Background:
Cigarette smoking induces alterations in DNA methylation in mononuclear cells in peripheral blood which persist over decades, however the impact on leukemia therapy is not well studied. Furthermore, smoking may increase the risk of cardiovascular events during TKI therapy. The aim of this study is to evaluate the impact of cigarette smoking on outcome in pts with newly diagnosed Ph+ALL treated with the combination of intensive therapy with a TKI and to describe a mouse model that will elucidate mechanistic aspects of our observations.
Methods:
From 04/2001 to 04/2018, 202 pts with newly diagnosed Ph+ALL who received the combination of intensive therapy (hyper-CVAD) with imatinib, dasatinib, or ponatinib were analyzed. Smoker was defined as prior history of smoking equal to or more than 1 pack-year (PY) history of smoking before the diagnosis of Ph+ALL. External validation was performed on independent dataset including 72 pts who were treated with the combination of intensive therapy and imatinib.
In order to identify the molecular features underlying cigarette smoking, we utilized a Ph+ ALL pt derived xenograft implanted into NOD-SCID IL2R gamma chain deficient mice. Prior to injection of leukemic cells by tail vein, mice were divided into smoking or nonsmoking groups. To model cigarette smoking, research cigarettes were burnt at a rate of 3 puffs per minute in a chambered smoking machine for 2 hours daily, 5 days per week for two weeks prior to implantation of human Ph+ ALL PDX. To determine if chemicals present in cigarettes (without combustion) are exerting direct effects on Ph+ ALL cells, 3 pts-derived Ph+ ALL cell lines (Z-181, Z-33 and Z-119) were cultured in the presence or absence of cigarette smoke condensate.
Results:
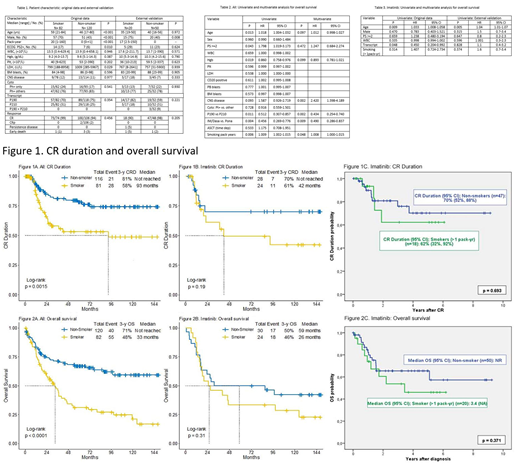
Pt characteristics are summarized in Table1; 54 (27%) were treated with HCVAD + imatinib; 72 (36%) with HCVAD + dasatinib; and 76 (38%) with HCVAD + ponatinib. The median follow-up was 77 months. 82 pts (41%) were identified as smokers (Table 1). The median PY of smoking was 20 (1-160). 5-year CR duration (CRD) was 53% and 78% in the smoker and non-smoker cohort, respectively (p=0.006) (Figure 1A); 5-year OS rates were 31% and 67%, respectively (p<0.001) (Figure 2A). Of the 54 pts who received HCVAD + imatinib, the 5-year CRD were 49% and 70%, respectively (p=0.202) (Figure 1B); the 5-year OS rates were 33% and 50%, respectively (p=0.218) (Figure 2B). Of the 72 pts who received HCVAD + dasatinib, the 5-year CR duration was 50% and 70% in the smoker and non-smoker cohort, respectively (p=0.249) (Figure 1C); the 5-year OS rate was 30% and 59%, respectively (p=0.013). Of the 76 pts who received HCVAD + ponatinib, the 5-year CRD were 63% and 93%, respectively (p=0.018); the 5-year OS rates were 23% and 91%, respectively (p<0.001). Multivariate Cox regression identified the presence of central nervous system disease (p=0.011; HR= 3.141), P190 transcript (p=0.001; HR=0.254), the achievement of 3-month CMR (p=0.014; HR=2.119), and smoking PY (p=0.001; HR=1.014) as prognostic factors for OS. Among 72 pts in the external validation cohort, 20 (28%) were smokers. Despite, the relatively small number of pts with only 20 smokers, there was a tendency of lower CR rates (90% vs 70%, p=0.69) and lower 4-year CRD rates (62% vs 70%, p=0.693) in smokers vs non-smokers (90% versus 98%; P=0.205). The median OS was 3.4 years and not reached (p=0.371), respectively. The tendency of worse outcome is consistent with our results of pts who received HCVAD + imatinib.
These trends were consistent in our mouse and cell line models. Mice became moribund roughly 60 days post Ph+ ALL PDX engraftment and leukemic burden was significantly higher in mice exposed to cigarette smoke (p= 0.003). In the presence of cigarette smoke condensate, high doses (2 uM) of imatinib were less effective in the cell lines compared to unexposed cells (p=0.002), supporting a role for a chemical component of cigarettes in promoting TKI resistance.
Conclusion:
Smoking is an independent poor prognostic factor of outcome in pts treated with chemotherapy and TKI, and can be accurately modeling in PDX bearing mice and cell lines. The best outcome was obtained in non-smokers treated with chemotherapy and ponatinib (5-year OS rate of 91%). The adverse effect of smoking on survival seems more prominent in pts who were treated with dasatinib and ponatinib. Smoking mechanisms, with an emphasis on DNA methylation changes, leading to this poor outcome are being studied.
Sasaki:Pfizer: Consultancy; Otsuka: Honoraria. Ravandi:Cyclacel LTD: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Selvita: Research Funding; Macrogenix: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Xencor: Consultancy, Research Funding. Short:Amgen: Honoraria; Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Kadia:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding. Konopleva:Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Kisoji: Consultancy, Honoraria; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding. Jain:Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. DiNardo:medimmune: Honoraria; agios: Consultancy, Honoraria; daiichi sankyo: Honoraria; jazz: Honoraria; syros: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; abbvie: Consultancy, Honoraria; celgene: Consultancy, Honoraria. Pemmaraju:abbvie: Consultancy, Honoraria, Research Funding; cellectis: Research Funding; celgene: Consultancy, Honoraria; samus: Research Funding; incyte: Consultancy, Research Funding; sagerstrong: Research Funding; affymetrix: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; novartis: Consultancy, Research Funding; plexxikon: Research Funding; Daiichi-Sankyo: Research Funding; mustangbio: Consultancy, Research Funding. Cortes:Novartis: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Merus: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; BiolineRx: Consultancy; Takeda: Consultancy, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria. O'Brien:Aptose Biosciences, Inc: Consultancy; Amgen: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Acerta: Research Funding; Alexion: Consultancy; Verastem: Consultancy; Janssen: Consultancy, Honoraria; Kite: Research Funding; GlaxoSmithKline: Consultancy; Gilead: Consultancy, Research Funding; Eisai: Consultancy; Celgene: Consultancy; Astellas: Consultancy; Vaniam Group LLC: Consultancy; TG Therapeutics: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Regeneron: Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding. Kantarjian:Pfizer: Honoraria, Research Funding; Cyclacel: Research Funding; Ariad: Research Funding; Novartis: Research Funding; Daiichi-Sankyo: Research Funding; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Agios: Honoraria, Research Funding; Immunogen: Research Funding; Amgen: Honoraria, Research Funding; Astex: Research Funding; Jazz Pharma: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria. Jabbour:Amgen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding; BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal