Introduction: Hepatotoxicity is a frequent and challenging adverse event in children with acute lymphoblastic leukemia (ALL), but patient factors that are predictive of hepatotoxicity are not well understood. We leveraged a data repository jointly developed by two pediatric oncology centers within the Leukemia Electronic Abstraction of Records [LEARN] Consortium to assess the landscape and determinants of liver dysfunction throughout ALL therapy in patients who were risk-stratified to receive either standard- or high-intensity treatment blocks.
Methods: The subjects were children ages 1-21 years who were treated for ALL between 2006-2014 at either Children's Hospital of Philadelphia or Texas Children's Hospital. Demographics, disease-related data, and every laboratory value collected during treatment were obtained by targeted manual abstraction and extensive semi-automated extraction of patient electronic medical record (EMR) data. To reduce cohort heterogeneity, we excluded patients who received non-standard ALL therapies. Patients were categorized as receiving either standard-intensity or high-intensity treatment for their first three blocks of therapy (Induction, Consolidation, Interim Maintenance 1 [IM1]) based on chemotherapeutic agents delivered in those blocks. Differences in laboratory value-determined hepatoxicity were then analyzed based on this categorization for all remaining phases of therapy. Hepatic lab values (AST [SGOT], ALT [SGPT], total bilirubin [t. bili], and conjugated bilirubin [c. bili]) were first normalized to the age-based upper limit of normal (ULN), and the median value was then determined. A multivariate mixed-effects linear regression model with random effects was used to identify differences in the treatment group medians and the following covariates: age, race/ethnicity, sex, BMI, and ALL immunophenotype. Laboratory values were classified by the CTCAE v5.0 grading system, with grade ≥ 3 considered 'elevated.'
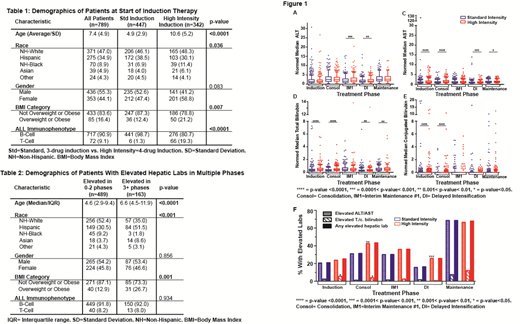
Results: 805 pediatric ALL patients were included in the analysis, representing 114,095 hepatic lab values (Table 1). Less than 10% of patients had elevated lab values at diagnosis. Throughout treatment, the majority of lab values fell within 1-2x ULN for age for both standard- and high-intensity treatment groups (Fig. 1a-d). The median hepatic lab values for the high-intensity group were slightly higher than the standard risk group across all treatment phases, and this difference was most consistently significant in Consolidation and Delayed Intensification. Among the four hepatic labs that were assessed, ALT had the most significant deviation above normal (up to 30x ULN, Fig 1a). Patients were more likely to have elevated transaminases during maintenance than prior to maintenance (Fig. 1e). Similarly, but to a lesser degree, patients were more likely to have elevated t. or c. bili during maintenance than prior to maintenance. Age, race, and BMI were correlated with elevated hepatic labs, with Hispanic and/or overweight patients more likely to have elevations in 3 or more phases of therapy (Table 2). However, no hepatic lab abnormalities were correlated with either overall or relapse-free survival.
Conclusions: This is the first comprehensive study of measures of hepatotoxicity in a large and uniformly-treated cohort of pediatric ALL. While significantly elevated hepatic labs are rare at diagnosis, they are common during ALL treatment and are seen more commonly in maintenance than in prior phases. Patients who are overweight and/or Hispanic are more likely to experience grade 3 or higher hepatoxicity. We observed no relationship between hepatotoxicity and relapse or survival. Further studies are ongoing to delineate the temporal correlation of liver function and chemotherapy dosing and administration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal