In this issue of Blood, Ramos et al demonstrate that the orthogonal interleukin-2 (IL-2)/IL-2 receptor β (IL-2Rβ) system selectively expands adoptively transferred regulatory T cells (Tregs) in vivo, leading to the amelioration of acute graft-versus-host disease (GVHD) without compromising the graft-versus-leukemia (GVL) effect in a mouse major histocompatibility complex (MHC) mismatch model.1
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for hematologic malignancies due to the graft-versus-tumor (GVT) effect. However, its success is limited by the occurrence of GVHD. Acute GVHD (aGVHD) is caused by alloreactive donor T cells recognizing and destroying host tissues.2 With GVHD being a major cause of nonrelapse mortality post–allo-HSCT, there remains a need to develop novel prophylaxis options that improve survival without relapse.
One emerging and promising prophylactic strategy is the use of Treg therapy. Preclinical models and early clinical trials showed that adoptive transfer of polyclonal Tregs efficiently ameliorate aGVHD while maintaining the GVT effect if used at high Treg to conventional T cell (Tcon) ratios (1:1).3,4 The limiting factor for a broader clinical application of Treg therapies is the low Treg numbers in the graft and the necessity of expansion for clinical use. The IL-2 dependence of Tregs for their homeostasis makes this cytokine an attractive target for in vivo Treg manipulation, and low-dose IL-2 has been used clinically to treat chronic GVHD.5 Notably, its pleiotropic nature affects different cell subsets in a dose-dependent manner, and at high doses, it can stimulate effector cells and potentially worsen alloimmune responses driving GVHD. The recently described orthogonal IL-2/IL-2Rβ system6,7 applied here reduces the need for extensive ex vivo Treg expansion and virtually eliminates IL-2 off-target effects.
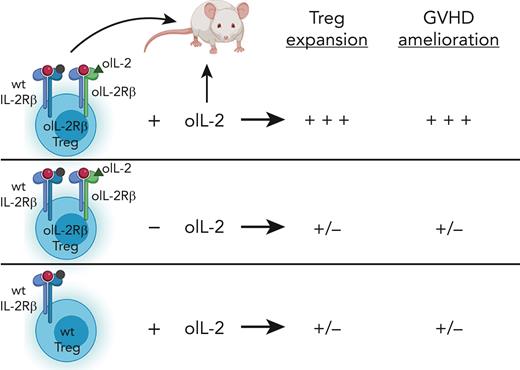
In this study, the authors evaluated genetically engineered Tregs, which can be selectively expanded in vivo as a novel, innovative method for aGVHD prophylaxis. Using a mouse MHC mismatch model for HSCT, they demonstrated that early adoptive transfer of Tregs expressing the orthogonal IL-2Rβ (oIL-2Rβ) enables their effective and selective expansion in vivo after administration of oIL-2. This expansion of oIL-2Rβ-expressing Tregs led to a near complete protection from lethal aGVHD at high Treg to Tcon (1:1) ratios and improved survival at suboptimal ratios (1:5) (see figure). The ability to significantly reduce the Treg numbers needed in the clinical setting would provide a major advance in terms of technical feasibility. Another advantage is the selectivity of the ortho system. Because the oIL-2Rβ only binds oIL-2 but not wild-type (WT) IL-2, off-target effects are significantly reduced.
Tregs expressing oIL-2Rβ significantly expand in vivo in the presence of oIL-2 and ameliorate aGVHD at a Tcon to Treg ratio of 5:1 in a mouse MHC mismatch model of HSCT. In contrast, unexpanded oIL-2Rβ Tregs (in the absence of oIL-2) and WT Tregs in the presence of oIL-2 do not expand and have virtually no effect on aGVHD. This figure was created with BioRender.com.
Tregs expressing oIL-2Rβ significantly expand in vivo in the presence of oIL-2 and ameliorate aGVHD at a Tcon to Treg ratio of 5:1 in a mouse MHC mismatch model of HSCT. In contrast, unexpanded oIL-2Rβ Tregs (in the absence of oIL-2) and WT Tregs in the presence of oIL-2 do not expand and have virtually no effect on aGVHD. This figure was created with BioRender.com.
A major concern in using Treg therapies is systemic immune suppression potentially leading to tumor relapse. Several previous studies have reported that polyclonal Treg transfers do not impair the GVT effect.3 Using 2 different tumor cell lines, the A20 lymphoma and the MLL-AF9 leukemia cell lines, the authors demonstrated that their engineered, preactivated, and expanded oIL-2Rβ Tregs maintained the GVT effect.
In an additional series of experiments, the authors analyzed the function of the expanded oIL-2Rβ Tregs early after allo-HSCT (day 7), given that high Treg levels are critical in the initial phase posttransplant for optimal effectiveness. Importantly, their homing ability to secondary lymphoid organs (SLOs) and aGVHD target tissues (gastrointestinal tract), shown by in vivo bioluminescent imaging, was retained. Furthermore, the authors found that on the protein level, expanded oIL-2Rβ Tregs maintained the expression of key suppressive and activation molecules. Transcriptionally, they upregulated genes related to Treg activation and suppressive function. Regarding the effect of oIL-2Rβ Treg expansion on donor effector/conventional T cells, they observed higher suppression of donor CD4+ and CD8+ T cell proliferation in peripheral blood and SLOs, which was associated with lower serum levels of inflammatory cytokines. The transcriptome of donor effector/conventional T cells revealed a downregulation of genes related to activation/proliferation after the expansion of oIL-2Rβ Tregs.
This initial report shows that early posttransplant adoptive transfer of oIL-2Rβ Tregs combined with their in vivo expansion is an elegant and promising approach to reduce aGVHD. As such, it contributes to a growing number of strategies being evaluated for GVHD prophylaxis.
Because a key component of this strategy involves in vivo expansion, this approach reduces the numbers of Tregs required for effective treatment. Importantly, the selective expansion directly targets only Tregs and therefore promises a high level of safety by minimizing IL-2 off-target effects. In addition, the increased half-life of oIL-2, compared with its WT counterpart, may further enhance therapeutic efficacy. Regardless, the strategy reported here requires an additional step of ex vivo manipulation. Nonetheless, such successful ex vivo Treg genetic engineering is an impressive accomplishment that could certainly benefit patients undergoing HSCTs. It would be exciting if a platform could be generated to create “off-the-shelf” oIL-2Rβ Tregs, bringing benefit to patients at a large number of medical centers.
The use of a human orthogonal IL-2/IL-2Rβ system has recently been evaluated in another therapeutic T cell modality, namely, in the context of chimeric antigen receptor (CAR) T cells,8 providing the basis for clinical trials using engineered oIL-2Rβ Tregs for GVHD prophylaxis. Nevertheless, Treg therapy alone may be insufficient to achieve optimal therapeutic efficacy, and, therefore, in combination with other prophylaxis strategies, it might lead to the best overall clinical outcomes.
Conflict-of-interest disclosure: D.W. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal