL-2Rβ Treg group treated with oIL-2 compared with the group treated with PBS (Figure 4D), with the upregulated genes being enriched in interferon gamma (IFNγ) signaling and the IL-2–STAT5 pathway (Figure 4E). oIL-2 stimulation of oIL-2Rβ Tregs in vivo in the context of aGVHD-upregulated genes correlated with Treg activation and immunosuppressive function. These upregulated genes were observed when compared with the group that received PBS (oIL-2Rβ Treg + PBS) and in the group that received oIL-2 in the absence of the oIL-2Rβ (tEGFR Tregs + oIL-2) (Figure 4C). Flow cytometry analysis showed a significant increase in the percentage of integrin αE (CD103) expression in the spleen and mLNs, and a significant decrease in the percentage of killer cell lectin-like receptor G1 (KLRG1) in the mLNs (Figure 4F; supplemental Figure 2B) of mice treated with oIL-2Rβ Tregs and oIL-2 compared with those treated with PBS. Altogether, these data demonstrate that oIL-2/oIL-2Rβ binding in Tregs induced an early expansion of the oIL-2Rβ Tregs without disrupting their ability to home to SLOs and aGVHD target tissues after allo-HSCT and induced intracellular signaling through the STAT5 pathway with an upregulation of genes related to Treg activation and suppression.
oIL-2 stimulation in oIL-2Rβ Tregs reveals upregulation of transcripts involved in activation and IL-2/STAT5 signaling during GVHD suppression. (A) Schematic representation of the workflow for the RNA-seq (created with BioRender.com). (B) PCA of transcriptome based on the top DEGs across oIL-2Rβ Tregs treated with oIL-2 (green) or PBS (blue) and tEGFR Tregs treated with oIL-2 (red). (C) Heatmap and hierarchical clustering of the differential expression from all the samples. Genes related with activation and Treg suppression are highlighted. Expression for each gene is scaled across single rows. (D) Volcano plots reveal significant and log2 fold change of transcripts from oIL-2Rβ Tregs treated with oIL-2 compared with PBS (left) or oIL-2 stimulation (right). Vertical dashed lines on volcano plots indicate a fold change of ±1.5; horizontal dashed line indicates an adjusted P = .05. (E) Gene set enrichment analysis of significant upregulated pathways (hallmark) shown in purple of oIL-2Rβ Tregs stimulated with oIL-2 compared with those stimulated with PBS. (F) The expression levels of different activator markers (CD62L, PD1, KLRG1, CD103, ICOS, and Lag3) on the FoxP3+ CD4+ GFP+ population were analyzed 7 days after allo-HSCT on cells in the spleen and mLNs. Pooled data from 3 independent experiments including 4 to 5 mice per group per each experiment. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 between indicated groups. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. FC, fold change; padj, adjusted P value.
oIL-2 stimulation in oIL-2Rβ Tregs reveals upregulation of transcripts involved in activation and IL-2/STAT5 signaling during GVHD suppression. (A) Schematic representation of the workflow for the RNA-seq (created with BioRender.com). (B) PCA of transcriptome based on the top DEGs across oIL-2Rβ Tregs treated with oIL-2 (green) or PBS (blue) and tEGFR Tregs treated with oIL-2 (red). (C) Heatmap and hierarchical clustering of the differential expression from all the samples. Genes related with activation and Treg suppression are highlighted. Expression for each gene is scaled across single rows. (D) Volcano plots reveal significant and log2 fold change of transcripts from oIL-2Rβ Tregs treated with oIL-2 compared with PBS (left) or oIL-2 stimulation (right). Vertical dashed lines on volcano plots indicate a fold change of ±1.5; horizontal dashed line indicates an adjusted P = .05. (E) Gene set enrichment analysis of significant upregulated pathways (hallmark) shown in purple of oIL-2Rβ Tregs stimulated with oIL-2 compared with those stimulated with PBS. (F) The expression levels of different activator markers (CD62L, PD1, KLRG1, CD103, ICOS, and Lag3) on the FoxP3+ CD4+ GFP+ population were analyzed 7 days after allo-HSCT on cells in the spleen and mLNs. Pooled data from 3 independent experiments including 4 to 5 mice per group per each experiment. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 between indicated groups. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. FC, fold change; padj, adjusted P value.
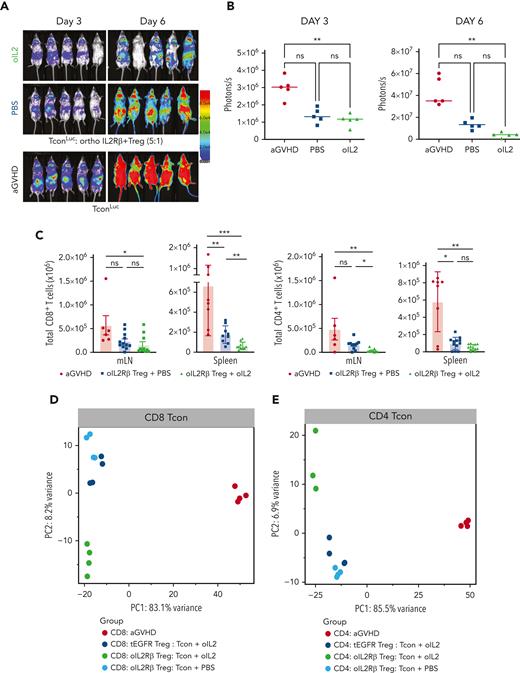
Early expansion of oIL-2Rβ Tregs with oIL-2 injections is associated with reduced donor Tcons
We used the same system described above with the exception that Tcons, not Tregs, were isolated from luc transgenic donors (Tconsluc+). A robust expansion of donor Tconsluc+ was observed after 3 and 6 days of Tcon infusion (Figure 5A). BLI of the Tconsluc+ in mice treated with oIL-2Rβ Tregs and oIL-2 showed a significant signal reduction reflective of a decrease in Tconluc+ proliferation when compared with that of those treated with PBS (Figure 5B). To further confirm the data obtained by BLI, we used a congenic marker system to distinguish Tregs from Tcons (CD45.1 vs CD45.2). A comparison of absolute Tcon numbers revealed that donor oIL-2Rβ Tregs with oIL-2 strongly suppressed the early expansion of both CD8+ and CD4+ T cells as measured in spleen, pLNs, and mLNs, except for CD8+ T cells in pLNs that were unaffected (Figure 5C; supplemental Figure 3A). Next, to evaluate the treatment effect on CD4+ and CD8+ Tcons, we performed RNA-seq on the SLOs (spleen and LNs) 7 days after allo-HSCT. PCA of the top 1000 most DEGs revealed a clear segregation of both CD8+ (variance of 83.1%) and CD4+ (variance of 85.5%) Tcon recovery from animals treated with Tregs compared with the Tcon recovery from the aGVHD group (without Tregs) (Figure 5D-E). Comparing Tcons in the presence of oIL-2Rβ Treg, we found a higher expression of genes associated with naïve T-cells (Foxo1, Tcf7, Foxp1, Il6ra, Sell, and Ccr7) and the immune response (CD8: Il10rb, Smad1, Eomes, Il4ra; CD4: Il21, Il27ra, and Il4ra), and a lower expression of genes associated with T-cell activation/effector differentiation (Icos, Batf, Ifng, Klrg1, Ifngr1, Prf1, Ctla4, Tnfrsf9, Il2ra, and Il18r1) (supplemental Figure 3B). The PC2 revealed a segregation of the Tcons treated with oIL-2Rβ Tregs and oIL-2 from Tcons treated with oIL-2Rβ Tregs and PBS and tEGFR Tregs and oIL-2, which contributed to 8.2% and 6.9% of the variance in CD8+ and CD4+ Tcons, respectively (Figure 5D-E).
oIL-2Rβ Treg and oIL-2 system reduces donor T-cell expansion and activation in vivo. (A) In vivo BLI data of Tconsluc+ from representative animals at day 3 (left) and 6 (right) after transplantation. (B) Quantification of Tconsluc+ luminescence from each mouse (p/s) per group (n = 5). (C) Absolute number of donor (H-2kb) CD4+ and CD8+ CD45.2+ cells in lymphoid tissues and spleen following transplantation. RNA-seq analysis of CD8+ (D) and CD4+ (E) Tcons recovered at day 7 after HSCT from animals treated with the indicated oIL-2Rβ (untreated, black; PBS, blue; oIL-2, green; tEGFR Treg + oIL-2, red.). (D-E) PCA of the transcriptome based on the top 1000 DEGs across all samples. (F) Proportion among CD4+ and CD8+ of naïve (CD44−CD62L+), central memory (CD44+CD62L+), and effector memory T cells (CD44+CD62L−) in the spleen of animals without (aGVHD) and with oIL-2Rβ Tregs treated with PBS and oIL-2 after day 7 following allo-HSCT and treatment. (G) Mean ± SD of the MFI of ICOS in the CD4+CD45.2+ Tcons (left) and CD8+CD45.2+ Tcons (right) in the spleen. Pooled data from 3 independent experiments including 4 to 5 mice per group per each experiment. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .001 between indicated groups. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis.
oIL-2Rβ Treg and oIL-2 system reduces donor T-cell expansion and activation in vivo. (A) In vivo BLI data of Tconsluc+ from representative animals at day 3 (left) and 6 (right) after transplantation. (B) Quantification of Tconsluc+ luminescence from each mouse (p/s) per group (n = 5). (C) Absolute number of donor (H-2kb) CD4+ and CD8+ CD45.2+ cells in lymphoid tissues and spleen following transplantation. RNA-seq analysis of CD8+ (D) and CD4+ (E) Tcons recovered at day 7 after HSCT from animals treated with the indicated oIL-2Rβ (untreated, black; PBS, blue; oIL-2, green; tEGFR Treg + oIL-2, red.). (D-E) PCA of the transcriptome based on the top 1000 DEGs across all samples. (F) Proportion among CD4+ and CD8+ of naïve (CD44−CD62L+), central memory (CD44+CD62L+), and effector memory T cells (CD44+CD62L−) in the spleen of animals without (aGVHD) and with oIL-2Rβ Tregs treated with PBS and oIL-2 after day 7 following allo-HSCT and treatment. (G) Mean ± SD of the MFI of ICOS in the CD4+CD45.2+ Tcons (left) and CD8+CD45.2+ Tcons (right) in the spleen. Pooled data from 3 independent experiments including 4 to 5 mice per group per each experiment. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .001 between indicated groups. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis.
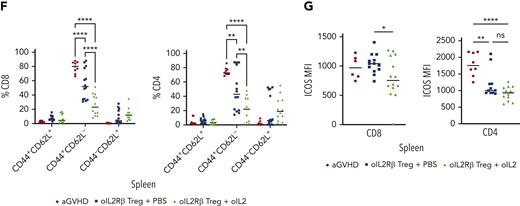
Using a uniform manifold approximation and projection dimension reduction model, we identified 3 major clusters in the spleen (supplemental Figure 4A). A significant decrease of effector memory (CD44+CD62L−) donor Tcons was observed between the groups that received oIL-2 compared with those that received PBS (P = .002 in CD4+ and P < .001 in CD8+) (Figure 5F). The frequencies of naïve and central memory T cells were unaffected (Figure 5F). In agreement with RNA-seq data, the mice that underwent transplantation with oIL-2Rβ Tregs showed a significant decrease in ICOS expression (mean fluorescence intensity) in the CD4+ T-cell population compared with the aGVHD group (P = .002 with PBS and P < .0001 with oIL-2). In the CD8+ T cells, we found a significant decrease (P = .04) of ICOS in the animals treated with oIL-2Rβ Tregs and oIL-2 compared with those treated with PBS (Figure 5G). At day 7 following allo-HCST, the concentration of several T cell–derived proinflammatory cytokines was elevated in the serum of aGVHD mice (without Treg). The administration of oIL-2Rβ Tregs with either oIL-2 or PBS reduced multiple proinflammatory cytokines compared with aGVHD mice (supplemental Figure 4B).
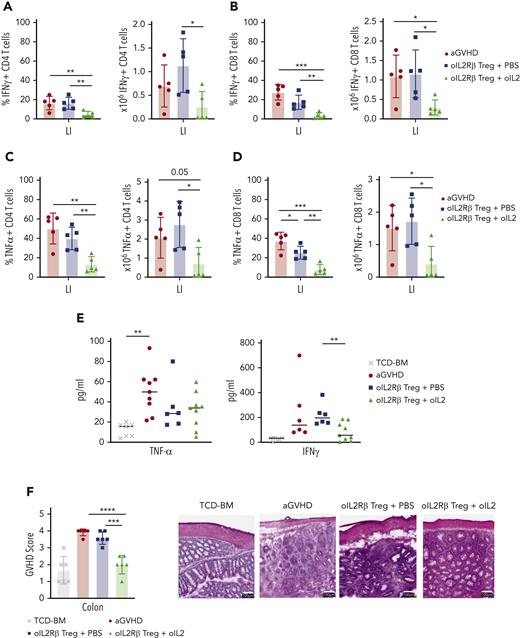
Mice treated with oIL-2Rβ Tregs and oIL-2 demonstrate a significant reduction of activated and proliferating colonic T cells
With enhanced Treg expansion and homing to the GIT in mice treated with oIL-2Rβ Tregs and oIL-2, we questioned whether there was significant protection of the colon. We harvested the colon and isolated lamina propia lymphocytes on day 14 after allo-HCST. Mice treated with oIL-2Rβ Tregs and oIL-2 compared with those treated with PBS demonstrated a significant reduction in the number and frequency of isolated CD4+ and CD8+ T cells able to produce the proinflammatory cytokines tumor necrosis factor α (TNFα) and IFNγ (absolute numbers: IFNγ CD4+ T cells, P = .017; IFNγ CD8+ T cells, P = .017; TNFα CD4+ T cells, P = .014; TNFα CD8+ T cells, P = .011) (Figure 6 A-D). In contrast, mice treated with oIL-2Rβ Treg and PBS only showed a significant decrease in the frequency of IFNγ producing CD8+ T cells (P = .04) (Figure 6D). Furthermore, we found a marked decrease in IFNγ levels in the PB of mice treated with oIL-2Rβ Tregs and oIL-2 compared with those treated with PBS on day 14 after allo-HSCT (Figure 6E). To directly evaluate colon integrity, we performed histological analysis with hematoxylin and eosin staining. The GVHD pathology score of the colon was significantly decreased in mice treated with oIL-2Rβ Tregs and oIL-2 when compared with those treated with PBS (P = .0002) and to aGVHD mice (P = .00001) (Figure 6F). Histological images demonstrate improved colon structure and integrity when mice were treated with oIL-2Rβ Tregs and oIL-2 compared with those treated with PBS (Figure 6F). Furthermore, we performed immunohistochemistry to evaluate the proliferation in the colon. The aGVHD mice showed higher CD4+ T-cell infiltration in the large intestine compared with the animals infused with oIL-2Rβ Tregs, a finding more pronounced in the mice injected with oIL-2 than in those injected with PBS (supplemental Figure 5). Ki67 expression was also evaluated, and lower expression was observed in the BM (without GVHD) and oIL-2 groups than in the aGVHD or oIL-2Rβ Tregs-PBS groups (supplemental Figure 5). Altogether, these data show that treatment with oIL-2Rβ Tregs and oIL-2 leads to enhanced suppression of Tcons in the GIT and an overall decrease in proliferation in the gut, which is associated with tissue repair.
oIL-2Rβ Tregs home to the GIT and potently suppress activated Tcons. BALB/c mice underwent transplantation with C57BL/6 BM and Tcons to induce aGVHD (n = 5) and were treated with oIL-2Rβ Tregs with daily injections of either PBS (n = 5) or oIL-2 (n = 5). The large intestine was harvested on day 14 for flow cytometry analysis. Data are representative of 2 independent experiments. Frequency (left) and absolute number (right) of INFγ+ CD4+ (A) and CD8+ (B) T cells. Frequency (left) and absolute number (right) of TNFα+ CD4+ (C) and CD8+ (D) T cells. (E) Concentration of TNFα and INFγ+ in PB on day 14 after allo-HSCT. (F) aGVHD pathology scores of the large intestine (LI) on day 14 after allo-HSCT (left). Representative histological images of the colon at original magnification ×10 following hematoxylin and eosin staining (right). Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. Error bars indicate the SD of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
oIL-2Rβ Tregs home to the GIT and potently suppress activated Tcons. BALB/c mice underwent transplantation with C57BL/6 BM and Tcons to induce aGVHD (n = 5) and were treated with oIL-2Rβ Tregs with daily injections of either PBS (n = 5) or oIL-2 (n = 5). The large intestine was harvested on day 14 for flow cytometry analysis. Data are representative of 2 independent experiments. Frequency (left) and absolute number (right) of INFγ+ CD4+ (A) and CD8+ (B) T cells. Frequency (left) and absolute number (right) of TNFα+ CD4+ (C) and CD8+ (D) T cells. (E) Concentration of TNFα and INFγ+ in PB on day 14 after allo-HSCT. (F) aGVHD pathology scores of the large intestine (LI) on day 14 after allo-HSCT (left). Representative histological images of the colon at original magnification ×10 following hematoxylin and eosin staining (right). Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. Error bars indicate the SD of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
oIL-2Rβ Treg and oIL-2 system permits the administration of lower numbers of Tregs to suppress GVHD lethality while maintaining the GVT response
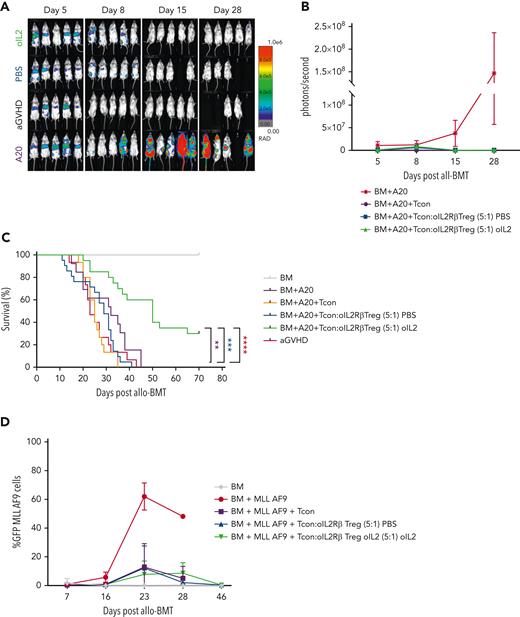
Improved Treg therapies will need to suppress GVHD without abrogating the GVT response to achieve the highly desired, GVHD-free, relapse-free survival. Therefore, we next examined whether the GVT activity of Tcons was maintained in the mice that underwent oIL-2Rβ Tregs transplant and were treated with oIL-2. BALB/c mice transplanted with TCD-BM from C57BL/6 animals and coinjected with A20luc/yfp lymphoma cells died before day 45 from tumor progression that was preceded by an increase in BLI signal intensity over time (Figure 7A). Mice that received Tcons were able to suppress A20luc/yfp growth but succumbed to aGVHD by day 35 (Figure 7A-C). When mice received oIL-2Rβ Treg transplant and were injected with either PBS or oIL-2, they showed an initial tumor signal like the BM plus lymphoma cells group at day 5 exhibiting engraftment of tumor cells (Figure 7A-B) but subsequently eradicated the lymphoma cells. Mice treated with oIL-2 showed enhanced aGVHD survival while simultaneously maintaining GVT responses, with a median survival of 50 days compared with 30 days in the mice treated with PBS, that succumbed to aGVHD by day 41 after allo-HCST (Figure 7A). No residual disease or relapse of leukemia was observed in the animals treated with oIL-2 at least 70 days after transplantation (supplemental Figure 6). To further confirm that oIL-2Rβ Tregs maintained GVT responses, we used the same allo-HCST model, but this time we measured the growth of a leukemia cell line MLL-AF9GFP+ in PB at multiple time points after allo-HCST. We found oIL-2Rβ Treg groups injected with PBS and oIL-2 suppressed tumor growth comparably to the Tcon group (Figure 7D). Overall, we show that oIL-2Rβ Tregs with oIL-2 allows for suppression of aGVHD with very low Treg numbers while maintaining GVT responses.
oIL-2Rβ Tregs maintain graft-versus-leukemia response. (A-C) Tumor growth and elimination (A-B) and survival (C) are shown for the BALB/c mice injected with A20luc/yfp+ (2 × 105) leukemia cells at the time of TCD BM transplantation with or without (purple) oIL-2Rβ Tregs and treated with oIL-2 (green) or PBS (blue). Tcon cells were administered 2 days after allo-BMT at a ratio of 5:1 (Tcon:Treg). (D) Frequency of enhanced GFP+ MLL-AF9 cells in the PB at various time points after allo-HSCT. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. Log-rank test was used to analyze survival curves. Error bars indicate the SD of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Results are pooled from 4 independent experiments with a total of 5 mice per group (oIL-2, n = 20; PBS, n = 21). Survival curves were plotted using the Kaplan-Meier method and compared using a log-rank test. allo-BMT, allogeneic bone marrow transplantation.
oIL-2Rβ Tregs maintain graft-versus-leukemia response. (A-C) Tumor growth and elimination (A-B) and survival (C) are shown for the BALB/c mice injected with A20luc/yfp+ (2 × 105) leukemia cells at the time of TCD BM transplantation with or without (purple) oIL-2Rβ Tregs and treated with oIL-2 (green) or PBS (blue). Tcon cells were administered 2 days after allo-BMT at a ratio of 5:1 (Tcon:Treg). (D) Frequency of enhanced GFP+ MLL-AF9 cells in the PB at various time points after allo-HSCT. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. Log-rank test was used to analyze survival curves. Error bars indicate the SD of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Results are pooled from 4 independent experiments with a total of 5 mice per group (oIL-2, n = 20; PBS, n = 21). Survival curves were plotted using the Kaplan-Meier method and compared using a log-rank test. allo-BMT, allogeneic bone marrow transplantation.
Discussion
Using a major histocompatibility complex mismatch model, we observed near complete protection from lethal aGVHD after transplantation of oIL-2Rβ Tregs and Tcons from C57BL/6 (H-2b) mice at a 1:1 ratio into lethally irradiated BALB/c (H-2d) animals injected with oIL-2 cytokine that was superior to the partial protection observed with PBS. When we stressed the model by decreasing the number of oIL-2Rβ Tregs transplanted with a 1:5 ratio of oIL-2Rβ Tregs:Tcons, only the mice that were injected with oIL-2 showed protection from lethal aGVHD. In our study we used a mouse serum albumin–fusion construct that substantially extended the IL-2 half-life from 5 to 50 hours in vivo and obviates receptor affinity differences for WT IL-2 and oIL-2. In recent publications, Zhang et al22 and Aspuria et al23 studied a human oIL-2 and IL-2Rβ system that enables specific in vivo control of chimeric antigen receptor T-cell expansion and activation with higher selectivity and affinity pairs. Preclinical and clinical studies have demonstrated that engineering the donor graft with a specific ratio of Tregs and Tcons can improve HSCT outcomes by reducing GVHD incidence and improving immune reconstitution.8,28
Tregs naturally home to sites of inflammation to modulate immune responses.29 To adequately suppress aGVHD, Tregs must travel to aGVHD target organs, which includes SLOs.30-32 We found that mice treated with oIL-2Rβ Tregsluc+ and injected with either oIL-2 or PBS showed similar migration patterns to SLOs. We recently showed, in a murine mixed hematopoietic chimerism model, that mice treated with oIL-2Rβ and injected with oIL-2 cytokine allowed for selective expansion of Tregs in vivo without stimulation of alloreactive T cells, allowing the establishment of organ transplantation tolerance.24 Here, using an allo-HCST model, we also observed selective expansion of the oIL-2Rβ donor Tregs when stimulated with oIL-2 early after transplant which led to significantly improved aGVHD suppression using suboptimal Treg numbers. The expanded Tregs persisted for 2 weeks and gradually decreased as observed in previously studies.28,33 To understand whether these transduced Tregs were dependent on oIL-2 supplementation, we used the same system without transplanted donor T cells (no GVHD), in which we observed that the withdrawal of oIL-2 after 28 days of injection did not significantly affect the presence of this population in the recipient and we were able to detect them by BLI after 100 days posttransplant (data not shown). Previous studies already showed that transplant recipients with luc+ Tregs had a persistent and stable BLI signal in SLOs over a 3-month period following transplantation.28 In our system, the Tregs express the 2 receptors, WT IL-2 and the oIL-2Rβ, which allows the proliferation/maintenance of this population in the presence of low IL-2.
aGVHD occurs in the setting of insufficient Treg numbers to adequately suppress alloreactive Tcon activation, proliferation, and homing.3 Thus, Treg therapy serves as an attractive aGVHD preventive approach.34,35 Nguyen et al observed that administration of Tregs 2 days before Tcon infusion led to a significant increase in donor Tcon suppression and improved survival of allo-HSCT murine recipients28 through expansion of donor Tregs in vivo in the absence of Tcons.28 Treg administration 2 days before Tcons, which was used in our studies, is now being investigated in clinical studies and has demonstrated a reduction of aGVHD compared with historical controls.10
In addition to early Treg infusion, Treg support via IL-2–based methods has been investigated. Low dose of IL-2, IL-2 muteins, and IL-2/anti–IL-2 complexes generated to preferentially bind and stimulate CD25-expressing cells promote Treg expansion because Tregs constitutively express CD25 but will also bind and stimulate immune cells that increase CD25 expression following activation.36,37 Whereas these methods have shown efficacy in some autoimmune models, the risk for alloreactive Tcon stimulation poses a threat to the therapeutic benefit in the allogeneic setting as demonstrated with GVHD exacerbation.20,38 Here, we show that combining this approach of early Treg infusion with the orthogonal system supports Treg expansion in vivo and increases GVHD survival.
Increasing the number of Tregs to suppress aGVHD may not be sufficient.39,40 The functional fitness of donor Tregs must be maintained after ex vivo and in vivo expansion. Tregs need to retain the expression of key phenotypic markers and preserve their suppression function. We show that oIL-2Rβ Tregs significantly increased levels of cell-surface antigens associated with Treg activation and suppression, particularly CTLA-4, ICOS, CD25, and GITR, following ex vivo expansion. Importantly, oIL-2Rβ Tregs maintained the expression of key suppression and activation molecules when measured on day 7 after allo-HSCT. At the protein level, oIL-2Rβ Tregs stimulated by oIL-2 or PBS appeared similar with some minor differences, whereas, at the transcriptomic level we found oIL-2Rβ Tregs stimulated by oIL-2 compared with oIL-2Rβ Tregs stimulated with PBS or tEGFR Tregs stimulated with oIL-2 upregulated genes involved in IL-2/STAT5 signaling and interferon responses. It is well described that IL-2/STAT5 signaling promotes the generation, survival, and suppressive function of Tregs.41,42 Recent data suggest that IFN-γ–producing Tregs favor the reinvigoration of the antitumor response,43 thus in the allo-HSCT setting would help to maintain the GVT response. Similar to previously published RNA-seq studies comparing oIL-2Rβ Tregs stimulated by oIL-2 vs WT IL-2, oIL-2 stimulation increased many IL-2–related genes with some unique genes not found in WT IL-2–stimulated Treg groups.24 In our studies, the groups stimulated in vivo with oIL-2 failed to upregulate the genes related to the oIL-2–STAT5 pathway because of the lack of the oIL-2Rβ chain expression (tEGFR Treg–oIL-2), confirming our previous work showing the specificity of this system.
Specifically, we found an upregulation of genes critical for Treg immune suppression including soluble factors, such as transforming growth factor β (Tgfb1) and IL-10 (Il10) and cell-surface molecules, such as OX40 (Tnfrsf4), GITR (Tnfrsf18), and CTLA-4 (Ctla4) in the animals treated with oIL-2 compared with those treated with PBS. Furthermore, oIL-2Rβ Tregs with either PBS or oIL-2 expressed genes consistent with an activated state of Tregs and genes involved in p53 and apoptotic pathways at day 7 after allo-HSCT. Similar data have been reported in transcriptomic studies done early after adoptive cell transfer, suggesting a possible explanation for the early expansion and contraction of Tregs in vivo.44 Data described here agree with our previous studies showing that oIL-2 potently induces activation of signals related to cell proliferation, survival, and immune regulation of oIL-2Rβ Tregs.24
A hallmark of aGVHD is the expansion of donor alloreactive T cells in the proinflammatory environment. The differentiation of this population into effector cells leads to tissue damage and an increase in and recruitment of inflammatory immune cell populations with cytokine dysregulation.45 Here, we observed a higher suppression of donor CD4+ and CD8+ T-cell expansion in the PB and SLOs, which was associated with lower serum levels of inflammatory cytokines in the mice that received oIL-2Rβ Treg transplants and were injected with oIL-2. The study of donor Tcon transcriptome during aGVHD showed suppression by oIL-2Rβ Treg/oIL-2 signaling through downregulation of genes related to activation/proliferation and an upregulation of genes associated with resting/naïve Tcon state such as CD62L (Sell), Il6ra, Foxo1, Tcf7, and Ccr7. Interestingly, we found a decrease in the transcript and protein levels of T-cell ICOS in both donor CD8+ and CD4+ T cells. In a recent work, ICOS was identified as a potential biomarker to visualize, by immune–positron emission tomography, the dynamics of activation, expansion, and tissue distribution of alloreactive donor Tcons in an aGVHD model.46 In addition, donor T-cell ICOS and host ICOS-ligand engagement are biologically relevant as known critical drivers of aGVHD.47 Furthermore, GIT damage is the leading cause of death in aGVHD because of high donor T-cell influx with elevated inflammatory cytokines.48 In our studies, only the animals treated with the oIL-2Rβ Treg/oIL-2 pair showed a significant decrease of the 2 main inflammatory cytokines, TNFα and IFNγ, on both CD8+ and CD4+ T cells in the colon with substantial improvements in GVHD pathology observed via histological analysis.
With the presence of greater numbers of Tregs in vivo, there is increased concern for generalized immunosuppression. Preclinical allo-HSCT models show polyclonal Treg transfers suppress aGVHD, but GVT responses have not been uniformly preserved.11,12,49 GVT is a major component of the overall beneficial effect of allo-HSCT in the treatment of hematological malignancies.50 In contrast, the transfer of unexpanded Tregs may better preserve GVT activity51 than that of ex vivo expanded Tregs that preactivated before infusion, increasing their potency. Whether engineered Tregs with enhanced potential for expansion or function pose a risk for GVT maintenance remains to be fully investigated. Our results show that mice treated with oIL-2Rβ Tregs and injected with oIL-2 demonstrate enhanced Tcon suppression with associated reduction of GVHD incidence and severity without affecting GVT responses directed against an A20 lymphoma and MLL-AF9 leukemia cells.
The oIL-2 system in Tregs showed enhanced expansion of Tregs in vivo early after allo-HSCT with significant aGVHD suppression. The early expansion of Tregs appears to be critical to suppress GVHD as we, using third-party Tregs, and others, using partially HLA-matched Tregs, have previously shown in vivo early peak expansion followed by a contraction phase.8,52 Future studies will need to investigate whether Treg persistence would be beneficial for aGVHD suppression and GVT maintenance and whether this could be achieved when combined with other strategies such as chimeric antigen receptors or via reinfusions of oIL-2Rβ Tregs. In conclusion, this is the first reported use of the oIL-2R and cytokine pair system in murine Treg to prevent aGVHD following allo-HCST. Selective Treg expansion early after transplantation, without undesired off-target effects, facilitates suppression of alloreactive Tcons, protection of aGVHD target organs, and overall improved clinical scores and survival in aGVHD, leading to improved outcomes in allo-HCST recipients.
Acknowledgments
The authors thank Xuhuai Ji at the Stanford Functional Genomics Facility for their excellent technical assistance in the genomic analysis.
This work was supported by grants from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases and NIH, National Heart, Lung, and Blood Institute (NHLBI) (grant numbers NIH R01 56067, R37 AI34495, R01 HL11879, R01 HL155114, P01 AI056299, T32 AI007313, and F30HL156312). This work was supported by grants from the NIH, NHLBI (P01 HL075462) (R.S.N.). Flow cytometry analysis and sorting were performed on instruments in the Stanford Shared FACS Facility purchased using a NIH S10 Shared Instrumentation Grant (S10RR027431-01).
Authorship
Contribution: T.L.R. and S.B.-W. conceived and designed the research studies and wrote the manuscript; S.B.-W., T.L.R., S.J., G.T., F.S., T.H., and P.-Y.L. conducted experiments; T.L.R., F.S., J.K.L., and A.S. analyzed data; B.K., L.L.S., and L.K.P. developed methodology and analyzed data; J.B. developed methodology and provided essential reagents; J.K.L., M.R., C.E., J.T., A.P.-M., J.E.W., and K.C.G. provided essential tools and intellectual input; and R.S.N. and B.R.B. supervised the research.
Conflict-of-interest disclosure: K.C.G. is the founder of Synthekine Therapeutics, which has licensed the ortho-2 technology. K.C.G. and L.K.P. are inventors on a patent application describing the ortho-2 system (biologically relevant orthogonal cytokine/receptor pairs, US patent no. 10,869,887B2). K.C.G., L.S., and L.K.P. are shareholders of Synthekine, a biotechnology company that has licensed the ortho–IL-2 technology. J.E.W. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics. B.R.B. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics; research funding from Carisma Therapeutics, Inc; and is a cofounder of Tmunity Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Division of Blood and Marrow Transplant and Cellular Therapy, Department of Pediatrics and the Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455; e-mail: blaza001@umn.edu; and Robert S. Negrin, Division of Blood and Marrow Transplantation and Cellular Therapy, Department of Medicine, Stanford University, 269 W. Campus Dr, CCSR, Room 2205, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
Author notes
∗T.L.R. and S.B.-W. contributed equally to this study and are joint first authors.
†R.S.N. and B.R.B. contributed equally to this study and are joint last authors.
Data are available on request from the corresponding authors, Bruce R. Blazar (blaza001@umn.edu) and Robert S. Negrin (negrs@stanford.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![oIL-2 selectively expands oIL-2Rβ transduced Tregs in vitro and enhances Treg suppressive function. (A) Representative flow cytometry plot displaying CD25+Foxp3+ Treg expression (left) and frequency (right) in NT, tEGFR, and oIL-2Rβ Tregs (right). (B) Representative histogram plots of tEGFR expression (left) and frequency (right) in NT, tEGFR Tregs, and oIL-2Rβ Tregs. (C) Heatmap and hierarchical clustering of the differential gene expression from fTregs, tEGFR Tregs, and oIL-2Rβ Tregs. Genes related with activation and Treg suppression are highlighted. Expression for each gene is scaled across single rows. (D) Heatmap displaying mean fluorescence intensity (MFI) of Treg markers CTLA-4, CD25, ICOS, Lag3, and Ki67 in Tregs freshly harvested (day 0 [D0]) or tEGFR Tregs and oIL-2Rβ Tregs on day 7 after harvest. (E) Absolute number of Tregs after 4-day stimulation with WT (1000 IU/mL) or oIL-2 (100 000 IU/mL). Data are representative of 4 independent experiments. (F) Percent suppression of CD4+ and CD8+ T cells after coculture with Tregs at 1:12 Treg:Tcon ratio. (G) Representative histograms show dilution of proliferation dye on CD4+ T cells after coculture with Tregs at 1:12 Treg:Tcon ratio. Data are representative of 3 independent experiments. Student t test with Bonferroni correction for multiple comparisons was used for statistical analysis. Error bars indicate the standard deviation (SD) of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CTV, CellTrace Violet; ns, no significance.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/11/10.1182_blood.2022018440/4/m_blood_bld-2022-018440-gr1.jpeg?Expires=1769260369&Signature=UhC2O52nyXNj-aF3Tou-PfljD-5yOa9aM4YskvuJFX-CLRVaq2BJWaINuzQ5uHlR1oV3q6ChMO5v6-zEFfCgRNS9t~ipkmAgUn1hQdHCGc9AWa7lHDcE9Rdf3WCN-gRPXWFGRUfVfRCIwGOvAWUFjwDYkGphd2cDaqfMsO4K1vwnSSPu22XwiV2ZO4SvXkp3fIF1mRngPJPSVQPOfskoN9GrFjxGdaupzasmmhQ1NzKalBZjmmK20ACRybWR2Iw0w6tqnJcP-FlCudaukL1GKBtmnvsVYAJ0nWxZg4OyAaUBQhakG~CdVFGYGCbYjS7vtH3CZzekeJH0HYgH0Ask4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Administration of oIL-2 expands oIL-2Rβ Tregs early after allo-HSCT. Expansion of donor oIL-2Rβ Tregluc+ cells in BALB/c recipients. After 8.8 Gy TBI, BALB/c mice received TCD BM + CD45.1+ Foxp3GFP+luc+ Tregs (1 × 106 or 0.2 × 106) that were transduced with oIL-2Rβ. To induce aGVHD, the mice were infused with 1 × 106 of C57BL/6 T cells on day 2. (A-B) Whole-body photons derived from Tregluc+ cells expanding in BALB/c recipients at days 7 and 14 after allo-HSCT. (A) The animals that received oIL-2 protein (n = 10) (top) and those that received PBS (n = 10) (bottom) are shown. (B) Quantification of whole-body photons using the 1:1 (top) and 5:1 (bottom) Tcon:Treg ratio. (C-D) Ex vivo BLI pictures of donor oIL-2Rβ Tregluc+ in the spleen, pLNs, mLNs, and GIT imaging. (C) The animals that received oIL-2 protein (top) and those injected with PBS at different time points after allo-HSCT (bottom) are shown. (D) Mean ± SD of total flux (photons per second [p/s]) obtained from each region of interest. Photons derived from Tregluc+ cells in the spleen (left) and mLNs (right) at days 2, 3, 5, and 7 after allo-HSCT. (E) PB, spleen, pLNs, and mLNs were recovered and analyzed by flow cytometry on day 7 after allo-HSCT. Pooled data from 3 independent experiments including 4 to 5 mice per group per each experiment. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Color bar represents signal intensity code over body surface area.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/11/10.1182_blood.2022018440/4/m_blood_bld-2022-018440-gr3.jpeg?Expires=1769260369&Signature=btnBjC3IdRj8Xj2g8EFIMKfNJM3-FLGqYdmgRJHr~vhq6QJjn66e9-ryZW3fg2aCe1AQnXj9s4DmZKdFPT16xQ8QOaXG1vJzNes8ZNNXOeq~VPggYcn3gVjhq-lOyZz9Xop2zyIo6OwFdyTAborEgk3RaJbENHE782HETQ4dl1ozJVNOXPs84-krBCFFrn9upoTDVBd6BcSCq3jeytg7d3Gdkw4W8nC2l2ygsw9jsTVgzRmfabA9B6kjN6RcXh28o7lyO9GZTIS-GFutVEs1V~1rzFFUkXZnzMxEw17crCweFVMJIh2UUNqK9dYM8YKO3Pow3sLiDmZ1k7aCXVsfKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal