Key Points
NGR-hTNF-α targets CD13+ tumor vessels and increases vascular permeability selectively in tumor/peritumoral areas of PCNSL.
R-CHOP preceded by low-dose NGR-hTNF was not associated with unexpected toxicity and resulted in tumor regression in 9 of 12 PCNSL patients.
Abstract
Patients with primary central nervous system lymphoma (PCNSL) are treated with high-dose methotrexate-based chemotherapy, which requires hospitalization and extensive expertise to manage related toxicity. The use of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) could overcome these difficulties, but blood-brain barrier (BBB) penetration of related drugs is poor. Tumor necrosis factor-α coupled with NGR (NGR-hTNF), a peptide targeting CD13+ vessels, induces endothelial permeabilization and improves tumor access of cytostatics. We tested the hypothesis that NGR-hTNF can break the BBB, thereby improving penetration and activity of R-CHOP in patients with relapsed/refractory PCNSL (NCT03536039). Patients received six R-CHOP21 courses, alone at the first course and preceded by NGR-hTNF (0.8 μg/m2) afterward. This trial included 2 phases: an “explorative phase” addressing the effect of NGR-hTNF on drug pharmacokinetic parameters and on vessel permeability, assessed by dynamic contrast-enhanced magnetic resonance imaging and 99mTc-diethylene-triamine-pentacetic acid–single-photon emission computed tomography, and the expression of CD13 on tumor tissue; and an “expansion phase” with overall response rate as the primary end point, in which the 2-stage Simon Minimax design was used. At the first stage, if ≥4 responses were observed among 12 patients, the study accrual would have continued (sample size, 28). Herein, we report results of the explorative phase and the first-stage analysis (n = 12). CD13 was expressed in tumor vessels of all cases. NGR-hTNF selectively increased vascular permeability in tumoral/peritumoral areas, without interfering with drug plasma/cerebrospinal fluid concentrations. The NGR-hTNF/R-CHOP combination was well tolerated: there were only 2 serious adverse events, and grade 4 toxicity was almost exclusively hematological, which were resolved without dose reductions or interruptions. NGR-hTNF/R-CHOP was active, with 9 confirmed responses (75%; 95% confidence interval, 51-99), 8 of which were complete. In conclusion, NGR-hTNF/R-CHOP was safe in these heavily pretreated patients. NGR-hTNF enhanced vascular permeability specifically in tumoral/peritumoral areas, which resulted in fast and sustained responses.
Introduction
Primary central nervous system lymphoma (PCNSL) is an aggressive malignancy with the peculiar clinical behavior of remaining confined to the CNS, with rare cases of extra-CNS dissemination.1 Accordingly, PCNSL is a stage IE disease with a diffuse large B-cell lymphoma (DLBCL) morphology in >95% of cases2 ; this represents a new entity called “primary diffuse large B-cell lymphoma of the CNS” in the 2017 World Health Organization classification of hematopoietic and lymphoid tumors.3 Compared with limited-stage extra-CNS DLBCL, patients with PCNSL have poorer survival figures, which have been attributed, at least in part, to the inefficacy of drugs currently used to treat extra-CNS DLBCL (ie, the R-CHOP regimen [rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone]) to cross the blood-brain barrier (BBB) and to achieve efficient tumor concentrations.4 This pharmacokinetic limitation coupled with the negative results of a randomized trial5 led to the exclusion of the CHOP regimen as part of first-line treatment of patients with PCNSL. Currently, patients with PCNSL are treated with high-dose methotrexate-based combinations, often in association with cytarabine, alkylating agents, and rituximab.6 The diffuse use of these modern combinations has significantly improved survival in patients with PCNSL, but these treatments require hospitalization and adequate direct experience, and are often burdened by relevant toxicity. Conversely, enhanced CNS delivery of R-CHOP could result in important advantages: this well-tolerated therapy, widely used in onco-hematologic centers, does not require hospitalization. Therefore, the use of IV agents capable of inducing a reversible BBB permeabilization and of enhancing tumor penetration of anticancer drugs is an attractive investigational approach in patients with PCNSL.
Using animal models of brain metastasis, it has been shown that IV administration of tumor necrosis factor (TNF), an inflammatory cytokine capable of altering endothelial cell–cell adhesion and barrier function, can induce selective BBB permeabilization and, consequently, can enhance tumor penetration of chemotherapeutic agents.7 Unfortunately, the use of TNF in patients with cancer is limited by prohibitive systemic toxicity, mainly due to vascular leakage syndrome resulting in hemodynamic instability, hypotension, and pulmonary edema.8 A growing body of evidence suggests that the therapeutic index of this cytokine can be enhanced by a vascular targeting approach. This can be achieved, for example, by fusing the N terminus of human TNF with CNGRCG, a tumor vasculature-homing peptide capable of recognizing an isoform of aminopeptidase N (CD13), a membrane-bound metalloproteinase, upregulated in angiogenic tumor blood vessels9,10 and barely or not at all expressed by normal blood vessels.11 The CNGRCG-TNF fusion protein made with human TNF (developed at the San Raffaele Scientific Institute of Milan, Italy, and called NGR-hTNF) allows the delivery of extremely low, yet pharmacologically active, doses of cytokine to the tumor vasculature, thereby avoiding systemic toxic reaction and counterregulatory mechanisms.12 Magnetic resonance imaging (MRI) measurements with blood pool contrast agent performed in lymphoma-bearing mice have shown that low-dose murine NGR-TNF can increase the leakage of the contrast agent from the vasculature to the tumor tissue, suggesting an increase in local vascular permeability.13 Furthermore, studies performed in melanoma and lymphoma animal models have shown that low-dose NGR-hTNF can locally enhance vascular permeability and increase the penetration of chemotherapeutic drugs in tumor tissues.8,10,12,14 NGR-hTNF has been tested in various phase 2 and 3 trials on different types of tumors, alone and in combination with various chemotherapeutic agents, with an excellent safety profile and evidence of activity.8 In a randomized trial of 400 patients with relapsed or refractory mesothelioma enrolled in 12 countries, although the primary end point was not reached, the addition of NGR-hTNF to the best investigator choice was associated with significantly improved survival in the subgroup of patients with refractory or early relapsed mesothelioma without increased toxicity.15
Based on these notions, we hypothesized that low-dose NGR-hTNF can alter the BBB and enhance tumor penetration and activity of R-CHOP in patients with PCNSL. As a part of a translational research program of NGR-hTNF, we designed a prospective phase 2 trial aimed to assess the feasibility and activity of R-CHOP chemoimmunotherapy preceded by BBB permeabilization with NGR-hTNF in patients with relapsed or refractory PCNSL. In a per-protocol planned “proof-of-principle” part of the trial, we investigated changes in the BBB permeability in the lymphomatous lesions and in the normal-appearing brain parenchyma by using dynamic contrast-enhanced MRI (DCE-MRI) and single-photon emission computed tomography (SPECT) in enrolled patients. Changes in the concentrations of R-CHOP drugs in plasma and cerebrospinal fluid (CSF) samples and expression of CD13, the target of NGR-hTNF, in vascular cells of diagnostic biopsy samples were also investigated as indicators of the specificity of the effect of NGR-hTNF on the tumor vasculature. Herein, we report the results of this “proof-of-concept” study as well as the per-protocol first-step analysis as an initial effort toward the development of a simple, manageable, and active treatment of patients with PCNSL, analogous to the treatment used worldwide of systemic DLBCL.
Patients and methods
Study population and selection criteria
The INGRID study is a single-arm, phase 2 trial focused on an experimental treatment consisting of 6 courses of conventional doses of R-CHOP21 preceded by NGR-hTNF infusion in HIV-negative patients with relapsed or refractory PCNSL (EUDRACT, 2014-001532-11; clinicaltrials.gov, NCT03536039). The trial has 2 distinct parts: (1) an exploratory phase focused on the feasibility of NGR-hTNF/R-CHOP and proof-of-principle of the effects of NGR-hTNF on vascular permeability in the first 10 enrolled patients; and (2) an expansion phase focused on activity and tolerability of the experimental treatment in the whole enrolled series. Selection criteria were: (1) histologically proven diagnosis of DLBCL according to the World Health Organization criteria16 ; (2) disease exclusively localized in the CNS, cranial nerves, meninges, and/or eyes both at first diagnosis and trial registration; (3) lymphoma relapsed after or refractory to previous chemotherapy containing high-dose methotrexate; (4) measurable disease; (5) age 18 to 80 years; and (6) Eastern Cooperative Oncology Group performance status score ≤3. Patients were excluded if they had: undergone previous organ transplant or other forms of immunosuppression; hepatitis B virus, hepatitis C virus, and/or HIV infections; or other malignancies. Any kind of consolidation therapy (ie, whole-brain radiotherapy [WBRT], autologous stem cell transplantation [ASCT], oral drug maintenance) during previous lines was admitted. Before trial registration, histopathological diagnostic specimens and neuroimaging examinations performed at diagnosis and relapse were centrally reviewed (M.P. and N.A., respectively), and patients were assessed by using physical and neurological examinations, hemogram and biochemical serum profiles, echocardiography, enhanced total-body computed tomography scan, bone marrow biopsy, contrast-enhanced brain MRI, CSF examination, ophthalmologic evaluation, and 18F-fluorodeoxyglucose positron emission tomography. Risk was defined according to the International Extranodal Lymphoma Study Group risk score.17 Written informed consent was obtained from each patient. This trial conformed to the Declaration of Helsinki and was approved by the institutional review board of the San Raffaele Scientific Institute of Milan, Italy.
Trial design and experimental treatment
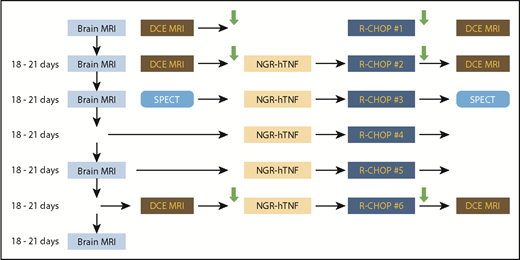
The design of the explorative phase is summarized in Figure 1. The first 10 enrolled patients received an initial course of R-CHOP (schedule given in the supplemental Data, available on the Blood Web site) that was not preceded by NGR-hTNF; this approach was used to explore the response to R-CHOP alone and as a comparator to establish the effect of NGR-hTNF on vascular permeability (as discussed later). The other 5 courses of R-CHOP were preceded by NGR-hTNF (0.8 µg/m2 delivered 2 hours before CHOP by a 1-hour infusion). The rationale for the timing and administration schedule of NGR-hTNF is summarized in the supplemental Data. Patients enrolled in the expansion phase (after the first 10) received the 6 courses of R-CHOP preceded by NGR-hTNF. Oral or IV acetaminophen/paracetamol at a dose of 1.000 mg was delivered as prophylaxis of infusion-related reactions, 30 to 60 minutes before starting each infusion of NGR-hTNF. No concomitant hydration was allowed during the NGR-hTNF infusion. Steroids, other than the 5 days of prednisone, were avoided, and, when clinically indicated, they were interrupted on the day of NGR-hTNF infusion. Therapy with proton pump inhibitors was avoided because these drugs can increase chromogranin levels, which can interfere with NGR-hTNF activity. H2-blockers (ie, ranitidine) were allowed as gastroprotective therapy.
Trial design. Enrolled patients received a first course of R-CHOP that was not preceded by NGR-hTNF, while the other 5 courses were preceded by NGR-hTNF. Each of the lines corresponds to a treatment course. The first column represents gadolinium-enhanced MRI (brain MRI) performed for response assessment; the second column represents cerebral DCE-MRI and SPECT performed before treatment course (day 0) and used as baseline data; the third and fourth columns represent treatment courses; the fifth column represents DCE-MRI and SPECT performed after treatment and used to assess changes in BBB permeability. Arrows represent collection of CSF and plasma samples.
Trial design. Enrolled patients received a first course of R-CHOP that was not preceded by NGR-hTNF, while the other 5 courses were preceded by NGR-hTNF. Each of the lines corresponds to a treatment course. The first column represents gadolinium-enhanced MRI (brain MRI) performed for response assessment; the second column represents cerebral DCE-MRI and SPECT performed before treatment course (day 0) and used as baseline data; the third and fourth columns represent treatment courses; the fifth column represents DCE-MRI and SPECT performed after treatment and used to assess changes in BBB permeability. Arrows represent collection of CSF and plasma samples.
Patients who completed the 6 planned courses and achieved a complete (CR) or partial (PR) response during explorative or expansion phases were evaluated for consolidative therapy. Per protocol, and according to previous treatments, WBRT 30-36 Gy, carmustine/thiotepa-conditioned ASCT, or oral lenalidomide maintenance were allowed.
Toxicity and response assessments
Treatment side effects were assessed separately for each chemotherapy course and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 3.0.18 The worst toxicity per organ, per patient was considered. Periodic specialist controls, electrocardiogram, troponin level determination, and echocardiography were performed before every treatment course to exclude cardiac toxicity. The impact of treatment on cognitive functions was not assessed by ad hoc tests.
All eligible patients were considered for response evaluation. Response was assessed by gadolinium-enhanced MRI of the brain performed on a 1.5-T scanner after the first, second, fourth, and sixth courses of treatment (Figure 1). In cases with concomitant positive CSF and/or vitreous, examination was performed after the second, fourth, and sixth courses. Response was defined according to the International Primary CNS Lymphoma Collaborative Group criteria19 : briefly, CR consisted of disappearance of all evidence of lymphoma; PR was a >50% decrease in tumor size; progressive disease (PD) was a >25% increase in tumor size or detection of any new lesion; and all other situations were considered as stable disease. As an important change in the International Primary CNS Lymphoma Collaborative Group criteria, a “response” was considered only whenever tumor regression was confirmed in 2 consecutive MRIs; accordingly, every response required a minimum duration of 6 to 8 weeks. Response after the first course of R-CHOP did not drive therapeutic decision, whereas patients with PD at any of the following MRIs were considered “off study” and treated according to institutional guidelines. The maximum response recorded from treatment start was considered for analyses. The duration of response was measured from the date of maximum response to the date of objective progression, death from any cause, or last visit of follow-up. After end of treatment, the disease was assessed every 3 months.
BBB permeability assessed by neuroimaging
Variations induced by NGR-hTNF in the BBB permeability at the level of the lymphomatous lesions, areas surrounding the tumor (perilesional area), and in the normal-appearing brain parenchyma were assessed by using DCE-MRI. DCE acquisition followed a standard protocol20 that also included conventional T1, T2, Flair, DWI, and dynamic susceptibility contrast perfusion sequences. As represented in Figure 1, DCE-MRI was performed within the conventional MRI study in day 0 (pretreatment, baseline data) and day 1 (posttreatment) of the first (R-CHOP alone), second (first course of NGR-hTNF/R-CHOP), and sixth (last course of NGR-hTNF/R-CHOP) treatment courses. In cases of multiple lesions, the largest one was considered. Data were suitable for analysis only in patients with responsive disease and measurable residual lesions. Postprocessing of DCE-MRI was performed by using Olea software; all dynamic images were corrected for motion artifacts and coregistered to a volumetric postcontrast T1 sequence. Results were expressed as Ktrans values, which were estimated as a pretreatment/posttreatment ratio and normalized by using contralateral white matter. Ktrans values obtained after the second course (after NGR-hTNF infusion) were compared with those obtained after the first course (without NGR-hTNF) to establish the effect of TNF on vascular permeability. Statistical significance was assessed by using Wilcoxon matched pairs test.
BBB permeability assessed by SPECT
Changes in BBB permeability induced by NGR-hTNF were also assessed by using brain scintigraphy. Because of its hydrophilic property, 99mTc-diethylene-triamine-pentacetic acid (99mTc-DTPA) penetrates only the disrupted BBB and spreads into the altered tissues. The amount of tracer’s uptake at the level of the brain lesions increases proportionally to the degree of vascular permeabilization. Brain scintigraphy was acquired twice (Figure 1), in basal condition, some days before the third course of treatment (median, 4 days; range, 1-6 days), and after the end of the third course; details of this procedure are summarized in the supplemental Data. A volume of interest of 30% of maximum uptake was drawn around the 99mTc-DTPA–positive area(s) by using an automatic isocontour method. Statistical significance of changes in volume of 99mTc-DTPA uptake between SPECTs performed in basal conditions (before NGR-hTNF) and after NGR-hTNF delivery was assessed by using Wilcoxon matched pairs test.
Expression of the target receptor of the CNGRCG peptide (CD13)
The CNGRCG moiety of NGR-hTNF can recognize a CD13 form expressed by tumor vessels, resulting in targeted delivery of TNF to the tumor vessels. The presence of this CD13 form in tumors was assessed by using immunohistochemical and immunofluorescence techniques on paraffin-embedded specimens of diagnostic tissue samples of enrolled patients; pericytes were detected with an anti–α-smooth muscle actin (αSMA) antibody (methodological details are given in the supplemental Data). CD13 expression did not condition patient registration in the trial or experimental treatment.
Drug concentrations in CSF and plasma samples
Changes in plasmatic and CSF concentrations of rituximab, cyclophosphamide, and doxorubicin, as well as in the CSF-to-plasma ratio potentially related to the treatment, were considered as surrogate parameters to establish the specificity of the vascular permeabilization effect of NGR-hTNF. Concentrations of these drugs were assessed on matched CSF and plasma samples collected on day 0 (baseline) and day 1 (1 hour after treatment) of the first (R-CHOP alone), second (first course of NGR-hTNF/R-CHOP), and sixth (last course of NGR-hTNF/R-CHOP) courses of treatment (Figure 1). CSF and plasma concentrations of rituximab were determined by using a validated enzyme-linked immunosorbent assay method (lower limit of quantification, 1.0 ng/mL). CSF and plasma concentrations of cyclophosphamide and doxorubicin were determined by using liquid-chromatography-tandem mass spectrometry methods (lower limit of quantification, 0.01 mg/L and 2.5 ng/mL, respectively).21 The statistical significance of differences between posttreatment drug concentrations achieved after the first course and after the second course was assessed by using Wilcoxon matched pairs test.
Statistical considerations
Feasibility of the NGR-hTNF/R-CHOP combination was the primary end point of the exploratory part of the trial, which required 10 enrolled patients. We considered feasibility as an indicator of toxicity and tolerability and regarded the proportion between delivered and planned treatment courses, treatment delays, interruptions and dose reductions due to toxicity, severe adverse events, and unexpected side effects. Treatment interruptions due to lack of tumor response were not considered to define feasibility. Secondary end points included overall response rate (ORR), changes in BBB permeability, expression of CD13, and changes in R-CHOP drugs’ pharmacokinetic parameters. In cases in which the experimental treatment would be safe and some tumor responses would be recorded, the chairman, after due multidisciplinary discussion, could propose to proceed with an open, noncomparative phase 2 trial, with ORR (CR and PR) as the primary end point. Accordingly, the 2-stage Simon Minimax design was used. The maximum ORR considered of low interest was 30% (rate reported in previous prospective trials focused on salvage treatment in patients with PCNSL performed at our institution22,23 ), and the minimum ORR considered of interest was 50%; to demonstrate that difference, a total of 28 patients was needed (one-sided test; type I error 0.10; power 0.9). At the first step, 12 patients (including the 10 patients of the exploratory phase) would be registered and, if at least 4 responses were observed, the study would have continued up to a total of 28 patients. Herein, we report results of both the explorative phase and the first step of the Simon Minimax model.
Results
Study population
Median age of the 12 assessed patients was 61 years (range, 41-68 years); 8 were male (Table 1). At trial registration, 11 patients had an intermediate to high International Extranodal Lymphoma Study Group risk score, with an Eastern Cooperative Oncology Group performance status score ≥2 in 7 patients, increased lactate dehydrogenase serum levels in 6, high CSF protein concentrations in 6, and involvement of deep areas of the brain in 5. All patients had brain parenchymal lesions, with concomitant intraocular disease in 1 patient; no patient had meningeal disease. Patients were heavily pretreated; 7 received ≥2 previous treatment lines, and 10 received also ASCT, WBRT, or both. Seven patients had refractory disease.
Patient characteristics
| Variables . | Number of patients . |
|---|---|
| Median age, y | 61 (range, 41-68) |
| Male:female | 2:1 |
| ECOG performance status >1 | 7 |
| High lactic dehydrogenase serum level | 6 |
| High CSF protein concentration* | 6 |
| Involvement of deep areas | 5 |
| IELSG risk score | |
| Low | 1 |
| Intermediate | 9 |
| High | 2 |
| Intraocular disease | 1 |
| Meningeal dissemination | 0 |
| Previous lines | |
| Previous lines ≥2 | 7 |
| Previous ASCT | 3 |
| Previous WBRT | 3 |
| Both ASCT + WBRT | 4 |
| Refractory to previous lines | 7 |
| Variables . | Number of patients . |
|---|---|
| Median age, y | 61 (range, 41-68) |
| Male:female | 2:1 |
| ECOG performance status >1 | 7 |
| High lactic dehydrogenase serum level | 6 |
| High CSF protein concentration* | 6 |
| Involvement of deep areas | 5 |
| IELSG risk score | |
| Low | 1 |
| Intermediate | 9 |
| High | 2 |
| Intraocular disease | 1 |
| Meningeal dissemination | 0 |
| Previous lines | |
| Previous lines ≥2 | 7 |
| Previous ASCT | 3 |
| Previous WBRT | 3 |
| Both ASCT + WBRT | 4 |
| Refractory to previous lines | 7 |
ECOG, Eastern Cooperative Oncology Group; IELSG, International Extranodal Lymphoma Study Group.
Lumbar puncture was contraindicated in 3 patients; CSF protein concentration was considered as unfavorable feature in IELSG risk score in these patients.
Feasibility and toxicity
Experimental treatment was well tolerated (Table 2); 62 (86%) of the 72 planned courses were delivered: 9 patients received the 6 planned courses, and treatment was interrupted due to PD in the other 3 patients. There were no cases of unexpected toxicity, and no patient required dose reductions. Treatment delay was recorded only in 3 (5%) courses due to cytopenia. Two severe adverse events were recorded, and both severe adverse events and grade 4 toxicities were resolved without particular complications. There was a single case of reaction to an NGR-hTNF infusion: a 65-year-old man affected by arterial hypertension in treatment from 10 years experienced transient grade 2 arterial hypertension during NGR-hTNF infusion at the first course. His infusion was interrupted by 15 minutes, the patient received symptomatic medication, and, as per protocol, he completed the infusion 1 hour later. The patient received 5 other courses of NGR-hTNF/RCHOP with per-protocol prophylaxis without experiencing infusion reactions. Five patients required blood/platelet transfusions (3 of them had received previous ASCT).
Tolerability and toxicity
| Variable, n % . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Neutropenia | 6 (10) | 6 (10) | 35 (56) | — |
| Thrombocytopenia | 21 (34) | 15 (24) | 11 (18) | — |
| Anemia | 47 (76) | 6 (10) | 1 (2) | — |
| Febrile neutropenia | — | 3 (5) | 1 (2)* | — |
| Hepatotoxicity | 7 (11) | 1 (2) | 1 (2) | — |
| Urinary tract infection | — | 1 (2) | — | — |
| Constipation | 1 (2) | — | — | — |
| Cardiotoxicity† | 1 (2)* | — | — | — |
| NGR-hTNF infusion reaction‡ | 1 (2) | — | — | — |
| Variable, n % . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Neutropenia | 6 (10) | 6 (10) | 35 (56) | — |
| Thrombocytopenia | 21 (34) | 15 (24) | 11 (18) | — |
| Anemia | 47 (76) | 6 (10) | 1 (2) | — |
| Febrile neutropenia | — | 3 (5) | 1 (2)* | — |
| Hepatotoxicity | 7 (11) | 1 (2) | 1 (2) | — |
| Urinary tract infection | — | 1 (2) | — | — |
| Constipation | 1 (2) | — | — | — |
| Cardiotoxicity† | 1 (2)* | — | — | — |
| NGR-hTNF infusion reaction‡ | 1 (2) | — | — | — |
All toxic events other than alopecia are reported. Denominator is the total number of delivered courses (n = 62).
Severe adverse events.
Transient grade 2 left ventricular ejection fraction reduction.
Transient grade 2 arterial hypertension.
Activity
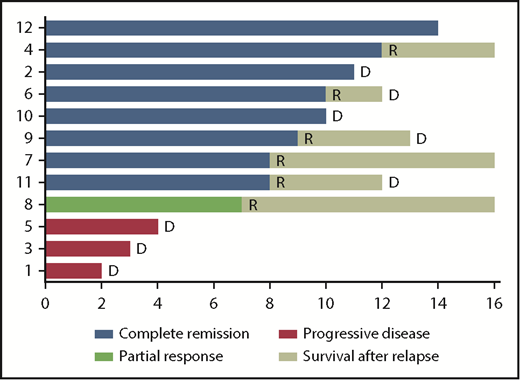
Per protocol, the first 10 enrolled patients received a course of R-CHOP without NGR/hTNF; after this course, 1 patient had PR, 7 had stable disease, and 2 experienced PD (details in supplemental Table 1). The best response to NGR-hTNF/R-CHOP combination was CR in 8 patients (examples in Figure 2) and PR in 1, with an ORR of 75% (95% confidence interval, 51-99); three patients experienced PD (Figure 3). Interestingly, one of the patients who experienced PD after the first course of R-CHOP (patient #8) achieved PR after NGR-hTNF/R-CHOP (after the second course of the experimental treatment), which was confirmed after the fourth and sixth courses. The predetermined activity threshold of the first-step analysis of at least 4 responses in the first 12 registered patients was largely achieved. The best response was achieved after the second course in 6 patients and after the fourth course in 3 patients. All the best responses were confirmed by a second MRI performed 6 weeks later. Consolidation in responding patients was WBRT in 2 patients, ASCT in 3, lenalidomide maintenance in 2, and combinations of these therapies in 2. Response lasted >6 months in all responders (median, 10 months; range, 7-14 months). Six responders experienced relapse at 7 to 12 months; 2 responders (patient #2 and patient #10 in Figure 3) died of complications related to progressive neurological impairment, without evidence of relapsing lymphoma. Four patients are alive at a median follow-up of 19 months (range, 14-28 months).
Examples of responses to R-CHOP preceded by NGR-hTNF. (A) Gadolinium-enhanced T1 weighted scan shows a large homogeneous enhancing lesion in the left frontal lobe (arrows) in a 62-year-old woman at the second relapse after high-dose methotrexate and after salvage high-dose ifosfamide-based therapy; disease was refractory to previous lines. (B) Tumor regression after 2 courses of experimental treatment. (C) Gadolinium-enhanced T1 weighted scan shows a large enhancing left temporal lesion (arrows) in a 65-year-old man at the second relapse after high-dose methotrexate and after salvage whole-brain irradiation. (D) Tumor regression after 2 courses of experimental treatment.
Examples of responses to R-CHOP preceded by NGR-hTNF. (A) Gadolinium-enhanced T1 weighted scan shows a large homogeneous enhancing lesion in the left frontal lobe (arrows) in a 62-year-old woman at the second relapse after high-dose methotrexate and after salvage high-dose ifosfamide-based therapy; disease was refractory to previous lines. (B) Tumor regression after 2 courses of experimental treatment. (C) Gadolinium-enhanced T1 weighted scan shows a large enhancing left temporal lesion (arrows) in a 65-year-old man at the second relapse after high-dose methotrexate and after salvage whole-brain irradiation. (D) Tumor regression after 2 courses of experimental treatment.
Swimmer plot of responses and duration of responses. The best response to NGR-hTNF/R-CHOP was complete in 8 patients (blue) and partial in 1 patient (green); 3 patients experienced progressive disease (red). Response lasted >6 months in all responders. Six responders experienced relapse (R) at 7 to 12 months; 2 responders (patient #2 and patient #10) died (D) of complications related to progressive neurological impairment, without evidence of relapsing lymphoma. Four patients (#4, #7, #8, and #12) are alive at 27, 19, 19, and 13 months from trial registration. Bars were cut at 16 months for clarity.
Swimmer plot of responses and duration of responses. The best response to NGR-hTNF/R-CHOP was complete in 8 patients (blue) and partial in 1 patient (green); 3 patients experienced progressive disease (red). Response lasted >6 months in all responders. Six responders experienced relapse (R) at 7 to 12 months; 2 responders (patient #2 and patient #10) died (D) of complications related to progressive neurological impairment, without evidence of relapsing lymphoma. Four patients (#4, #7, #8, and #12) are alive at 27, 19, 19, and 13 months from trial registration. Bars were cut at 16 months for clarity.
BBB permeability assessed by neuroimaging
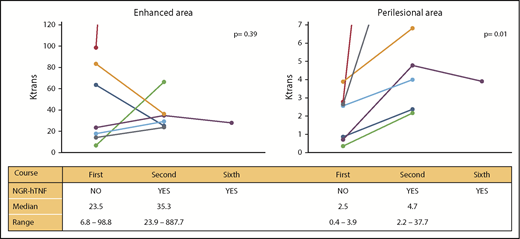
Per protocol, vascular permeability changes were assessed in the 7 patients with responsive and measurable disease (see Patients and methods). The 7 patients were suitable for analysis after the first and second courses, whereas only 1 patient who experienced partial tumor regression as the best response was assessable after the sixth course as the other 6 patients had CR at that time. DCE-MRI analysis showed that vascular permeability was increased after the first NGR-hTNF infusion (Figure 4). This effect was more evident in perilesional areas. The median (range) Ktrans of contrast-enhanced areas after the first course of R-CHOP (without NGR-hTNF) was 23.5 (6.8-98.8) and rose to 35.3 (23.9-887.7; P = .39) after the second course (NGR-hTNF/R-CHOP); increase of Ktrans values after NGR-hTNF/R-CHOP was observed in 5 of the 7 analyzed patients. In perilesional areas, baseline values (R-CHOP alone) were lower (median, 2.5; range, 0.4-3.9) but significantly increased to 4.7 (range, 2.2-37.7; P = .01) after NGR-hTNF infusion in the second course; this increasing effect was confirmed in all 7 assessed patients.
Changes in BBB permeability assessed by DCE-MRI in responders. Changes in the enhanced areas are represented on the left and in the perilesional areas on the right; results are expressed in Ktrans. Values at the first course (without NGR-hTNF) and second and sixth courses (with NGR-hTNF) for each patient are linked with a line. Data were suitable for analysis only in patients with responsive disease and measurable residual lesions. Median and range values per subgroup are reported at the bottom of each graphic.
Changes in BBB permeability assessed by DCE-MRI in responders. Changes in the enhanced areas are represented on the left and in the perilesional areas on the right; results are expressed in Ktrans. Values at the first course (without NGR-hTNF) and second and sixth courses (with NGR-hTNF) for each patient are linked with a line. Data were suitable for analysis only in patients with responsive disease and measurable residual lesions. Median and range values per subgroup are reported at the bottom of each graphic.
In the single patient assessed after the sixth course, Ktrans values were similar to those recorded after the second course, both in enhanced and perilesional areas, which suggests a sustained effect of NGR-hTNF.
BBB permeability assessed by SPECT
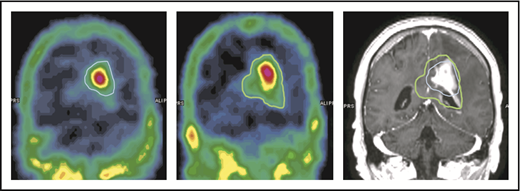
The capability of NGR-hTNF to increase vascular permeability in tumor and perilesional areas was confirmed by results of SPECT studies. Quantitative analysis showed an increase in the extent of the 99mTc-DTPA–positive region(s) in all the investigated cases (an example is given in Figure 5). The median volume of ≥30% 99mTc-DTPA uptake (volume of interest) measured before and after the infusion of NGR-hTNF/R-CHOP was 26 cm3 (range, 5-67 cm3) and 40 cm3 (range, 10-92 cm3), respectively (P = .028). There was a median volume increase of 45%, with a range of 14% to 87%.
An example of increase of 99mTc-DTPA uptake after the infusion of NGR-hTNF followed by R-CHOP at the third course of treatment. The volume of ≥30% 99mTc-DTPA uptake is contoured in 2 SPECT studies performed before (left image, blue line) and after (middle image, green line) administration of NGR-hTNF and R-CHOP. Comparison of contoured volumes are represented in the gadolinium-enhanced T1-weighted MRI showing the tumor (right image). The volume of interest before and after NGR-hTNF/R-CHOP delivery was 22 cm3 and 40 cm3, respectively.
An example of increase of 99mTc-DTPA uptake after the infusion of NGR-hTNF followed by R-CHOP at the third course of treatment. The volume of ≥30% 99mTc-DTPA uptake is contoured in 2 SPECT studies performed before (left image, blue line) and after (middle image, green line) administration of NGR-hTNF and R-CHOP. Comparison of contoured volumes are represented in the gadolinium-enhanced T1-weighted MRI showing the tumor (right image). The volume of interest before and after NGR-hTNF/R-CHOP delivery was 22 cm3 and 40 cm3, respectively.
Expression of the target receptor of the CNGRCG peptide (CD13)
Immunohistochemical staining revealed the presence of CD13 in diagnostic brain biopsy specimens of the 12 registered patients; stained vessels in most instances showed narrowed lumina with irregular outlines (Figure 6A). Immunohistochemical and confocal immunofluorescence analysis of tissue sections stained with an anti-CD13 polyclonal antibody and with anti-αSMA (a marker of pericytes) antibody showed that most stained vessels lacked a pericyte layer (Figure 6B), likely corresponding to immature vessels. Three-dimensional projections of more mature vessels showed that CD13 was expressed on the luminal side of tumor vessels (Figure 6C-D), which is accessible to NGR-hTNF delivered by the IV route.
Expression of CD13 by tumor vasculature. (A) Immunohistochemical analysis of CD13 expression within the lymphomatous component of diagnostic brain biopsy sample of an enrolled patient. Staining was performed by using the anti-CD13 monoclonal antibody SP187 alone (brown signal, 400×). (B) Immunohistochemical analysis of CD13 and αSMA (a marker of pericytes). The costaining was performed with the anti-CD13 monoclonal antibody SP187 (brown) and the anti-αSMA monoclonal antibody 1A4 (red). Black arrows indicate CD13-positive vessels; red arrows indicate αSMA-positive perivascular cells (scale bar, 20 µm; 630×). Left panels: representative photograph of areas with large vessels with pericyte coverage (red) and some microvessels, showing CD13 staining (brown) also in the absence of pericytes. Right: representative photograph of an area with several CD13-positive structures, likely corresponding to microvessels (brown). (C-D) Confocal immunofluorescence analysis of a tissue section stained with a polyclonal anti-CD13 antibody (green) and with the anti-αSMA antibody 1A4 (red) (400×; scale bar, 50 µm). Inset: Three-dimensional projection of CD13 and αSMA staining of a mature vessel (asterisk) (400×; scale bar, 25 µm) showing that CD13 was expressed on the luminal side of the vessels.
Expression of CD13 by tumor vasculature. (A) Immunohistochemical analysis of CD13 expression within the lymphomatous component of diagnostic brain biopsy sample of an enrolled patient. Staining was performed by using the anti-CD13 monoclonal antibody SP187 alone (brown signal, 400×). (B) Immunohistochemical analysis of CD13 and αSMA (a marker of pericytes). The costaining was performed with the anti-CD13 monoclonal antibody SP187 (brown) and the anti-αSMA monoclonal antibody 1A4 (red). Black arrows indicate CD13-positive vessels; red arrows indicate αSMA-positive perivascular cells (scale bar, 20 µm; 630×). Left panels: representative photograph of areas with large vessels with pericyte coverage (red) and some microvessels, showing CD13 staining (brown) also in the absence of pericytes. Right: representative photograph of an area with several CD13-positive structures, likely corresponding to microvessels (brown). (C-D) Confocal immunofluorescence analysis of a tissue section stained with a polyclonal anti-CD13 antibody (green) and with the anti-αSMA antibody 1A4 (red) (400×; scale bar, 50 µm). Inset: Three-dimensional projection of CD13 and αSMA staining of a mature vessel (asterisk) (400×; scale bar, 25 µm) showing that CD13 was expressed on the luminal side of the vessels.
Drugs concentrations in CSF and plasma samples
The effect of NGR-hTNF was specific for the tumor area as suggested by the fact that this drug did not change plasmatic or CSF levels of R-CHOP drugs. Indeed, a significant effect of NGR-hTNF on plasma concentrations of doxorubicin and cyclophosphamide was not observed, as drug concentrations in the baseline samples collected before each course were invariably undetectable (data not shown), and concentrations in samples collected after each course were stable from the first to the sixth course (Table 3). In line with the well-known prolonged terminal half-life of rituximab (up to 80 days),24 a progressive increase in the basal (from 16.6 ± 14.1 ng/mL to 73.6 ± 43.8 ng/mL) and peak (from 45.4 ± 17.0 ng/mL to 110.2 ± 93.4 ng/mL) plasma concentrations of this antibody was found from the first to the sixth course. As expected, doxorubicin and rituximab were not detected in CSF samples, whereas detected CSF levels and the CSF/plasma ratio of cyclophosphamide did not change after NGR-hTNF delivery.
Concentrations of doxorubicin, cyclophosphamide, and rituximab in plasma and CSF samples
| Drug concentrations . | Without NGR-hTNF* . | After NGR-hTNF† . | P . |
|---|---|---|---|
| Plasma | |||
| Doxorubicin, ng/mL | 29.6 ± 7.4 | 26.0 ± 6.7 | .43 |
| Cyclophosphamide, mg/L | 26.3 ± 7.7 | 27.8 ± 7.9 | .17 |
| Rituximab, ng/mL | 45.4 ± 17.0 | 69.1 ± 13.4 | .04 |
| CSF | |||
| Doxorubicin, ng/mL | <2.5 (all samples) | <2.5 (all samples) | |
| Cyclophosphamide, mg/L | 14.1 ± 3.5 | 15.5 ± 4.8 | .27 |
| Rituximab, ng/mL | <1.0 (all samples) | <1.0 (all samples) | |
| CSF/plasma ratio‡ | |||
| Cyclophosphamide, % | 60 ± 20 | 62 ± 19 | .73 |
| Drug concentrations . | Without NGR-hTNF* . | After NGR-hTNF† . | P . |
|---|---|---|---|
| Plasma | |||
| Doxorubicin, ng/mL | 29.6 ± 7.4 | 26.0 ± 6.7 | .43 |
| Cyclophosphamide, mg/L | 26.3 ± 7.7 | 27.8 ± 7.9 | .17 |
| Rituximab, ng/mL | 45.4 ± 17.0 | 69.1 ± 13.4 | .04 |
| CSF | |||
| Doxorubicin, ng/mL | <2.5 (all samples) | <2.5 (all samples) | |
| Cyclophosphamide, mg/L | 14.1 ± 3.5 | 15.5 ± 4.8 | .27 |
| Rituximab, ng/mL | <1.0 (all samples) | <1.0 (all samples) | |
| CSF/plasma ratio‡ | |||
| Cyclophosphamide, % | 60 ± 20 | 62 ± 19 | .73 |
Samples collected after the first course of treatment (ie, after R-CHOP without NGR-hTNF).
Samples collected after the second course of treatment (ie, after NGR-hTNF followed by R-CHOP).
The ratio was not estimated for doxorubicin and rituximab because CSF concentrations resulted below the lower limit of quantification.
Discussion
To the best of our knowledge, this study is the first prospective trial focused on the feasibility and activity of R-CHOP in patients with relapsed or refractory PCNSL. Importantly, this trial develops an innovative strategy for increasing the BBB permeability and drug penetration in tumor and perilesional areas. This strategy exploited the use of NGR-hTNF, a TNF-α derivative capable of targeting tumor blood vessels and increasing endothelial permeability. Our results indicate that the NGR-hTNF/R-CHOP combination is feasible and well tolerated, even in heavily pretreated patients. Results of DCE-MRI and SPECT studies confirmed that NGR-hTNF selectively enhances the vascular permeability in the tumor and peritumoral areas. The tumor specificity of NGR-hTNF effects is also supported by the lack of changes in concentrations of R-CHOP drugs in plasma and CSF samples, which bona fide excludes nonspecific effects of this cytokine on CSF filtration in choroidal plexus and drug pharmacokinetics. Importantly, neuroimaging and histopathological data were consistent with activity of this experimental strategy. In fact, 9 of 12 assessed patients achieved fast and prominent tumor regression after NGR-hTNF/R-CHOP treatment, which was complete in 8 and allowed the use of consolidation therapies in all responsive patients. Efficacy of NGR-hTNF/R-CHOP on tumor cells growing in compartments such as the eye or leptomeninges remains to be defined because none of the 12 treated patients had meningeal disease at trial registration, whereas only 1 patient had subretinal disease; interestingly, the latter patient did not experience ocular relapse after 27 months from trial registration.
This trial exhibits a few limitations. In particular, sample size of this part of the trial (n = 12) seems small. However, this study group is appropriate to demonstrate as proof-of-principle the effects of NGR-hTNF on tumor BBB. This proof of concept is substantiated by use of well-standardized, modern diagnostic techniques and the consistency of radiological, pharmacological, histological, and clinical data, which enable reliable inferences to be made about BBB changes induced by NGR-hTNF. Although the current article is mostly focused on the biological implications of this innovative approach, the achievement of the predetermined activity threshold of the first step of the Simon Minimax design (at least 4 responses in the first 12 registered patients) suggests a promising activity of this strategy and prompts us to complete the planned accrual (n = 28).
Previous studies that focused on NGR-hTNF and its synergistic effects with chemotherapeutic agents both in animal models and patients with solid tumors have shown that the selectivity of NGR-hTNF for tumor vessels requires interaction with specific receptors.12,14,25 In particular, NGR-hTNF, at low doses, may potentially engage high avidity interactions with CD13, TNF-R1, and TNF-R2 on endothelial cells that express these receptors (ie, angiogenic endothelial cells)26 but not with endothelial cells lacking CD13, as in normal tissues. Importantly, findings of the present trial are in line with these facts: the effects of NGR-hTNF were more evident in tumor and peritumoral areas where expression of CD13 by the tumor vessels was confirmed by immunohistochemistry and immunofluorescence techniques.
The NGR-hTNF/R-CHOP combination was safe: unexpected toxicities were not recorded and, importantly, dose intensity was maintained in all cases. Hematological toxicity was well controlled with granulocyte colony-stimulating factor and antibiotics, and only a few patients previously treated with ASCT needed blood/platelet transfusions. These figures are in line with the good tolerability of NGR-hTNF reported in previous clinical trials, both when used alone27,28 or in combination with chemotherapeutic agents.29 When doses up to 60 μg/m2 have been used, chills and fever were the most frequently observed toxicities. In the current trial, a single case of transient infusion reaction (ie, grade 2 arterial hypertension) was recorded. Importantly, in line with previous trials,29 the combination of NGR-hTNF with doxorubicin was not associated with severe cardiovascular events.
Published experience with R-CHOP in patients with PCNSL is anecdotal, mostly due to the diffuse belief that CNS bioavailability of related drugs is poor. A few, available pre-rituximab clinical studies including >20 patients support this notion, which is in line with undetectable concentrations of assessed drugs in CSF samples collected after the first R-CHOP course (without NGR-hTNF) in the present series (Table 3). When used as upfront treatment, CHOP chemotherapy was associated with a low response rate and did not contribute to improved disease control in combination with high-dose, methotrexate-based chemotherapy or with WBRT, with a 2-year overall survival after CHOP-WBRT of only 20% to 40%.5,30-32 Studies focused on CHOP ± rituximab in patients with relapsed or refractory PCNSL do not exist; however, disappointing results reported as first-line treatment suggest that CHOP ± rituximab should be inactive as salvage therapy. In line with these reports, response achieved after R-CHOP alone in the first 10 enrolled patients was insignificant; most patients had stable or progressive disease, which excludes bona fide that responses reached later on by the same patients were exclusively due to R-CHOP activity. Conversely, fast and consistent tumor regression recorded in 9 of the 12 assessed patients suggests that the addition of NGR-hTNF results in improved activity of R-CHOP combination. However, these results should be taken into account with caution because PCNSL exhibits important molecular and biological differences with respect to systemic DLBCL, and some of them are related per se to a poorer efficacy of R-CHOP. In particular, most cases of PCNSL have an activated B-cell–like phenotype,2 a subtype of DLBCL less sensitive to R-CHOP, and exhibit frequent genetic alterations of NF-κB and components of the Toll-like receptor signaling pathway (MYD88, CARD11, and CD79B), which is a component of the proximal B-cell receptor signaling pathway.33,34 These genetic alterations activate the related pathways, increase NF-κB activity, and, importantly, are more commonly associated with the less sensitive activated B-cell–like phenotype. Conversely, the genetic signature of PCNSL closely resembles that of primary testicular lymphoma,35 an entity that usually exhibits excellent outcome when treated with R-CHOP and suitable CNS and testicular prophylaxes.36 Accordingly, and as planned by the protocol, the encouraging activity and excellent safety profile of NGR-hTNF/RCHOP, recorded in a group of heavily pretreated patients, deserve to be addressed in a larger number of patients, and completion of the estimated accrual of the INGRID trial is warranted.
In conclusion, low-dose NGR-hTNF exerted relevant effects on vascular permeability in patients with relapsed or refractory PCNSL. These effects were specific in tumor and peritumoral areas, and were consistently shown by standardized DCE-MRI, SPECT, and plasma/CSF pharmacokinetics studies. NGR-hTNF/R-CHOP combination was well tolerated and followed by fast and prominent tumor regression in 9 of the 12 assessed patients. Accrual completion of this trial is warranted, and, in the case of positive results, this innovative approach deserves to be addressed in first-line treatment prospective trials.
Preliminary results of this trial have been presented at the 2018 annual meeting of the American Society of Clinical Oncology, Chicago, IL, 1-5 June 2018; and presented at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors appreciate the excellent technical assistance and sustained scientific collaboration of Angelo Diffidenti and Maria Colia (Research Nurses of the Lymphoma Unit, San Raffaele Scientific Institute, Milan, Italy), Stefano Orezzi (Neuroradiology Unit, San Raffaele Scientific Institute), Daniela De Lorenzo (Datamanger and Study Coordinator Office of the Lymphoma Unit, San Raffaele Scientific Institute), Anna Chiara (Radiotherapy and Tomotherapy Unit, San Raffaele Scientific Institute), and Elisabetta Miserocchi and Giulio Modorati (Ophthalmology Unit, San Raffaele Scientific Institute). They also acknowledge the hematologists and oncologists of the Department of Onco-Hematology, San Raffaele Scientific Institute, for their excellent clinical assistance. The authors are indebted to the enrolled patients and their families of the INGRID trial for their generous commitment.
The INGRID trial was performed without commercial funding. It was supported by a grant from the Leukemia and Lymphoma Society (A.J.M.F., grant 6510-17) and, in part, by a grant from the Associazione Italiana Ricerca control il Cancro (A.C., grant IG-19220). NGR-hTNF was kindly provided by Molmed SpA (Milan, Italy).
Authorship
Contribution: A.J.M.F. was responsible for study conception, design and supervision; A.J.M.F., G.C., L.P., A.C., D.C., F. Ciceri, and C.B. developed the methodology; E.S. was responsible for administrative support and data management; A.N. was responsible for statistics; T.C., M.F., S.G., M.S., S.P., and C.C. were responsible for treatment of patients; G.M.C. and N.A. performed neuroimaging assessment and analysis; F.F. performed SPECT assessment and analysis; D.C. performed pharmacology assessments and analysis; M.P., A.C., and F. Curnis were responsible for histopathology, immunohistochemistry, and immunofluorescence; T.C. and P.L. performed acquisition of clinical data; A.J.M.F., T.C., A.C., N.A., and A.N. were responsible for analysis and interpretation of data; A.J.M.F., A.C., N.A., and M.P. wrote the manuscript; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: C.B. reports employment and equity ownership from MolMed SpA, and A.C. reports consultancy to MolMed SpA, during the conduct of the study. A.C. and F. Curnis are inventors of a patent on NGR-hTNF. The remaining authors declare no competing financial interests.
Correspondence: Andrés J. M. Ferreri, Lymphoma Unit, Department of Onco-hematology, IRCCS San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: ferreri.andres@hsr.it.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal