Key Points
BIVV001 is a novel fusion protein that provides fourfold longer hemostatic control than rFVIII in preclinical hemophilia A models.
BIVV001 has the potential to allow for more optimal, extended protection against all bleeding types in patients with severe hemophilia A.
Abstract
Factor VIII (FVIII) replacement products enable comprehensive care in hemophilia A. Treatment goals in severe hemophilia A are expanding beyond low annualized bleed rates to include long-term outcomes associated with high sustained FVIII levels. Endogenous von Willebrand factor (VWF) stabilizes and protects FVIII from degradation and clearance, but it also subjects FVIII to a half-life ceiling of ∼15 to 19 hours. Increasing recombinant FVIII (rFVIII) half-life further is ultimately dependent upon uncoupling rFVIII from endogenous VWF. We have developed a new class of FVIII replacement, rFVIIIFc-VWF-XTEN (BIVV001), that is physically decoupled from endogenous VWF and has enhanced pharmacokinetic properties compared with all previous FVIII products. BIVV001 was bioengineered as a unique fusion protein consisting of a VWF-DʹD3 domain fused to rFVIII via immunoglobulin-G1 Fc domains and 2 XTEN polypeptides (Amunix Pharmaceuticals, Inc, Mountain View, CA). Plasma FVIII half-life after BIVV001 administration in mice and monkeys was 25 to 31 hours and 33 to 34 hours, respectively, representing a three- to fourfold increase in FVIII half-life. Our results showed that multifaceted protein engineering, far beyond a few amino acid substitutions, could significantly improve rFVIII pharmacokinetic properties while maintaining hemostatic function. BIVV001 is the first rFVIII with the potential to significantly change the treatment paradigm for severe hemophilia A by providing optimal protection against all bleed types, with less frequent doses. The protein engineering methods described herein can also be applied to other complex proteins.
Introduction
Patients with severe hemophilia A (HemA) have endogenous plasma factor VIII (FVIII) levels of <1% and experience recurrent bleeds that can be treated and prevented by episodic and prophylactic FVIII replacement, respectively.1,2 Extended half-life recombinant FVIII (rFVIII) therapies3,4 (eg, rFVIII Fc fusion protein [rFVIIIFc]), have reduced prophylactic dose frequencies from 3 to 4 times per week to 2 times weekly.5-9 Still, residual treatment burden often persists with existing products,10,11 and further improvements in the pharmacokinetics (PK) of rFVIII remains an unmet need.
Treatment goals in severe HemA are expanding beyond targeting low annualized bleed rates12 and now include outcomes, such as prolonged joint protection and improvements in patient-reported quality of life.12-14 Meeting these goals requires high sustained plasma FVIII levels over long periods.13,15-17 Our goal was to develop an FVIII replacement therapy for patients with severe HemA that would not only permit once-weekly (or longer interval) prophylactic dosing, but would also maintain high FVIII levels.
In plasma, 95% to 98% of FVIII circulates in a tight complex with von Willebrand factor (VWF), which stabilizes FVIII and modulates its plasma half-life by protecting it from degradation.18-20 This interaction sets an upper limit on the circulating half-life of FVIII, ultimately subjecting FVIII to the VWF clearance pathway.21 All approved FVIII (plasma-derived, recombinant, standard, and extended half-life) molecules interact with plasma VWF with similar affinity as native FVIII and are therefore subjected to the VWF-imposed half-life ceiling.22 We hypothesized that this half-life ceiling could be overcome by engineering a stabilized FVIII that does not bind to circulating VWF.
Herein, we present the protein engineering and nonclinical assessment of rFVIIIFc-VWF-XTEN (BIVV001). BIVV001 was designed with novel shielding from rapid plasma clearance and represents a new class of FVIII replacement product that breaks VWF-imposed half-life limitations. The safety, tolerability, and PK of BIVV001 have recently been evaluated in a first-in-human trial (clinicaltrials.gov #NCT03205163).23
Methods
Additional methodology is described in the supplemental Methods (available on the Blood Web site) and includes descriptions of the 1-stage clotting and chromogenic assays, plasmid cloning, expression and purification, size-exclusion chromatography, thrombin digestion studies, PK studies in mice and monkeys, surface plasma resonance and the Octet system, in silico immunogenicity, and XTEN technology (Amunix Pharmaceuticals, Inc, Mountain View, CA).
In-house–generated, B-domain–deleted (BDD) rFVIII and full-length rFVIII (Advate; Takeda Pharmaceuticals USA, Inc, Deerfield, IL) were used in these studies as comparators to BIVV001. rFVIII was purchased and reconstituted according to the manufacturer’s guidelines.
In vitro efficacy studies
Whole-blood clotting assay
The effects of BIVV001 and rFVIII on whole-blood clotting time were assessed using rotational thromboelastometry (ROTEM) and the activation by a trace amount of tissue factor (EXTEM) assay, as previously described.24,25 The EXTEM assay was used because of its relevance to bleeding in humans.26,27 In brief, congenitally FVIII-deficient whole blood from a HemA human blood donor was obtained with written informed consent and spiked with increasing concentrations of BIVV001 and rFVIII, in duplicate, to achieve FVIII levels of 0% to 60% (measured by the 1-stage activity assay [supplemental Methods]). Spiked whole blood was then subjected to EXTEM analysis with a 1:2000 dilution of the EXTEM reagent in Tris/bovine serum albumin buffer as the trigger for coagulation. Similar ROTEM experiments using the nonactivated method and reagent were performed, using BIVV001- and rFVIII-spiked (1%-100%) whole blood from HemA mice.
Thrombin generation assay
The procoagulant activity of BIVV001 and rFVIII was assessed by measuring peak thrombin concentration and endogenous thrombin potential (the net amount of thrombin that test plasma can generate) by using the calibrated automated thrombogram method described by Hemker et al28 and standard assay protocol and reagents from Thrombinoscope, BV (Stago, Maastricht, The Netherlands).29 Congenitally FVIII-deficient plasma was spiked with BIVV001 and rFVIII (1.6%-100% FVIII activities), as described for ROTEM. Final concentrations of reagents were 1 pM tissue factor and 4 µM phospholipids for assay wells and 630 nM thrombin calibrator for calibration wells.
Factor Xa generation assay
The procoagulant properties of BIVV001 and rFVIII as precursors to activated FVIII in the Xase complex were assessed using the activated factor X (FXa) generation assay, as previously described.24,30,31 Human α-thrombin, factor IXa (FIXa), factor X (FX), and FXa were purchased from Haematologic Technologies (Essex Junction, VT). Recombinant hirudin, CHA-XAChrom, and the FXa fluorogenic substrate, Pefachrome 6034, were obtained from Enzyme Research Laboratories (South Bend, IN). Fresh platelets were isolated from human whole blood obtained with consent through an in-house blood donor program and processed the same day. In brief, BDD-rFVIII or rFVIIIFc-VWF-XTEN was first activated with α-thrombin for ∼5 minutes, then the reaction was stopped with hirudin. Reaction product was then mixed in duplicate with FIXa in the presence of Ca2+, and resting or synthetic peptide SFLLRN-activated platelets. Xase complex activity was quantified by calculating Km values for the conversion of FX to FXa using an FXa-specific chromogenic assay at 405 nm. Kinetic parameters were determined for each independent run and then averaged. The 405-nm absorbance data were acquired with the BioTek Synergy 2 multimode microplate reader. The Gen5 application supplied with the plate reader was used for data analysis.30
In vivo efficacy studies
All animal protocols were approved by an independent institutional animal care and use committee. All in vivo efficacy experiments were performed blinded with respect to drug administration and outcome measurements.
Acute blood loss tail-clip model
The acute hemostatic efficacies of BIVV001 and rFVIII were evaluated in an acute blood loss mouse model (supplemental Figure 1), as previously described.32 In brief, the tails of anesthetized male HemA mice (age, 8-12 weeks) were immersed in 37°C saline for 10 minutes to dilate the lateral tail vein. BIVV001 (37.5, 75, and 150 IU/kg; n = 14 for each dose), equivalent doses of rFVIII (n = 14 for each dose), and vehicle (n = 14) were administered via the tail vein. Five minutes later, the distal 5 mm of the tail was clipped, and shed blood was collected into blood collection tubes for 30 minutes. Blood loss was quantified gravimetrically by measuring the increase in blood collection tube weight (assumption: 1 g increased weight = 1 mL blood loss). To account for estimated evaporation during the 30-minute sampling period, 0.1 mL was added to the final weight of calculated blood loss. Normal C57BL/6 mice (n = 15) served as a positive control. Blood loss was compared between groups by using the Kolmogorov-Smirnov test. The dose of rFVIII and BIVV001 required to protect 50% of mice from blood loss (defined as less than or equal to the mean volume of blood lost by C57BL/6 mice) was calculated as previously described.33
Tail vein transection bleeding model
The tail vein transection (TVT) bleeding model (supplemental Figure 1) was performed as previously described,32,34 except that HemA mice (n = 15-20 per dose group) received single-dose BIVV001 (1.2-120 IU/kg, intravenously) or equivalent doses of rFVIII at 96 and 24 hours, respectively, before lateral TVT. Vehicle was administered to a control group of HemA mice at 24 hours before injury. The log-rank (Mantel-Cox) test was used to compare 24-hour survival rates of BIVV001 and rFVIII treatment groups with those of vehicle-treated mice.
Results
Constructs used in the development of BIVV001
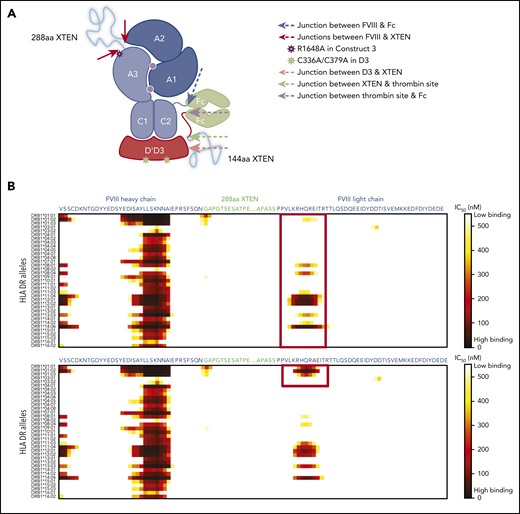
The following 4 constructs were used: (1) rFVIIIFc-VWF(D′D3), (2) rFVIIIFc-VWF-XTEN, (3) rFVIIIFc-VWF-XTEN, and (4) rFVIIIFc-VWF-XTEN (BIVV001). They are described in the supplemental Methods, and their development is illustrated in Figure 1A.
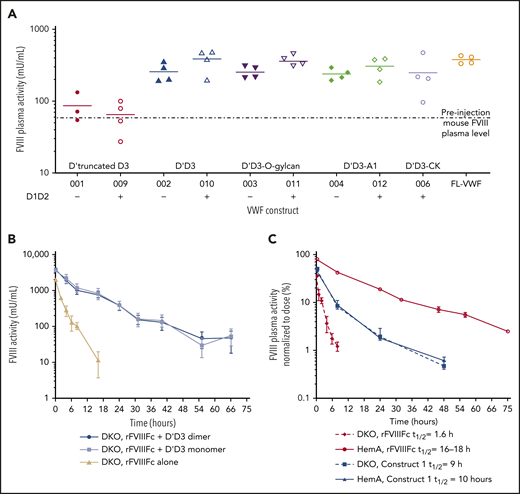
Construct 1: rFVIIIFc-VWF(DʹD3) heterodimer
VWF fragments and constructs were screened in VWF knockout mice to identify a minimal VWF region that stabilizes FVIII and may prevent FVIII binding to endogenous VWF (supplemental Methods; supplemental Figure 2A). Constructs expressing the complete D′D3 domain with the VWF propeptide (D1D2; ie, VWF-010) conferred the highest levels of endogenous FVIII, comparable to full-length VWF (FL-VWF; Figure 2A; supplemental Figure 2B). Constructs expressing a truncated D′D3 (VWF-001) provided limited protection, suggesting that the full D′D3 is needed for FVIII stabilization (Figure 2A). We also evaluated the need for the D1D2 domain, given that this fragment is necessary for appropriate DʹD3 folding in VWF.35 Based on surface plasma resonance experiments, the FVIII binding affinities of DʹD3 constructs with a D1D2 domain in cis (VWF-017; equilibrium dissociation constant [KD] = 2.35E−10) were numerically higher than when the D1D2 domain was in trans (VWF-016+VWF-046; KD = 3.91E−09) or absent (VWF-016; KD = 2.57E−08; supplemental Figure 3A-C). A DʹD3 construct with a C1099A/C1142A double mutation (VWF-014; KD = 1.5E−08) had similar FVIII affinity relative to its wild type (WT) counterpart (VWF-016) without the D1D2 domain (supplemental Figure 3B-C). However, the C1099A/C1142A double mutation with D1D2 in cis (VWF-015; KD = 2.38E−9) affinity was lower than VWF-017 but better than VWF-016. Also, in double-knockout (DKO) mice PK studies, both monomeric (C1099A/C1142A; VWF-013) and WTdimeric DʹD3 (with D1D2 in cis; VWF-010) fragments were comparable in protecting rFVIIIFc (Figure 2B). Collectively, these results provided the rationale for fusing D1D2DʹD3 (1-1240aa) with the C1099A/C1142A mutations to rFVIIIFc to generate construct 1 (Figure 1A). Construct 1 lacked binding to FL-VWF (supplemental Figure 4).
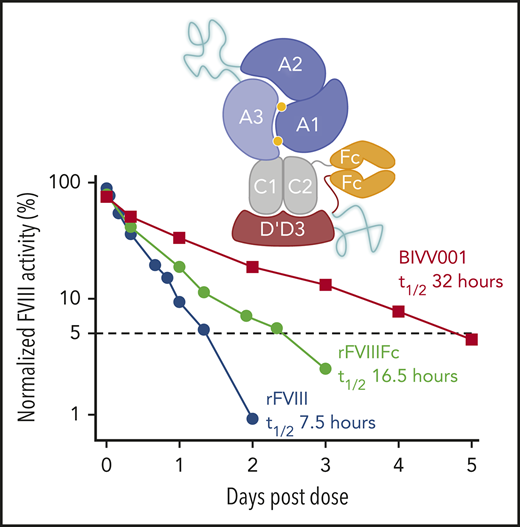
Development of BIVV001. (A) Construct 1: rFVIIIFc-VWF(DʹD3). rFVIIIFc is stabilized by covalently attaching a D1D2DʹD3 (C1099A/C1142A) domain of VWF through immunoglobulin-G1 Fc molecules, which prevents interaction of rFVIIIFc with endogenous full-length VWF. A R1648A mutation in FVIII prevents FVIII processing into heavy and light chains. Construct 2: rFVIIIFc-VWF-XTEN. One 288-amino-acid XTEN polypeptide is inserted into the B-domain region of FVIII, a second 144-amino-acid XTEN polypeptide is inserted between DʹD3 and Fc. Construct 3: rFVIIIFc-VWF-XTEN. The LVPR thrombin site located between DʹD3 and Fc on constructs 1 and 2 was replaced with an FVIII acidic region 2 (a2) thrombin site. Construct 4: rFVIIIFc-VWF-XTEN (BIVV001). Three amino acid residues (GAP) from the FVIII/XTEN junction and 9 amino acids (PPVLKRHQA) from the FVIII B-domain linker were removed to avoid potential MHCII-binding sites. (B) BIVV001 is generated by coexpressing 2 polypeptide chains in HEK293 cells, a human cell line. Purified component parts of BIVV001 were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; BioRad stain-free gel; 4%-20%) under reduced (R) and nonreducing (NR) conditions. See supplemental Methods for more details. FVIII-XTEN-Fc portion is expressed in the first chain (band F8; NR and R lane) and DʹD3-XTEN-Fc portion (band VD; R lane) is expressed in the second chain. The 2 chains are held together by disulfide bonds in the Fc region (heterodimer band [HD]; NR lane). The D1D2 (propeptide) is removed during intracellular processing. Size-exclusion chromatography (SEC) was performed using high-performance liquid chromatography (1260 Bioinert) with a BEH450 column and run isocratically at 0.3 mL/min for 18 minutes. (See supplemental Methods for more details.) The SEC profile of BIVV001 under nonreducing conditions is shown; the predicted molecular mass of BIVV001 is ∼312 kDa, but, because of the presence of XTEN, it has a large hydrodynamic radius and elutes before 670 kDa. MW, molecular weight; PACE, paired basic amino acid cleaving enzyme; Tyr, tyrosine.
Development of BIVV001. (A) Construct 1: rFVIIIFc-VWF(DʹD3). rFVIIIFc is stabilized by covalently attaching a D1D2DʹD3 (C1099A/C1142A) domain of VWF through immunoglobulin-G1 Fc molecules, which prevents interaction of rFVIIIFc with endogenous full-length VWF. A R1648A mutation in FVIII prevents FVIII processing into heavy and light chains. Construct 2: rFVIIIFc-VWF-XTEN. One 288-amino-acid XTEN polypeptide is inserted into the B-domain region of FVIII, a second 144-amino-acid XTEN polypeptide is inserted between DʹD3 and Fc. Construct 3: rFVIIIFc-VWF-XTEN. The LVPR thrombin site located between DʹD3 and Fc on constructs 1 and 2 was replaced with an FVIII acidic region 2 (a2) thrombin site. Construct 4: rFVIIIFc-VWF-XTEN (BIVV001). Three amino acid residues (GAP) from the FVIII/XTEN junction and 9 amino acids (PPVLKRHQA) from the FVIII B-domain linker were removed to avoid potential MHCII-binding sites. (B) BIVV001 is generated by coexpressing 2 polypeptide chains in HEK293 cells, a human cell line. Purified component parts of BIVV001 were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; BioRad stain-free gel; 4%-20%) under reduced (R) and nonreducing (NR) conditions. See supplemental Methods for more details. FVIII-XTEN-Fc portion is expressed in the first chain (band F8; NR and R lane) and DʹD3-XTEN-Fc portion (band VD; R lane) is expressed in the second chain. The 2 chains are held together by disulfide bonds in the Fc region (heterodimer band [HD]; NR lane). The D1D2 (propeptide) is removed during intracellular processing. Size-exclusion chromatography (SEC) was performed using high-performance liquid chromatography (1260 Bioinert) with a BEH450 column and run isocratically at 0.3 mL/min for 18 minutes. (See supplemental Methods for more details.) The SEC profile of BIVV001 under nonreducing conditions is shown; the predicted molecular mass of BIVV001 is ∼312 kDa, but, because of the presence of XTEN, it has a large hydrodynamic radius and elutes before 670 kDa. MW, molecular weight; PACE, paired basic amino acid cleaving enzyme; Tyr, tyrosine.
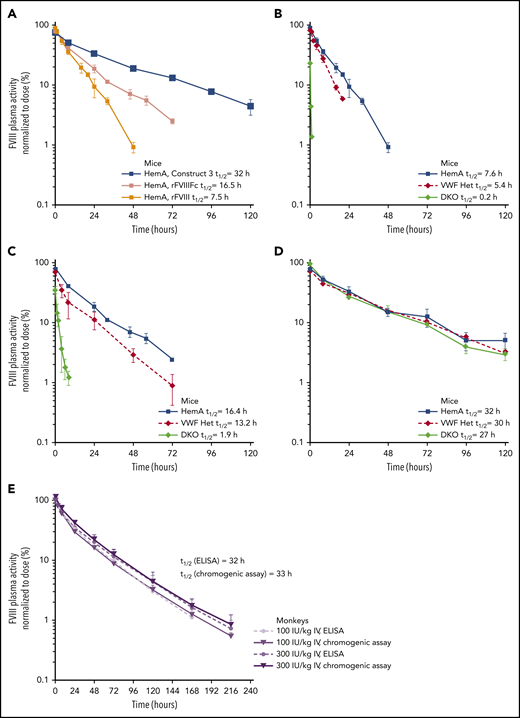
The half-lives of construct 1 and rFVIIIFc were evaluated in HemA and DKO mice (Figure 2C). The half-life of rFVIIIFc, an FVIII molecule stabilized by VWF, was 16 to 18 hours in HemA mice (ie, mice with normal endogenous VWF) and 1.6 hours in DKO mice (ie, no endogenous VWF). The PK profiles and half-lives of construct 1 were identical in HemA and DKO mice (half-life = 9-10 hours). The half-life of construct 1 was longer than that of rFVIIIFc in DKO mice but shorter than rFVIIIFc in HemA mice.
Expression and PK properties of the VWF D′D3 domain. (A) Plasmids expressing VWF fragments were administered to VWF-knockout mice by hydrodynamic injection (HDI),60 to identify the VWF domains necessary for optimal endogenous FVIII stabilization. Plasma FVIII activity was measured in an FVIII chromogenic activity assay before and 48 hours after DNA injection. Individual data points are shown with the horizontal lines depicting the mean. (B) The rFVIIIFc half-life was compared in FVIII/VWF DKO mice expressing VWF-010 and VWF-013 to determine whether the dimeric (VWF-010) or monomeric (VWF-013: D1D2DʹD3C1099A/C1142A) DʹD3 is comparable in protecting FVIII. rFVIIIFc (200 IU/kg) was administered via the tail vein, and the plasma FVIII activity was measured with the chromogenic assay. FVIII activity in mU/mL was plotted against time. Data are means ± SD; n = 4 mice per time point. (C) The PKs of rFVIIIFc-VWF(DʹD3; 200 IU/kg IV) were evaluated in HemA and DKO mice, using a methodology similar to those described in panel B. Plasma FVIII activity was normalized to the dose of factor administered and was plotted against time. Factor half-life (t1/2) was calculated by noncompartmental modeling using WinNonLin, version 5.2, (Pharsight Corp, Mountain View, CA). Data are means ± SD; n = 4 mice per time point.
Expression and PK properties of the VWF D′D3 domain. (A) Plasmids expressing VWF fragments were administered to VWF-knockout mice by hydrodynamic injection (HDI),60 to identify the VWF domains necessary for optimal endogenous FVIII stabilization. Plasma FVIII activity was measured in an FVIII chromogenic activity assay before and 48 hours after DNA injection. Individual data points are shown with the horizontal lines depicting the mean. (B) The rFVIIIFc half-life was compared in FVIII/VWF DKO mice expressing VWF-010 and VWF-013 to determine whether the dimeric (VWF-010) or monomeric (VWF-013: D1D2DʹD3C1099A/C1142A) DʹD3 is comparable in protecting FVIII. rFVIIIFc (200 IU/kg) was administered via the tail vein, and the plasma FVIII activity was measured with the chromogenic assay. FVIII activity in mU/mL was plotted against time. Data are means ± SD; n = 4 mice per time point. (C) The PKs of rFVIIIFc-VWF(DʹD3; 200 IU/kg IV) were evaluated in HemA and DKO mice, using a methodology similar to those described in panel B. Plasma FVIII activity was normalized to the dose of factor administered and was plotted against time. Factor half-life (t1/2) was calculated by noncompartmental modeling using WinNonLin, version 5.2, (Pharsight Corp, Mountain View, CA). Data are means ± SD; n = 4 mice per time point.
Construct 2: XTEN introduction
Approximately 400 different constructs with varying lengths, types, and combinations of XTEN36,37 insertions to construct 1 were evaluated for their impact on FVIII-specific activity and PK properties, to improve the rFVIIIFc-VWF(D′D3) half-life (data not shown). In brief, the combination of a 288-residue XTEN inserted between the A2 and A3 domain of FVIII (ie, replacing most of the natural B-domain region, between residues S743 and Q1638 of human full-length FVIII)38-41 and a 144-residue XTEN as a linker between the DʹD3 and Fc domain provided maximum improvement in PK, with minimal impact on FVIII activity. The resultant construct 2 had an FVIII half-life in HemA mice of 35 hours (supplemental Figure 5), approximately fourfold longer than that of rFVIII.
Construct 3: insertion of thrombin cleavage sites
A thrombin cleavage site was incorporated between Fc and the DʹD3-XTEN linker to permit release of DʹD3-XTEN from rFVIIIFc upon thrombin activation. Thrombin cleavage rates of various thrombin cleavable sequences (LVPR,42 FVIII acidic regions 1, 2, and 3) were evaluated using Bio-Layer interferometry (Octet). The FVIII acidic region 2 (a2) sequence demonstrated the fastest thrombin cleavage rate compared with other sites. Thus, the a2 sequence was incorporated in construct 3, replacing the LVPR sequence, which was present in constructs 1 and 2, resulting in better specific activity (>30% better) than construct 2. Data for thrombin cleavage experiments using construct 4 (hereafter referred to as BIVV001) are presented in supplemental Figure 6B,C.
VWF independence
The PK of Construct 3 was evaluated in HemA, DKO, and VWF Het mice and compared with the PK of 2 other rFVIII molecules (BDD-rFVIII and rFVIIIFc) in the same strains (Figure 3A-D; supplemental Table 1). In HemA mice, construct 3 had a fourfold longer half-life compared with rFVIII and an approximately twofold longer half-life compared with rFVIIIFc (Figure 3A; supplemental Table 1). The half-life of rFVIII in HemA mice was 7.6 hours (Figure 3B; supplemental Table 1), whereas the half-life of rFVIIIFc was 16.4 hours. In VWF Het and DKO mice, rFVIII (Figure 3B; supplemental Table 1) and rFVIIIFc (Figure 3C; supplemental Table 1) half-lives were shorter than in HemA mice. In contrast, the half-life of construct 3 was similar in all 3 mouse strains; thus, confirming that the PK of construct 3 is independent of endogenous VWF levels (Figure 3D; supplemental Table 1).
PK profiles of constructs 3 and 4. PK profiles were evaluated using methods described in Figure 2. (A) The PK of construct 3 was compared with rFVIIIFc and rFVIII in HemA mice. The PK of rFVIII (B), rFVIIIFc (C), and construct 3 (D) were compared in HemA, VWF heterozygous (VWF Het), and FVIII/VWF DKO mice. Doses from 125 to 200 IU/kg were used for IV injection, and plasma FVIII activity was normalized to the dose of factor administered and was plotted against time. Half-life (t1/2) was calculated by noncompartmental modeling using WinNonLin, version 5.2 (Pharsight Corp, Mountain View, CA). Data are means ± SD; n = 3 to 4 animals per group for all mouse studies. (E) The PK of BIVV001 (construct 4; 100 and 300 IU/kg IV) was evaluated in cynomolgus monkeys. FVIII activity was measured with a capture chromogenic assay (Act) or enzyme-linked immunosorbent assay (ELISA) (Ag). For capture chromogenic assay, a biotinylated anti-XTEN monoclonal antibody was used to capture BIVV001 and prevent interference from endogenous FVIII. For ELISA, antihuman FVIII (GMA-8023) was used to capture BIVV001 and prevent interference from endogenous FVIII. Data are means ± SD; n = 4 per group with 4 animals per time point.
PK profiles of constructs 3 and 4. PK profiles were evaluated using methods described in Figure 2. (A) The PK of construct 3 was compared with rFVIIIFc and rFVIII in HemA mice. The PK of rFVIII (B), rFVIIIFc (C), and construct 3 (D) were compared in HemA, VWF heterozygous (VWF Het), and FVIII/VWF DKO mice. Doses from 125 to 200 IU/kg were used for IV injection, and plasma FVIII activity was normalized to the dose of factor administered and was plotted against time. Half-life (t1/2) was calculated by noncompartmental modeling using WinNonLin, version 5.2 (Pharsight Corp, Mountain View, CA). Data are means ± SD; n = 3 to 4 animals per group for all mouse studies. (E) The PK of BIVV001 (construct 4; 100 and 300 IU/kg IV) was evaluated in cynomolgus monkeys. FVIII activity was measured with a capture chromogenic assay (Act) or enzyme-linked immunosorbent assay (ELISA) (Ag). For capture chromogenic assay, a biotinylated anti-XTEN monoclonal antibody was used to capture BIVV001 and prevent interference from endogenous FVIII. For ELISA, antihuman FVIII (GMA-8023) was used to capture BIVV001 and prevent interference from endogenous FVIII. Data are means ± SD; n = 4 per group with 4 animals per time point.
BIVV001
In silico immunogenicity prediction
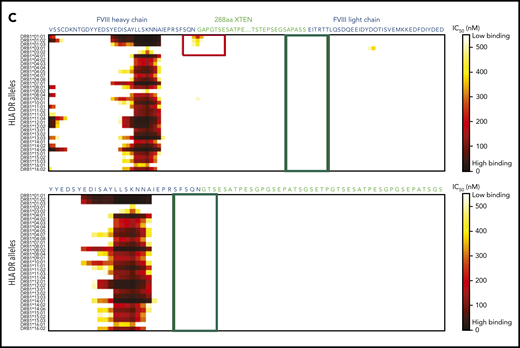
In construct 3, the following 2 regions displayed probability of high-affinity binding to some major histocompatibility complex class II (MHCII) DR alleles: (1) the FVIII-R1648A mutation and (2) the junction between the FVIII heavy chain and XTEN (Figure 4A-C). These sites were modified by deleting GAP residues from the XTEN and PPVLKRHQR1648A from the native FVIII B-domain linker, without introducing additional mutations (Figure 4B-C). Removal of the potential MHCII binding sites (supplemental Table 2) resulted in the final construct, BIVV001 (Figure 1A-B). BIVV001 has XTEN inserted between amino acids N745 and E1649 of FVIII (mature FVIII numbering) and is secreted as a single-chain FVIII43 rather than as a dual-chain molecule because it lacks the R1648 processing site (Figure 1A-B). Similar in silico analysis predicted a lack of MHCII binding to any portion of the XTEN (Figure 4C), consistent with the XTEN design36,37 and predicted that no MHCII binding epitopes were generated by the C1099A/C1142A mutations or the other junctions (supplemental Figure 7).
HLA DR allele binding analysis of novel mutations and junctions in construct 3 to identify potential neoepitopes. (A) Construct 3, rFVIIIFc-VWF-XTEN, contains an R1648A mutation in the FVIII chain, introduced to avoid cleavage at R1648 and prevent intracellular processing into heavy and light chains. In the D3 domain, amino acids Cys1099 and Cys1142 were mutated to Ala to prevent DʹD3 dimerization. (B,C) Amino acid binding predictions to various HLA DR alleles were evaluated with the NetMHCIIpan, version 3.0, method, as described previously.61 Binding predictions are depicted before (B; red box; top) and after (B; red box, bottom) mutating WT-R1648 into R1648A in construct 3. After deleting the 9 amino acid residues from the FVIII B-domain linker that captures R1648A (C; green box, top), and before (C; red box; top; green box, bottom) deleting the GAP residues at the FVIII-XTEN junction. GAP residues were from XhoI restriction enzyme site, originally introduced to allow XTEN insertion into FVIII during cloning. IC50, half-maximal inhibitory concentration.
HLA DR allele binding analysis of novel mutations and junctions in construct 3 to identify potential neoepitopes. (A) Construct 3, rFVIIIFc-VWF-XTEN, contains an R1648A mutation in the FVIII chain, introduced to avoid cleavage at R1648 and prevent intracellular processing into heavy and light chains. In the D3 domain, amino acids Cys1099 and Cys1142 were mutated to Ala to prevent DʹD3 dimerization. (B,C) Amino acid binding predictions to various HLA DR alleles were evaluated with the NetMHCIIpan, version 3.0, method, as described previously.61 Binding predictions are depicted before (B; red box; top) and after (B; red box, bottom) mutating WT-R1648 into R1648A in construct 3. After deleting the 9 amino acid residues from the FVIII B-domain linker that captures R1648A (C; green box, top), and before (C; red box; top; green box, bottom) deleting the GAP residues at the FVIII-XTEN junction. GAP residues were from XhoI restriction enzyme site, originally introduced to allow XTEN insertion into FVIII during cloning. IC50, half-maximal inhibitory concentration.
Binding of BIVV001 to VWF
Surface plasma resonance experiments confirmed that DʹD3 (as a surrogate for FL-VWF) did not bind to BIVV001 after XTEN insertions and removal of 9 B-domain residues (supplemental Figure 8), suggesting that BIVV001 is most likely shielded from binding FL-VWF in vivo. A Fab fragment generated from an anti–FVIII-A1 domain antibody (Fab8004) showed similar binding affinity to those of all tested FVIII variants (supplemental Figure 9), confirming that the lack of binding of free DʹD3 to BIVV001 is not due to generalized shielding of the FVIII component of BIVV001 by the XTEN polypeptides or attached DʹD3.
BIVV001 PK: large-scale manufacturing material
In HemA mice, the half-lives of BIVV001 at 3 intravenous (IV) dose levels (25, 50, and 100 IU/kg) were 31, 30, and 25 hours, respectively, based on chromogenic activity assays (supplemental Figure 10; supplemental Table 3), which represents a three- to fourfold longer half-life than that of rFVIII. In cynomolgus monkeys, BIVV001 half-life was ∼33 hours, based on a BIVV001-specific capture chromogenic activity assay and 32 hours based on the enzyme-linked immunosorbent assay (Figure 3E; supplemental Table 4), which are approximately fourfold longer than reported for rFVIII in the same species.44
BIVV001 efficacy studies
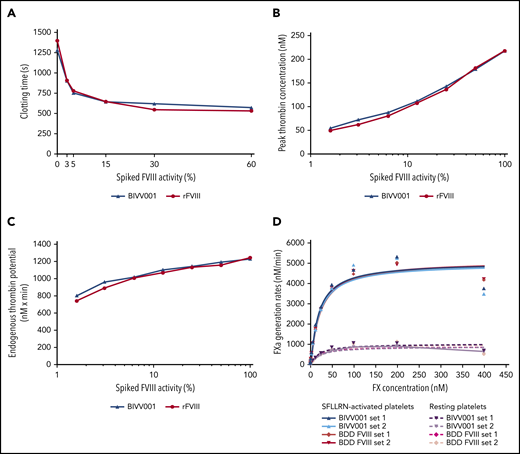
BIVV001 molar specific activity is comparable to other FVIII products when measured by a chromogenic assay (1.75 IU/pmol; standard deviation [SD] ± 0.07), but it is about two- to threefold lower by the 1-stage clotting assay (0.7 IU/pmol; SD ± 0.01).45 An array of in vitro and in vivo efficacy experiments (discussed below) confirmed that the BIVV001 hemostatic efficacy correlated well with the rFVIII efficacy when BIVV001 potency was assigned by an activated partial thromboplastin time–based 1-stage assay. In vitro hemostatic potential was evaluated by using a whole-blood clotting assay (ROTEM), thrombin generation assay, FXa generation assay (ie, Xase complex activity), and thrombin digestion kinetics. BIVV001 and rFVIII at equivalent activity levels (determined by the 1-stage assay) elicited similar dose-dependent shortening of HemA human donor (n = 1) whole-blood clotting time, peak thrombin generation, and endogenous thrombin potential (Figure 5A-C). Comparable ROTEM data were obtained using whole blood from HemA mice (n = 4; supplemental Figure 11). In the Xase generation assay, both BIVV001 and rFVIII elicited similar rates of FXa production (Figure 5D).
In vitro hemostatic potential of BIVV001. (A) The effect of BIVV001 on whole-blood clotting time was assessed by using ROTEM and the extrinsically activated test with tissue factor assay. Whole blood from a blood donor with HemA was spiked with increasing concentrations of BIVV001 or rFVIII to obtain FVIII activities of 0% to 60% (based on a 1-stage activity assay). The procoagulant activity of BIVV001 and rFVIII was assessed by measuring peak thrombin concentration (B) and endogenous thrombin potential (C) using a thrombin generation assay. Congenitally FVIII-deficient plasma was spiked with BIVV001 and rFVIII (FVIII activities of 1.6%-100%). Representative curves are shown for ROTEM and thrombin generation assay. (D) The procoagulant properties of BIVV001 and BDD FVIII as precursors to the Xase complex (FVIIIa-FIXa) were assessed using an FXa generation assay, as previously described.24,30,31 Xase complex Km values were calculated by measuring the conversion of FX to FXa, by using an FXa-specific chromogenic assay. Xase activity was assessed in the presence of Ca2+ and resting (R) or SFLLRN-activated human platelets (in duplicate).
In vitro hemostatic potential of BIVV001. (A) The effect of BIVV001 on whole-blood clotting time was assessed by using ROTEM and the extrinsically activated test with tissue factor assay. Whole blood from a blood donor with HemA was spiked with increasing concentrations of BIVV001 or rFVIII to obtain FVIII activities of 0% to 60% (based on a 1-stage activity assay). The procoagulant activity of BIVV001 and rFVIII was assessed by measuring peak thrombin concentration (B) and endogenous thrombin potential (C) using a thrombin generation assay. Congenitally FVIII-deficient plasma was spiked with BIVV001 and rFVIII (FVIII activities of 1.6%-100%). Representative curves are shown for ROTEM and thrombin generation assay. (D) The procoagulant properties of BIVV001 and BDD FVIII as precursors to the Xase complex (FVIIIa-FIXa) were assessed using an FXa generation assay, as previously described.24,30,31 Xase complex Km values were calculated by measuring the conversion of FX to FXa, by using an FXa-specific chromogenic assay. Xase activity was assessed in the presence of Ca2+ and resting (R) or SFLLRN-activated human platelets (in duplicate).
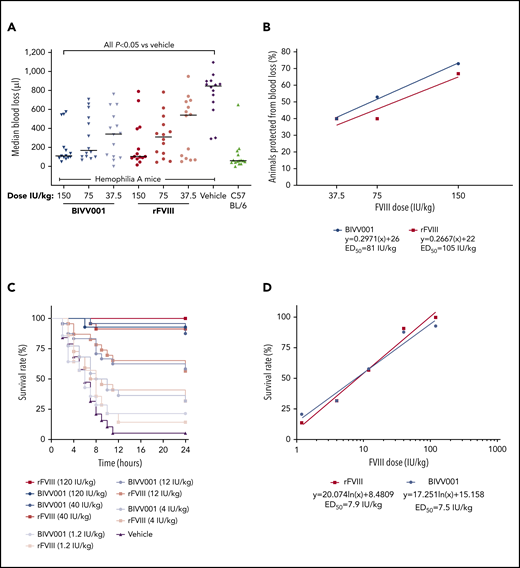
BIVV001 and rFVIII demonstrated similar dose-dependent, acute in vivo hemostatic efficacy in the tail-clip model (Figure 6A-B). Blood loss after tail clip was significantly decreased by all doses of BIVV001 and rFVIII evaluated, compared with that of vehicle-treated HemA mice (P ≤ .05). There were no significant differences in blood loss between BIVV001 and rFVIII (P = .3338). At the 150-IU/kg dose, BIVV001 and rFVIII reduced blood loss by 73% and 67%, respectively, compared with vehicle-treated HemA mice. The 50% effective dose (ED50) value in this model was 81 IU/kg for BIVV001 and 105 IU/kg for rFVIII (Figure 6B).
Efficacy of BIVV001 in HemA mice. (A,B) Acute hemostatic efficacies of BIVV001 (37.5, 75, and 150 IU/kg; all IV and n = 14 for each dose), equivalent doses of rFVIII (n = 14 for each dose), and vehicle (n = 14) were evaluated in HemA mice in an acute-blood-loss, tail-clip model. C57BL/6 mice (n = 15) served as the positive control. (A) Individual median blood loss over 30 minutes was quantified gravimetrically and compared between groups with the Kolmogorov-Smirnov t test. Horizontal bars are medians. (B) The state of being “protected” was granted if blood loss was less than or equal to the mean volume of blood lost by C57BL/6 mice in the tail-clip model. The ED50 was the dose of each treatment that achieved protection from acute bleeding in 50% of treated animals. ED50 values were 81 IU/kg for BIVV001 and 105 IU/kg for rFVIII. (C) The effect of prophylactic single-dose BIVV001 (1.2-120 IU/kg; IV), equivalent doses of rFVIII, and vehicle on 24-hour survival rates of HemA mice (n = 15-20 per dose group) after tail vein transection were assessed. BIVV001 and rFVIII were administered at 96 and 24 hours, respectively, before tail vein transection. (D) Twenty-four-hour survival rates after BIVV001 and rFVIII administration were compared with those obtained with vehicle (log-rank Mantel-Cox test). FVIII led to significantly higher survival rates at 24 hours after TVT (P < .05) compared with vehicle. Direct survival comparison showed no significant difference between BIVV001 and rFVIII, indicating that BIVV001 is comparable to rFVIII; n = 15 to 20 mice per group. Similar survival protection ED50 values for BIVV001 and rFVIII were calculated by the nonlinear fit equation.
Efficacy of BIVV001 in HemA mice. (A,B) Acute hemostatic efficacies of BIVV001 (37.5, 75, and 150 IU/kg; all IV and n = 14 for each dose), equivalent doses of rFVIII (n = 14 for each dose), and vehicle (n = 14) were evaluated in HemA mice in an acute-blood-loss, tail-clip model. C57BL/6 mice (n = 15) served as the positive control. (A) Individual median blood loss over 30 minutes was quantified gravimetrically and compared between groups with the Kolmogorov-Smirnov t test. Horizontal bars are medians. (B) The state of being “protected” was granted if blood loss was less than or equal to the mean volume of blood lost by C57BL/6 mice in the tail-clip model. The ED50 was the dose of each treatment that achieved protection from acute bleeding in 50% of treated animals. ED50 values were 81 IU/kg for BIVV001 and 105 IU/kg for rFVIII. (C) The effect of prophylactic single-dose BIVV001 (1.2-120 IU/kg; IV), equivalent doses of rFVIII, and vehicle on 24-hour survival rates of HemA mice (n = 15-20 per dose group) after tail vein transection were assessed. BIVV001 and rFVIII were administered at 96 and 24 hours, respectively, before tail vein transection. (D) Twenty-four-hour survival rates after BIVV001 and rFVIII administration were compared with those obtained with vehicle (log-rank Mantel-Cox test). FVIII led to significantly higher survival rates at 24 hours after TVT (P < .05) compared with vehicle. Direct survival comparison showed no significant difference between BIVV001 and rFVIII, indicating that BIVV001 is comparable to rFVIII; n = 15 to 20 mice per group. Similar survival protection ED50 values for BIVV001 and rFVIII were calculated by the nonlinear fit equation.
In the TVT model, pretreatment of HemA mice with BIVV001 and rFVIIIFc at 96 and 24 hours, respectively, provided similar dose-dependent protection from the lethal effects of TVT assessed at 24 hours after injury (Figure 6C-D). Survival rates were significantly higher for mice that were pretreated with BIVV001 and rFVIII compared with those pretreated with vehicle (Figure 6C-D; P < .05). There were no differences in survival rates between BIVV001 and rFVIII at the doses evaluated. The ED50 values for BIVV001 (7.5 IU/kg) and rFVIII (7.9 IU/kg) were similar.
Discussion
In the past, researchers have focused on maintaining or improving FVIII-VWF binding (eg, fusing a VWF-targeting nanobody to FVIII46 ) to stabilize FVIII in plasma and improve FVIII half-life.22,47 However, this interaction also constrains FVIII half-life to a maximum of ∼15 to 19 hours and confounds development of FVIII replacement products that address the unmet need of high sustained FVIII activity without burdensome dosing frequencies.13-15,22,48 BIVV001 is designed to circulate in blood independent of endogenous VWF and represents a new class of FVIII replacement product that breaks the VWF-imposed half-life ceiling.
Yee and colleagues49 demonstrated that VWF DʹD3 domain administration is sufficient to stabilize FVIII in VWF-deficient mice, but the same fragment does not prevent FVIII binding to FL-VWF in mice with normal levels of endogenous VWF. We bioengineered a fusion protein with a covalently linked DʹD3 domain of VWF that both stabilized rFVIII in circulation and most likely prevented in vivo association of rFVIII with endogenous VWF. We recombinantly fused rFVIIIFc (an FVIII replacement molecule that utilizes the Fc receptor–mediated recycling pathway24,32,50 to extend FVIII activity) to a VWF DʹD3 fragment. Biolayer interferometry revealed a lack of rFVIIIFc-VWF(D′D3; construct 1) binding to an FL-VWF–coated sensor, confirming shielding from FL-VWF. Our internal data demonstrate that FL-VWF could displace DʹD3 from FVIII because DʹD3 has an ∼20-fold lower affinity for FVIII than does FL-VWF.
Our hypothesis that a DʹD3 domain would prevent FVIII interaction with endogenous VWF was supported by the results of the PK studies of construct 1 and rFVIIIFc in HemA and DKO mice. The construct 1 half-life was longer in DKO mice than that of rFVIIIFc, demonstrating that DʹD3 stabilized FVIII in the absence of VWF. Conversely, construct 1 had a shorter half-life than rFVIIIFc in HemA mice, implying that DʹD3 offers less protection from plasma clearance than FL-VWF. However, the similar half-life observed for construct 1 in both the presence (HemA) and absence (DKO) of endogenous VWF confirmed that rFVIII in construct 1 does not interact with VWF. Thus, the DʹD3 domain retains partial stabilization/protective function of VWF and creates an intramolecular interaction with FVIII that cannot be displaced by excess endogenous VWF. To improve the affinity of the DʹD3 fragment for FVIII, the D1D2 was retained in cis, which promotes DʹD3 disulfide bond formation and domain folding.35 The FVIII affinity of WT-dimeric D′D3 (VWF-017) was higher than monomeric double-mutant D′D3 (VWF-015); however, both forms of D′D3 elicited similar rFVIIIFc half-life improvements in DKO PK experiments. Thus, we incorporated mutations in the rFVIIIFc-DʹD3 fusion protein to avoid dimerization. Given that DʹD3 only partially protects FVIII, whereas FL-VWF fully stabilizes FVIII,20 we introduced the R1680A mutation to prevent dissociation of the FVIII heavy and light chain in the absence of FL-VWF binding. The R1680 residue was later deleted.
After extensive screening, 2 XTEN polypeptides, 1 in place of the FVIII B-domain region (connecting heavy and light chains) and the other as a DʹD3 and Fc linker, were introduced to further enhance fusion protein (construct 2) half-life. Construct 3 PK data in mice confirm that the insertion of XTEN enhanced the PK properties of the fusion protein but did not impact FVIII shielding by DʹD3. Final construct (BIVV001) half-life was ∼2 times the duration of the reported half-life of FL-VWF in humans (∼15-16 hours).51 In monkeys, BIVV001 increased plasma FVIII half-life approximately fourfold, from 8 hours44 to 32 to 33 hours, representing the first FVIII molecule with a half-life >30 hours in this species.
An additional FVIII a2 thrombin site was introduced between D′D3-XTEN and Fc to enable efficient release of D′D3-XTEN from rFVIIIFc after thrombin activation. As B-domain XTEN is flanked by natural FVIII thrombin cleavage sites (a2 on the N terminus and a3 on the C terminus), it is also released from the fusion protein upon thrombin activation. Thus, BIVV001 activation results in a molecule that is similar to activated rFVIIIFc, which has been extensively characterized,24,32 with long-term efficacy and safety demonstrated in clinical trials.52-54 BIVV001 is composed of natural amino acids (including XTEN), and thus, the fragments generated after thrombin activation are assumed to be degraded by regular protein degradation processes. It is expected that DʹD3-XTEN released upon thrombin cleavage would not interfere with endogenous VWF function, because DʹD3 has lower affinity for FVIII than does FL-VWF and would present at much lower concentrations than VWF.
We used in silico immunogenicity prediction tools to prevent potential MHCII neoepitope creation and further refine protein design, which is the first example of such engineering to generate a molecule that has entered clinical trials. Although animal models are often used to determine the antigenic potential of a protein,55 such models are not highly predictive and cannot be incorporated during the initial stages of protein design when multiple constructs need to be screened rapidly. In silico antigenicity assessment methods avoid some of these issues but are not infallible. For the final construct, BIVV001, we modified amino acid sequences that had the potential to bind MHCII receptors. The R1680 site (both WT and mutated R1680A) demonstrated potential to bind MHC; hence, we removed 9 amino acid residues from the B-domain, generating a final construct with only 5 amino acids from FVIII B domain (SFSQN). Neither XTEN nor D3 C1099A/C1142A demonstrated high-affinity MHCII binding capacity. BIVV001 is in development for previously untreated and treated HemA patients, the former of which are at risk of developing neutralizing antidrug antibodies (inhibitors) to FVIII replacement products. BIVV001 is a significantly modified protein with unknown risk of eliciting inhibitor development. However, BIVV001 is composed of endogenous protein domains (DʹD3 and Fc) to which people have been tolerized, an FVIII sequence that is similar to other rFVIII products, XTEN polypeptides that lack secondary structure, and hydrophobic residues to minimize association with MHC receptors. Furthermore, in a phase 1b/2a clinical trial of recombinant human growth factor-XTEN protein, no significant immune responses to XTEN were observed.56 Thus, although the immunogenic potential of BIVV001 has yet to be evaluated in phase 3 trials, we predict it to be similar to other rFVIII replacement products.
As with another single-chain rFVIII molecule (Afystla57,58 ), BIVV001 has a two- to threefold difference in specific activity between 1-stage and chromogenic assays. The lower activity observed using the 1-stage method could be attributed to the 2 thrombin cleavage steps required to fully activate single-chain FVIII43,58 or to subtle differences in thrombin cleavage surrounding the XTEN and/or dissociation of XTEN/DʹD3 upon activation. This in turn is affected by thrombin concentration in the system and thrombin incubation time before Xase complex assembly. Unlike the 1-stage method, activity derived from a chromogenic method is generally independent of activation kinetics. Therefore, similar molar-specific activity by the chromogenic assay demonstrates that BIVV001 is fully functional once it is activated and is comparable to chromogenic specific activity of other FVIII molecules. Various in vitro and in vivo efficacy experiments performed in this study confirmed that BIVV001 potency is best assigned by the 1-stage assay to maintain the expected efficacy of conventional FVIII. In both the tail-clip and TVT models, BIVV001 had an ED50 similar to rFVIII and, most notably, the 4-fold longer half-life of BIVV001 translated into 4-fold longer protection from bleeding than was afforded by rFVIII.
Our results demonstrate how knowledge of biological systems can be used to guide protein engineering of novel, multidomain fusion proteins. Multiple considerations were incorporated, which included dimeric Fc for half-life extension and to position DʹD3 for optimal FVIII binding, and DʹD3 fusion to FVIII to create an intramolecular interaction preventing FVIII association with endogenous VWF and overcoming the VWF-imposed half-life ceiling. Precise placement of the XTEN and DʹD3-XTEN moieties and incorporation of optimal thrombin cleavage sites permitted participation of BIVV001 in the clotting cascade while maintaining natural regulation of FVIII activity.
In summary, we have developed a new class of FVIII replacement product that overcomes the VWF-imposed half-life ceiling. High sustained FVIII activity associated with BIVV001 administration may provide optimal bleed protection with less frequent dosing for patients with HemA. Phase 1/2A safety and tolerability and PK of single-23 and repeat-59 dose BIVV001 have been evaluated in subjects with severe HemA. BIVV001 clinical efficacy and safety will be assessed in future phase 3 trials.
Qualified researchers may request access to data sets that were generated and/or analyzed during the current study, by e-mail to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Francis John Golder (JK Associates, Inc, a member of the Fishawack Group of Companies) for editorial assistance in the development of the manuscript.
This study, including the editorial assistance, was funded by Sanofi.
Authorship
Contribution: E.S.C., T.L., J.K., H.J., and R.P. designed the study; E.S.C., T.L., J.K., S.P.-W., B.Y., Q.L., D.D., N.M., J.L., A.M.H., D.R., and Zhan Liu performed the research; E.S.C., T.L., J.K., B.Y., Q.L., A.I., A.G., C.F., M.T., T.C., R.M., T.M.D., Zhiqian Liu, O.M., L.Z., and V.S. contributed vital new reagents or analytic tools; E.S.C., T.L., J.K., S.P.-W., B.Y., Q.L., J.S., Zhan Liu, A.v.d.F., B.M., H.J., G.F.P., J.S., J.M.S., and R.P. analyzed the data; E.S.C., T.L., J.S., and R.P. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: E.S.C., T.L., S.P.W., Q.L., N.M., J.L., D.D., A.H., A.I., Zhan Liu, A.V.D.F., A.G., J.S., B.M. and R.P. are current employees of Sanofi and shareholders. M.T. and T.D. are current employees of Biogen and stock holders. J.K., C.F., H.J., Zhiqian Liu, G.F.P., R.M., and T.C. are former employees of Biogen. B.Y., D.R., J.M.S., and O.M. are former employees of Bioverativ, a Sanofi company. V.S. is an employee of Amunix Pharmaceuticals.
Correspondence: Ekta Seth Chhabra, Sanofi, 225 Second Ave, Waltham, MA 02451; e-mail: ekta.sethchhabra@sanofi.com.
REFERENCES
Author notes
E.S.C. and T.L. contributed equally to this study.

![Development of BIVV001. (A) Construct 1: rFVIIIFc-VWF(DʹD3). rFVIIIFc is stabilized by covalently attaching a D1D2DʹD3 (C1099A/C1142A) domain of VWF through immunoglobulin-G1 Fc molecules, which prevents interaction of rFVIIIFc with endogenous full-length VWF. A R1648A mutation in FVIII prevents FVIII processing into heavy and light chains. Construct 2: rFVIIIFc-VWF-XTEN. One 288-amino-acid XTEN polypeptide is inserted into the B-domain region of FVIII, a second 144-amino-acid XTEN polypeptide is inserted between DʹD3 and Fc. Construct 3: rFVIIIFc-VWF-XTEN. The LVPR thrombin site located between DʹD3 and Fc on constructs 1 and 2 was replaced with an FVIII acidic region 2 (a2) thrombin site. Construct 4: rFVIIIFc-VWF-XTEN (BIVV001). Three amino acid residues (GAP) from the FVIII/XTEN junction and 9 amino acids (PPVLKRHQA) from the FVIII B-domain linker were removed to avoid potential MHCII-binding sites. (B) BIVV001 is generated by coexpressing 2 polypeptide chains in HEK293 cells, a human cell line. Purified component parts of BIVV001 were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; BioRad stain-free gel; 4%-20%) under reduced (R) and nonreducing (NR) conditions. See supplemental Methods for more details. FVIII-XTEN-Fc portion is expressed in the first chain (band F8; NR and R lane) and DʹD3-XTEN-Fc portion (band VD; R lane) is expressed in the second chain. The 2 chains are held together by disulfide bonds in the Fc region (heterodimer band [HD]; NR lane). The D1D2 (propeptide) is removed during intracellular processing. Size-exclusion chromatography (SEC) was performed using high-performance liquid chromatography (1260 Bioinert) with a BEH450 column and run isocratically at 0.3 mL/min for 18 minutes. (See supplemental Methods for more details.) The SEC profile of BIVV001 under nonreducing conditions is shown; the predicted molecular mass of BIVV001 is ∼312 kDa, but, because of the presence of XTEN, it has a large hydrodynamic radius and elutes before 670 kDa. MW, molecular weight; PACE, paired basic amino acid cleaving enzyme; Tyr, tyrosine.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/135/17/10.1182_blood.2019001292/1/m_bloodbld2019001292f1.png?Expires=1765010120&Signature=X9lvfOXkNu-jlCujgb7D~6nf4LiTCgVSeM1qyT8mZ9gZN9B4fChzm6ljTg8f9fVOTgRB7GEYGztSPcOj6ly3UkMt7qLmR9bsPSzvH3EPvtaBZXuHn9Px04VUdUdnUhRqTKKEip4KtsjzO-5~iBP5z-B527eamLiqo1wsdf0BVZLQ-2VbxNbLghM9Aq2Yz4hJnRopgmdHYgkeFdXoTShFaouatL1Wl1nbjS2h5sdVu1kUmP63QKy0k3bdbgvL6EgW8D9IQNECWqU9g49OiL3hyCgJPgDjOIMhXsapt~uVicvF-xM~c39ec99Ws2l8Q67VNyJ-EmRZ7mQ3GJbB~LoQIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)