Key Points
Midostaurin plus intensive chemotherapy can be safely administered in older FLT3-ITD-positive patients with AML.
Compared with historical controls, midostaurin significantly improved event-free survival in older and younger FLT3-ITD positive patients with AML.
Abstract
Patients with acute myeloid leukemia (AML) and a FLT3 internal tandem duplication (ITD) have poor outcomes to current treatment. A phase 2 hypothesis-generating trial was conducted to determine whether the addition of the multitargeted kinase inhibitor midostaurin to intensive chemotherapy followed by allogeneic hematopoietic cell transplantation (alloHCT) and single-agent maintenance therapy of 12 months is feasible and favorably influences event-free survival (EFS) compared with historical controls. Patients 18 to 70 years of age with newly diagnosed AML and centrally confirmed FLT3-ITD were eligible: 284 patients were treated, including 198 younger (18-60 years) and 86 older (61-70 years) patients. Complete remission (CR) rate, including CR with incomplete hematological recovery (CRi) after induction therapy, was 76.4% (younger, 75.8%; older, 77.9%). The majority of patients in CR/CRi proceeded to alloHCT (72.4%). Maintenance therapy was started in 97 patients (34%): 75 after alloHCT and 22 after consolidation with high-dose cytarabine (HiDAC). Median time receiving maintenance therapy was 9 months after alloHCT and 10.5 months after HiDAC; premature termination was mainly a result of nonrelapse causes (gastrointestinal toxicity and infections). EFS and overall survival at 2 years were 39% (95% confidence interval [CI], 33%-47%) and 34% (95% CI, 24%-47%) and 53% (95% CI, 46%-61%) and 46% (95% CI, 35%-59%) in younger and older patients, respectively. EFS was evaluated in comparison with 415 historical controls treated within 5 prospective trials. Propensity score-weighted analysis revealed a significant improvement of EFS by midostaurin (hazard ratio [HR], 0.58; 95% CI, 0.48-0.70; P < .001) overall and in older patients (HR, 0.42; 95% CI, 0.29-0.61). The study was registered at www.clinicaltrials.gov as #NCT01477606.
Introduction
Internal tandem duplications (ITDs) of the FMS-like tyrosine kinase 3 gene (FLT3) occur in ∼20% to 30% of adult patients with acute myeloid leukemia (AML), with a decreasing prevalence in older patients.1,2 In younger adults, FLT3-ITDs are among the most frequently found mutations in AML.3,4 These ITDs result in amino acid sequence changes with intact coding frames.5 This leads to constitutive activation of the receptor tyrosine kinase and its downstream signaling pathways, resulting in dysregulation of cellular proliferation.5 ITDs are located in exons 14 and 15 of the FLT3 gene and show a broad variation in the position of their insertion sites, as well as in number and sizes of the duplicated fragments.6 FLT3-ITDs confer an unfavorable prognosis because of lower complete remission (CR) and high relapse rates translating into inferior overall survival (OS).6-9 In particular, high (>0.5) mutant-to-wild-type (WT) allelic ratios and insertion sites in the tyrosine kinase domain-1 (TKD1) of the gene confer an unfavorable prognosis.6-9 Whether a concomitant nucleophosmin-1 (NPM1) mutation adds to prognostication in AML with FLT3-ITD is still a matter of debate.7,9,10 The role of allogeneic hematopoietic cell transplantation (alloHCT) in first CR to overcome the negative effect of FLT3-ITD, especially in the context of low allelic ratios on survival, remains controversial.7,9
According to the results of the double-blinded randomized CALGB 10603/RATIFY study, midostaurin, a multitargeted kinase inhibitor, was approved in combination with intensive chemotherapy and as maintenance therapy by the European Medicines Agency for adult patients with AML exhibiting an activating FLT3 mutation. Midostaurin was approved by the US Food and Drug Administration as well.11 Although the patient population in the RATIFY study11 comprised only younger adults (18-59 years), the midostaurin approvals have no upper age limit.
The aim of this prospective, multiinstitutional, phase 2 trial (German-Austrian AML Study Group [AMLSG] 16-10) was to determine the effect of midostaurin plus intensive chemotherapy followed by alloHCT and single-agent midostaurin maintenance therapy in adult patients with FLT3-ITD-positive AML, and to compare the primary end point event-free survival (EFS) to historical controls. Beyond the results from the RATIFY trial,11 this study provides important data with regard to the feasibility and efficacy of midostaurin in older patients (60-70 years) and the use of the inhibitor as maintenance after alloHCT.
Patients and methods
Patients
Fast molecular screening including FLT3-ITD was performed in patients with newly diagnosed AML within the AMLSG BiO study.1 Between June 2012 and May 2016, 431 adult patients aged 18 to 70 years with FLT3-ITD-positive AML were eligible for the AMLSG 16-10 study, and 292 (68%) were enrolled. Patients enrolled had lower white blood cell (WBC) counts (odds ratio [OR] for 10-fold increment, 0.44; 95% confidence interval [CI], 0.30-0.65), were more frequently female (OR, 1.57; 95% CI, 1.03-2.40), and were younger (OR, 0.80 for a 10-year difference in age; 95% CI, 0.66-0.96; supplemental Table 1, available on the Blood Web site). Diagnoses included de novo AML, secondary AML with a preceding history of myelodysplastic syndrome or myeloproliferative neoplasms, and therapy-related AML.12 Patients with acute promyelocytic leukemia and core-binding factor AML, as well as patients with concomitant renal (creatinine >1.5× upper normal serum level), liver (aspartate aminotransferase or ALP >2.5× upper normal serum level), or cardiac dysfunction (New York Heart Association III/IV); uncontrolled infection; primary coagulation disturbance; or performance status (Eastern Cooperative Oncology Group) >2 were excluded. The protocol was approved by the lead Ethics Review Committee and registered at clinicaltrialsregister.eu (EudraCT Number: 2011-003168-63) and clinicaltrials.gov (ClinicalTrials.gov identifier: NCT01477606). Written informed consent was obtained from all patients.
The historical controls comprise all AML cases with FLT3-ITD from 5 previous AMLSG trials recruiting between 1993 and 2008 at the same centers also involved in the AMLSG 16-10 trial.13-17 Treatment in all patients consisted of induction therapy with idarubicin, cytarabine, etoposide, and up to 4 cycles of high-dose cytarabine-based consolidation therapy. AlloHCT in first CR was performed on investigators discretion.
Genetic analyses
Screening for FLT3 mutations was performed centrally in the 2 AMLSG reference laboratories at Ulm University Hospital and Hannover Medical School, using the standardized clinical trial assay from the RATIFY study.11 An FLT3 ITD allelic ratio ≥0.05, as determined by DNA fragment analysis, was considered positive. Leukemia samples were also analyzed for mutations in FLT3 TKD at codons D835/I836 and NPM1, as previously described.13,18 Chromosome banding analysis was performed centrally; karyotypes were designated according to the International System for Human Cytogenetic Nomenclature.19
Study design
Induction therapy
All patients received 1 induction cycle according to the 7+3 scheme.11 Midostaurin was administered orally 50 mg twice daily, starting on day 8, until 48 hours before the start of the subsequent chemotherapy cycle. Patients achieving partial remission could receive an optional second cycle of induction therapy identical to the first cycle.
Consolidation therapy
Patients who achieved a complete remission (CR) or CR with incomplete hematological recovery (CRi) received consolidation therapy. AlloHCT from a matched related or unrelated donor was intended for all patients (1 consolidation cycle before alloHCT was optional). In case alloHCT was not possible, patients received up to 4 cycles of high-dose cytarabine (HiDAC). Cytarabine was administered in a dose of 3 g/m2 (patients >65 years: 1 g/m2) twice daily on days 1, 3, and 5. Midostaurin was administered orally in a dose of 50 mg twice daily, starting on day 6, until 48 hours before start of conditioning therapy for alloHCT or 48 hours before start of subsequent consolidation chemotherapy.
Maintenance therapy
Midostaurin maintenance was intended in all patients either after alloHCT or after HiDAC. Midostaurin was given orally in a dose of 50 mg twice daily for 365 days. After consolidation, therapy with HiDAC midostaurin was continued after the last applied cycle. After alloHCT, midostaurin was started at the earliest 30 days and at the latest 100 days after transplantation.
Throughout the study, regular biobanking was performed for future pharmacokinetic and pharmacodynamic studies.
Definition of response criteria, survival end points, and hematologic recovery
In accordance with standard criteria, CR was defined as less than 5% bone marrow blasts, an absolute neutrophil count of ≥1.0 × 109/L, a platelet count of ≥100 × 109/L, no blasts in the peripheral blood, and no extramedullary leukemia; CRi was defined as CR except for residual neutropenia (neutrophils, <1.0 × 109/L) or thrombocytopenia (platelets, <100 × 109/L).20 Relapse was defined as more than 5% bone marrow blasts unrelated to recovery from the preceding course of chemotherapy or new extramedullary leukemia in patients with previously documented CR.
EFS, OS, cumulative incidence of relapse (CIR), and cumulative incidence of death were defined according to the ELN-2010 recommendations (for the definition, see supplemental Appendix 1).20
Times to WBC, neutrophil, and platelet recovery were measured from the first day of chemotherapy of each cycle until the first day with values ≥1, ≥0.5, and ≥20 × 109/L for WBC, neutrophils, and platelets, respectively. Toxicities were defined and graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0.
Sample size planning and statistical analysis
The study was planned as single-group phase 2 study in comparison with historical controls of FLT3-ITD-positive patients with AML from previous trials.7,13-17 Initial sample size calculation with n = 142 (first cohort) was based on an estimated improvement of EFS after 2 years from 25% to 37.5%, using a 2-sided logrank test at a significance level of 5% and a power of 90%. After accrual of the target patient number of n = 142 (first cohort), distribution according to patients’ age showed that about two-thirds of patients were younger (18-60 years), and one-third older (>60 years). To better define the effect of midostaurin in older patients and the relative value of alloHCT in first CR, the study was amended with a doubling of sample size, including a second cohort of 142 patients and an adaptation of objectives and statistical testing with a global as well as subgroup assessments in younger and older patients based on a Bonferroni-based chain procedure. In addition, based on pharmacokinetic modeling of drug-drug interactions showing an increase of midostaurin plasma concentrations by more than 10-fold (90% CI, 7.4-14.5) in case of comedication with the potent CYP3A4 inhibitor ketoconazole,21 the amendment also implemented a dose reduction of midostaurin to 25 mg every other day in case of comedication with strong CYP3A4 inhibitors.
After recruitment of cohort 2, an additional amendment with a sample size increase to 440 patients and inclusion of OS as key secondary end point was performed, and dose reduction of midostaurin in case of comedication with strong CYP3A4 inhibitors was omitted.
The focus of this interim analysis of the first 2 cohorts of the study was to evaluate results in younger and older patients with respect to feasibility and safety, including a comparison based on historical controls, using propensity scores. We applied the inverse probability of treatment weighting method22 and used the stabilized and trimmed version of the weights23 and a robust variance estimator for the resulting Cox proportional hazards model to account for the sample weights. Propensity scores were calculated using a logistic regression model in which the cohort status was regressed on age, sex, NPM1 mutational status, WBC counts, and percentage of bone marrow blasts, as these variables have been reported as important prognostic factors.7,9,11 To account for missing data in bone marrow blasts, missing value imputation using chained equations was applied.24
Pairwise comparisons between patient subgroups were performed by the Mann-Whitney or Kruskal-Wallis test for continuous variables, and by Fisher’s exact test for categorical variables. Univariable and multivariable logistic regression models were applied to investigate the influence of covariates on response to induction therapy. The primary end point of the study was EFS; secondary end points were OS, relapse-free survival, therapy-related toxicity, and their correlation with the study drug. The median duration of follow-up was calculated by the reverse Kaplan-Meier estimate25 ; the Kaplan-Meier method was used to estimate the distributions of EFS and OS.26 Estimation of CIR and cumulative incidence of death and tests of equality across groups were performed according to Gray.27 Estimates of survival probabilities with respect to maintenance therapy after alloHCT were computed using the landmark method.28
All statistical analyses were performed with the statistical software environment R, version 3.2.1, using the R packages rms, version 4.3-1, and cmprsk, version 2.2-2.29
Results
Patients and baseline characteristics
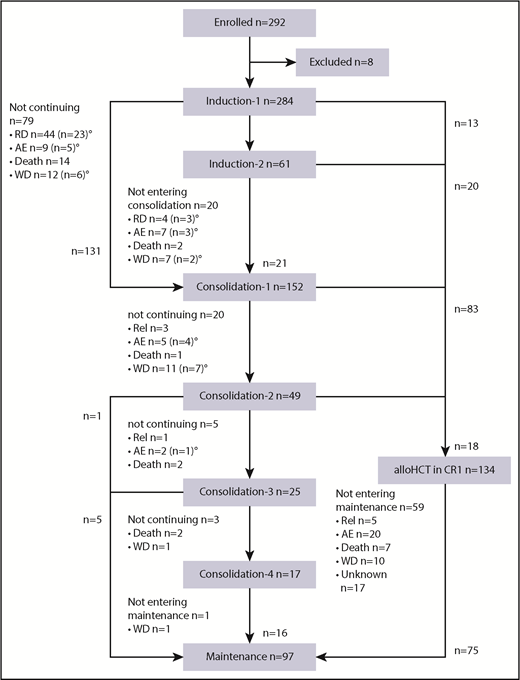
Of 292 patients enrolled, 8 patients (3%) were excluded from the analysis: 6 because of violation of inclusion/exclusion criteria and 2 because of early (within 5 days) withdrawal of informed consent (Figure 1). Of 284 patients receiving induction therapy, 279 patients (98%) received at least 1 dose of midostaurin; 5 patients did not receive midostaurin because of central nervous system hemorrhage (n = 2), sepsis (n = 1), respiratory failure (n = 1), and myocardial infarction (n = 1). Table 1 shows patient demographics and presents laboratory and genetic characteristics of all 284 patients according to age subgroups (younger, 18-60 years; older, 61-70 years), as well as of 415 historical controls. Compared with historical controls, patients in the AMLSG 16-10 study were older and presented more frequently with secondary AML.
CONSORT diagram. RD, refractory disease; AE, adverse event; WD, patient’s withdrawal; Rel, relapse. °Patients proceeding to alloHCT outside the protocol in first CR (n = 39) or with RD (n = 15).
CONSORT diagram. RD, refractory disease; AE, adverse event; WD, patient’s withdrawal; Rel, relapse. °Patients proceeding to alloHCT outside the protocol in first CR (n = 39) or with RD (n = 15).
Patient and disease characteristics of the AMLSG 16-10 study and the historical control according to age
| . | AMLSG 16-10 . | Historical controls . | ||||
|---|---|---|---|---|---|---|
| . | All patients (n = 284) . | Younger, 18 to 60 yr (n = 198) . | Older, 61 to 70 yr (n = 86) . | All patients (n = 415)* . | Younger, 18 to 60 yr (n = 353) . | Older, 61 to 70 yr (n = 62) . |
| Age, years | ||||||
| Median (range) | 54.1 (18-70) | 50.3 (18-60) | 65.2 (61-70) | 50.5 (18-70) | 47.2 (18-60) | 66.4 (61-70) |
| ECOG performance status | ||||||
| 0, n (%) | 106 (38.7) | 77 (40.5) | 29 (34.5) | 92 (22.2) | 82 (23.2) | 10 (16.1) |
| >0, n (%) | 168 (61.3) | 113 (59.5) | 55 (65.5) | 323 (77.8) | 271 (76.8) | 52 (83.9) |
| Missing | 10 | 8 | 2 | 0 | 0 | 0 |
| Male sex, No. (%) | 120 (42) | 77 (39) | 43 (50) | 193 (46.5) | 157 (44.5) | 36 (58.1) |
| WBC, 109/L | ||||||
| Median (range) | 43.3 (1.0-879) | 45.4 (1.1-879) | 40.4 (1.0-854) | 44.8 (0.2-439) | 41.4 (0.2-427) | 66.7 (1.2-439) |
| Missing | 6 | 3 | 3 | 3 | 2 | 1 |
| Hemoglobin, g/dL | ||||||
| Median (range) | 8.9 (4.1-18.1) | 8.9 (4.1-18.1) | 8.9 (5.4-15.0) | 9.0 (3.1-14.6) | 9.0 (3.1-14.6) | 9.4 (7.0-13.9) |
| Missing | 6 | 3 | 3 | 3 | 2 | 1 |
| Platelets, 109/L | ||||||
| Median (range) | 58.5 (5-333) | 59 (8-328) | 58 (5-333) | 58 (6-734) | 58 (8-734) | 65 (6-358) |
| Missing | 6 | 3 | 3 | 2 | 2 | 0 |
| Bone marrow blasts, %† | ||||||
| Median (range) | 85 (0-100) | 85 (0-100) | 85 (7-100) | 85 (2-100) | 85 (2-100) | 89 (10-100) |
| Missing | 36 | 21 | 15 | 25 | 20 | 5 |
| Peripheral blood blasts, %† | ||||||
| Median (range) | 52 (0-99) | 53 (0-99) | 48 (0-98) | 62 (0-100) | 61 (1-100) | 73 (0-100) |
| Missing | 24 | 14 | 10 | 33 | 30 | 3 |
| 2010 ELN risk classification, n (%)20 | ||||||
| Favorable | 2 (1) | 2 (2.6) | 6 (1.4) | 5 (1.4) | 1 (1.6) | |
| Intermediate-1 | 187 (71.7) | 141 (77) | 46 (59) | 315 (75.9) | 271 (76.8) | 44 (71) |
| Intermediate-2 | 57 (21.8) | 35 (19.1) | 22 (28.2) | 72 (17.4) | 56 (15.9) | 16 (25.8) |
| Adverse | 15 (5.3) | 7 (3.8) | 8 (10.3) | 22 (5.3) | 21 (6.0) | 1 (1.6) |
| AML type | ||||||
| De novo AML, n (%) | 245 (88) | 178 (92) | 67 (79) | 396 (95.5) | 339 (96) | 57 (93) |
| s-AML, n (%) | 21 (8) | 8 (4) | 13 (15) | 6 (1.5) | 4 (1) | 2 (3) |
| t-AML, n (%) | 13 (5) | 8 (4) | 5 (6) | 12 (3) | 10 (3) | 2 (3) |
| Missing | 5 | 4 | 1 | 1 | 0 | 1 |
| Mutated NPM1 | ||||||
| n (%) | 165 (58) | 119 (60) | 46 (53) | 230 (56.5) | 197 (57.1) | 33 (53.2) |
| Missing | 0 | 0 | 0 | 8 | 8 | 0 |
| FLT3-ITD allelic ratio | ||||||
| <0.5 | 134 (47) | 91 (46) | 43 (50) | 131 (43.0) | 125 (43.4) | 6 (35.3) |
| ≥0.5 | 149 (53) | 106 (54) | 43 (50) | 174 (57.0) | 163 (56.6) | 11 (64.7) |
| Missing | 1 | 1 | 0 | 110 | 65 | 45 |
| FLT3 TKD mutation | ||||||
| n (%) | 10 (4) | 8 (4) | 2 (2) | 16 (4.1) | 15 (4.5) | 1 (1.5) |
| Missing | 0 | 0 | 0 | 2 | 1 | 1 |
| . | AMLSG 16-10 . | Historical controls . | ||||
|---|---|---|---|---|---|---|
| . | All patients (n = 284) . | Younger, 18 to 60 yr (n = 198) . | Older, 61 to 70 yr (n = 86) . | All patients (n = 415)* . | Younger, 18 to 60 yr (n = 353) . | Older, 61 to 70 yr (n = 62) . |
| Age, years | ||||||
| Median (range) | 54.1 (18-70) | 50.3 (18-60) | 65.2 (61-70) | 50.5 (18-70) | 47.2 (18-60) | 66.4 (61-70) |
| ECOG performance status | ||||||
| 0, n (%) | 106 (38.7) | 77 (40.5) | 29 (34.5) | 92 (22.2) | 82 (23.2) | 10 (16.1) |
| >0, n (%) | 168 (61.3) | 113 (59.5) | 55 (65.5) | 323 (77.8) | 271 (76.8) | 52 (83.9) |
| Missing | 10 | 8 | 2 | 0 | 0 | 0 |
| Male sex, No. (%) | 120 (42) | 77 (39) | 43 (50) | 193 (46.5) | 157 (44.5) | 36 (58.1) |
| WBC, 109/L | ||||||
| Median (range) | 43.3 (1.0-879) | 45.4 (1.1-879) | 40.4 (1.0-854) | 44.8 (0.2-439) | 41.4 (0.2-427) | 66.7 (1.2-439) |
| Missing | 6 | 3 | 3 | 3 | 2 | 1 |
| Hemoglobin, g/dL | ||||||
| Median (range) | 8.9 (4.1-18.1) | 8.9 (4.1-18.1) | 8.9 (5.4-15.0) | 9.0 (3.1-14.6) | 9.0 (3.1-14.6) | 9.4 (7.0-13.9) |
| Missing | 6 | 3 | 3 | 3 | 2 | 1 |
| Platelets, 109/L | ||||||
| Median (range) | 58.5 (5-333) | 59 (8-328) | 58 (5-333) | 58 (6-734) | 58 (8-734) | 65 (6-358) |
| Missing | 6 | 3 | 3 | 2 | 2 | 0 |
| Bone marrow blasts, %† | ||||||
| Median (range) | 85 (0-100) | 85 (0-100) | 85 (7-100) | 85 (2-100) | 85 (2-100) | 89 (10-100) |
| Missing | 36 | 21 | 15 | 25 | 20 | 5 |
| Peripheral blood blasts, %† | ||||||
| Median (range) | 52 (0-99) | 53 (0-99) | 48 (0-98) | 62 (0-100) | 61 (1-100) | 73 (0-100) |
| Missing | 24 | 14 | 10 | 33 | 30 | 3 |
| 2010 ELN risk classification, n (%)20 | ||||||
| Favorable | 2 (1) | 2 (2.6) | 6 (1.4) | 5 (1.4) | 1 (1.6) | |
| Intermediate-1 | 187 (71.7) | 141 (77) | 46 (59) | 315 (75.9) | 271 (76.8) | 44 (71) |
| Intermediate-2 | 57 (21.8) | 35 (19.1) | 22 (28.2) | 72 (17.4) | 56 (15.9) | 16 (25.8) |
| Adverse | 15 (5.3) | 7 (3.8) | 8 (10.3) | 22 (5.3) | 21 (6.0) | 1 (1.6) |
| AML type | ||||||
| De novo AML, n (%) | 245 (88) | 178 (92) | 67 (79) | 396 (95.5) | 339 (96) | 57 (93) |
| s-AML, n (%) | 21 (8) | 8 (4) | 13 (15) | 6 (1.5) | 4 (1) | 2 (3) |
| t-AML, n (%) | 13 (5) | 8 (4) | 5 (6) | 12 (3) | 10 (3) | 2 (3) |
| Missing | 5 | 4 | 1 | 1 | 0 | 1 |
| Mutated NPM1 | ||||||
| n (%) | 165 (58) | 119 (60) | 46 (53) | 230 (56.5) | 197 (57.1) | 33 (53.2) |
| Missing | 0 | 0 | 0 | 8 | 8 | 0 |
| FLT3-ITD allelic ratio | ||||||
| <0.5 | 134 (47) | 91 (46) | 43 (50) | 131 (43.0) | 125 (43.4) | 6 (35.3) |
| ≥0.5 | 149 (53) | 106 (54) | 43 (50) | 174 (57.0) | 163 (56.6) | 11 (64.7) |
| Missing | 1 | 1 | 0 | 110 | 65 | 45 |
| FLT3 TKD mutation | ||||||
| n (%) | 10 (4) | 8 (4) | 2 (2) | 16 (4.1) | 15 (4.5) | 1 (1.5) |
| Missing | 0 | 0 | 0 | 2 | 1 | 1 |
ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet; ITD, internal tandem duplication; NPM1, nucleophosmin-1; s-AML, AML after previous myelodysplastic syndrome or myeloproliferative neoplasm; t-AML, therapy-related AML.
Clinical trials distribution; AMLHD9316 (n = 29), AMLHD98-A17 (n = 121); AMLHD98B13 (n = 23), AMLSG 06-0414 (n = 39), and AMLSG 07-0415 (n = 203).
In case of bone marrow blasts <20%, diagnosis of AML was established on the basis of extramedullary disease or peripheral blood blasts >20%
Induction therapy
After first induction cycle, 172 (60.6%) of 284 patients achieved a CR/CRi and 54 patients a partial remission, 44 patients had refractory disease, and 14 patients died. Of 61 patients who received a second induction cycle (response to first induction cycle: 51 in partial remission, 10 in CR), 55 patients achieved a CR/CRi, 4 had RD, and 2 died. Overall, 217 (76.4%) patients achieved a CR/CRi, 51 (18%) had refractory disease, and 16 (5.6%) died during induction therapy (supplemental Table 2). CR/CRi rates were comparable in younger and older patients (P = .76), high and low FLT3-ITD allelic ratios (P = .48), and cohort 1 and cohort 2 (P = .40), but were favorably affected by the presence of mutant NPM1 (P < .001). The rate of deaths during induction therapy was significantly higher in older (10.5%) compared with younger (3.5%) patients (P = .03). The rate of death during induction therapy in older patients dropped from 15.7% in cohort 1 to 2.4% in cohort 2, whereas in younger patients, no relevant difference was noted (2.1% and 5.3%, respectively).
The rates of observed adverse events grade 3 and higher were in the expected range of intensive induction chemotherapy, as previously reported,11 except for fever in neutropenia, which may be a result of underreporting (Table 2). Cardiac adverse events were significantly, and pulmonary and upper respiratory tract adverse events were in trend, more common in older compared with younger patients (Table 2). Dose modification during first induction therapy, including dose reduction and intermittent or permanent dose suspension, was documented in 55% of the patients (n = 157). Dose reduction resulting from toxicity was reported in 49% (cohort 1, 52%, n = 74; cohort 2, 45%, n = 64) and resulting from comedication in 6.5% (cohort 2, 13%, n = 19).
Toxicities grade 3 or higher according to MedDRA category coding plus fever in neutropenia during first induction therapy
| . | All patients (n = 284) . | Younger, 18-60 yr (n = 198) . | Older, 61-70 yr (n = 86) . | P . |
|---|---|---|---|---|
| Infection, n (%) | 168 (59) | 118 (60) | 50 (58) | .90 |
| Febrile neutropenia, n (%) | 100 (35) | 69 (35) | 31 (36) | .89 |
| Gastrointestinal, n (%) | 70 (25) | 45 (23) | 25 (29) | .46 |
| Metabolic/Laboratory, n (%) | 61 (21) | 44 (22) | 17 (20) | .75 |
| Cardiac total, n (%) | 37 (13) | 18 (6) | 19 (22) | .004 |
| Cardiac general, n (%) | 21 (7) | 11 (4) | 10 (12) | .09 |
| Cardiac arrhythmia, n (%) | 16 (6) | 7 (2) | 9 (10) | .03 |
| Pulmonary/upper respiratory, n (%) | 25 (9) | 13 (7) | 12 (14) | .07 |
| Pain, n (%) | 23 (8) | 17 (9) | 6 (7) | .81 |
| Constitutional, n (%) | 18 (6) | 12 (6) | 6 (7) | .79 |
| Renal/Genitourinary, n (%) | 17 (6) | 13 (7) | 4 (5) | .79 |
| Hemorrhage/bleeding, n (%) | 17 (6) | 9 (5) | 8 (9) | .17 |
| Hepatobiliary/pancreas, n (%) | 8 (3) | 5 (3) | 3 (3) | .71 |
| Neurologic, n (%) | 9 (3) | 6 (3) | 3 (3) | .99 |
| Rash, n (%) | 5 (2) | 4 (2) | 1 (1) | .99 |
| Other, n (%) | 18 (6) | 10 (5) | 8 (9) | .19 |
| . | All patients (n = 284) . | Younger, 18-60 yr (n = 198) . | Older, 61-70 yr (n = 86) . | P . |
|---|---|---|---|---|
| Infection, n (%) | 168 (59) | 118 (60) | 50 (58) | .90 |
| Febrile neutropenia, n (%) | 100 (35) | 69 (35) | 31 (36) | .89 |
| Gastrointestinal, n (%) | 70 (25) | 45 (23) | 25 (29) | .46 |
| Metabolic/Laboratory, n (%) | 61 (21) | 44 (22) | 17 (20) | .75 |
| Cardiac total, n (%) | 37 (13) | 18 (6) | 19 (22) | .004 |
| Cardiac general, n (%) | 21 (7) | 11 (4) | 10 (12) | .09 |
| Cardiac arrhythmia, n (%) | 16 (6) | 7 (2) | 9 (10) | .03 |
| Pulmonary/upper respiratory, n (%) | 25 (9) | 13 (7) | 12 (14) | .07 |
| Pain, n (%) | 23 (8) | 17 (9) | 6 (7) | .81 |
| Constitutional, n (%) | 18 (6) | 12 (6) | 6 (7) | .79 |
| Renal/Genitourinary, n (%) | 17 (6) | 13 (7) | 4 (5) | .79 |
| Hemorrhage/bleeding, n (%) | 17 (6) | 9 (5) | 8 (9) | .17 |
| Hepatobiliary/pancreas, n (%) | 8 (3) | 5 (3) | 3 (3) | .71 |
| Neurologic, n (%) | 9 (3) | 6 (3) | 3 (3) | .99 |
| Rash, n (%) | 5 (2) | 4 (2) | 1 (1) | .99 |
| Other, n (%) | 18 (6) | 10 (5) | 8 (9) | .19 |
MedRA, Medical Dictionary for Regulatory Activities.
Consolidation therapy
According to protocol, alloHCT was intended in all patients achieving CR/CRi and was actually performed in 134 patients (Figure 1); alloHCT was performed after first induction cycle (n = 13), after 2 induction cycles (n = 20), and after 1 (n = 83) or 2 (n = 18) consolidation cycles. In addition, 23, 16, 15, and 19 patients received alloHCT in first CR/CRi after induction therapy outside the protocol, with refractory disease after induction therapy but first CR/CRi after salvage therapy, with refractory disease after induction therapy and after relapse, respectively. Overall, 207 (72.9%) of 284 patients proceeded to alloHCT (age 18-60 years, n = 155 [78%]; age 61-70 years, n = 52 [60%]). The distribution of donor type was matched related donor in 55 patients (27%; age 18-60 years, n = 44 [28%]; age 61-70 years, n = 11 [21%]) and matched unrelated donor in 152 patients (73%; age 18-60 years, n = 111 [72%]; age 61-70 years, n = 41 [79%]).
In 40 patients not proceeding to an alloHCT within the protocol, 9, 6, 8, and 17 patients received 1, 2, 3, and 4 cycles HiDAC consolidation therapy, respectively. The time between stop of midostaurin and start of subsequent chemotherapy (including second induction and consolidation cycles) was documented with 2 days in 258 cycles and only 1 day in 17 cycles.
In the historical control, 237 (57.1%) patients received alloHCT overall, and of 286 patients achieving a CR/CRi after induction therapy, 122 (42.8%) proceeded to alloHCT in first CR/CRi. The distribution of donor type was matched-related donor in 85 (35.9%; age 18-60 years, n = 80 [35.9%]; age 61-70 years, n = 5 [35.7%]) and matched-unrelated donor in 152 (64.1%) patients (age 18-60 years, n = 143 [64.1%]; age 61-70 years, n = 9 [64.3%]).
Maintenance therapy
Maintenance therapy was started in 97 (34%) of 284 patients, in 75 (56%) of 134 after alloHCT, and in 22 (55%) of 40 after HiDAC consolidation. Reasons for not proceeding to maintenance therapy after alloHCT (n = 59) or HiDAC (n = 18) were death in 7 (12%) and 5 patients (28%), relapse in 5 (8%) and 4 (22%) patients, adverse events in 20 (34%) and 2 (11%) patients, patients’ decision in 10 (17%) and 5 (28%) patients, and unknown in 17 (29%) and 2 (11%) patients, respectively. Maintenance therapy was started in 55 (56%) of 99 younger and in 20 (57%) of 35 older patients receiving alloHCT within the protocol. Maintenance therapy was started after alloHCT in median at day 71 (31-100 range). In the majority of patients (58.7% alloHCT; 72.7% HiDAC), maintenance therapy was terminated early after a median of 9 and 10.5 months after alloHCT and HiDAC (Table 3), respectively, with no difference between younger and older patients. The causes “relapse” and “other reasons” to terminate maintenance therapy were significantly more frequent in patients after HiDAC (Table 3). In Table 4, the adverse events grade 3 or above reported at least once during maintenance phase are summarized. Gastrointestinal adverse events (70%) were reported most commonly, followed by infections (51%) and blood count changes (46%). In particular, gastrointestinal, infections, blood count changes, pain, allergies, and dermatological and renal adverse events were more commonly observed in patients starting maintenance therapy after alloHCT, whereas a trend toward more cardiac arrhythmias was observed after HiDAC consolidation.
Time receiving maintenance therapy, and reasons for early termination
| . | All patients (n = 97) . | alloHCT (n = 75) . | HiDAC (n = 22) . | P . |
|---|---|---|---|---|
| Months, median (range) | 9 (1-13) | 9 (1-13) | 10.5 (1-12) | .82 |
| Early termination, n (%) | ||||
| Total | 60 (61.8) | 44 (58.7) | 16 (72.7) | .32 |
| Death | 1 (1.7) | 1 (2.3) | 0 | .99 |
| IC/patients’ wish | 11 (18.3) | 11 (25) | 0 | .06 |
| Midostaurin toxicity | 28 (46.6) | 24 (54.55) | 4 (25) | .29 |
| Other reasons* | 7 (11.7) | 2 (4.55)* | 5 (31.25)† | .006 |
| Relapse | 13 (21.7) | 6 (13.6) | 7 (43.75) | .009 |
| . | All patients (n = 97) . | alloHCT (n = 75) . | HiDAC (n = 22) . | P . |
|---|---|---|---|---|
| Months, median (range) | 9 (1-13) | 9 (1-13) | 10.5 (1-12) | .82 |
| Early termination, n (%) | ||||
| Total | 60 (61.8) | 44 (58.7) | 16 (72.7) | .32 |
| Death | 1 (1.7) | 1 (2.3) | 0 | .99 |
| IC/patients’ wish | 11 (18.3) | 11 (25) | 0 | .06 |
| Midostaurin toxicity | 28 (46.6) | 24 (54.55) | 4 (25) | .29 |
| Other reasons* | 7 (11.7) | 2 (4.55)* | 5 (31.25)† | .006 |
| Relapse | 13 (21.7) | 6 (13.6) | 7 (43.75) | .009 |
IC, withdrawal of informed consent.
Thrombocytopenia, lung cancer.
Molecular relapse, myelodysplastic syndrome, accidental mistake in midostaurin intake (n = 2), lost to follow-up.
Toxicities grade 3 or above, according to MedRA category coding occurring at least once during maintenance therapy
| . | All patients (n = 97), n (%) . | After alloHCT (n = 75), n (%) . | After HiDAC (n = 22), n (%) . | P . |
|---|---|---|---|---|
| Gastrointestinal | 68 (70) | 60 (80) | 8 (36) | .0001 |
| Infection | 49 (51) | 42 (56) | 7 (32) | .06 |
| Febrile neutropenia | 14 (14) | 10 (13) | 4 (18) | .73 |
| Blood/marrow | 46 (47) | 39 (52) | 1 (5) | <.0001 |
| Pain | 37 (38) | 34 (45) | 3 (14) | <.0001 |
| Constitutional | 35 (36) | 29 (39) | 6 (27) | .45 |
| Allergy/immunology | 33 (34) | 32 (43) | 1 (5) | .006 |
| Metabolic/laboratory | 37 (38) | 35 (47) | 2 (9) | .15 |
| Dermatological | 29 (30) | 27 (36) | 2 (9) | .02 |
| Neurologic | 24 (25) | 20 (27) | 4 (18) | .58 |
| Renal/genitourinary | 23 (24) | 23 (31) | .001 | |
| Pulmonary/upper respiratory | 16 (16) | 15 (20) | 1 (5) | .11 |
| Musculoskeletal/soft tissue | 13 (13) | 11 (15) | 2 (9) | .73 |
| Ocular/visual | 13 (13) | 12 (16) | 1 (5) | .29 |
| Cardiac general | 13 (13) | 11 (15) | 2 (9) | .72 |
| Hemorrhage/bleeding | 7 (7) | 7 (9) | .34 | |
| Auditory/ear | 7 (7) | 7 (9) | .34 | |
| Cardiac arrhythmia | 5 (5) | 2 (3) | 3 (14) | .07 |
| Hepatobiliary/pancreas | 4 (4) | 4 (5) | .57 | |
| Secondary malignancy | 2 (2) | 2 (3) | .99 | |
| Other | 18 (19) | 16 (21) | 2 (9) | .35 |
| . | All patients (n = 97), n (%) . | After alloHCT (n = 75), n (%) . | After HiDAC (n = 22), n (%) . | P . |
|---|---|---|---|---|
| Gastrointestinal | 68 (70) | 60 (80) | 8 (36) | .0001 |
| Infection | 49 (51) | 42 (56) | 7 (32) | .06 |
| Febrile neutropenia | 14 (14) | 10 (13) | 4 (18) | .73 |
| Blood/marrow | 46 (47) | 39 (52) | 1 (5) | <.0001 |
| Pain | 37 (38) | 34 (45) | 3 (14) | <.0001 |
| Constitutional | 35 (36) | 29 (39) | 6 (27) | .45 |
| Allergy/immunology | 33 (34) | 32 (43) | 1 (5) | .006 |
| Metabolic/laboratory | 37 (38) | 35 (47) | 2 (9) | .15 |
| Dermatological | 29 (30) | 27 (36) | 2 (9) | .02 |
| Neurologic | 24 (25) | 20 (27) | 4 (18) | .58 |
| Renal/genitourinary | 23 (24) | 23 (31) | .001 | |
| Pulmonary/upper respiratory | 16 (16) | 15 (20) | 1 (5) | .11 |
| Musculoskeletal/soft tissue | 13 (13) | 11 (15) | 2 (9) | .73 |
| Ocular/visual | 13 (13) | 12 (16) | 1 (5) | .29 |
| Cardiac general | 13 (13) | 11 (15) | 2 (9) | .72 |
| Hemorrhage/bleeding | 7 (7) | 7 (9) | .34 | |
| Auditory/ear | 7 (7) | 7 (9) | .34 | |
| Cardiac arrhythmia | 5 (5) | 2 (3) | 3 (14) | .07 |
| Hepatobiliary/pancreas | 4 (4) | 4 (5) | .57 | |
| Secondary malignancy | 2 (2) | 2 (3) | .99 | |
| Other | 18 (19) | 16 (21) | 2 (9) | .35 |
EFS, OS, and CIR
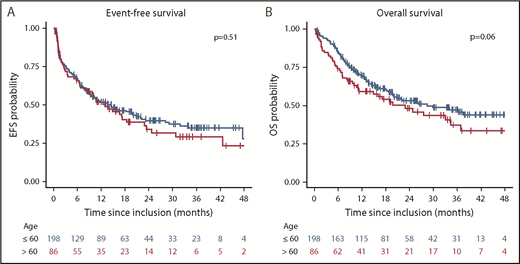
The median follow-up calculated by the reverse Kaplan-Meier method was 28.9 months (95% CI, 25.0-33.6 months). Median and 2-year EFS and OS were 13.2 months (95% CI, 10.0-18.3 months) and 26.0 months (95% CI, 18.9-37.0 months) and 37.7% (95% CI, 32%-44.3%) and 50.9% (95% CI, 44.9%-57.6%), respectively. There was no difference in EFS according to age group (P = .51), but a trend toward better OS in younger patients in univariable (P = .06; Figure 2) and multivariable (P = .07; supplemental Table 3) analysis. There was no difference between cohort 1 and cohort 2 in EFS (P = .85) and OS (P = .96). EFS and OS were significantly better in patients with a concomitant NPM1 mutation (logrank test; P < .0001 and P = .04, respectively), whereas no difference was identified in EFS and OS, according to dichotomized FLT3-ITD allelic ratio (logrank test; P = .52 and P = .74, respectively). In patients achieving a first CR/CRi (n = 217) within the protocol, CIR at 2 years in younger and older patients receiving alloHCT was low, at 18.1% (95% CI, 10.8%-25.4%) and 17.6% (95% CI, 2.7%-32.6%), respectively. Higher CIR rates were observed in patients receiving HiDAC, with 39.2% (95% CI, 20.3%-58.1%) and 56.4% (95% CI, 34.8%-78.1%) in younger and older patients, respectively (supplemental Figure 1). No difference in cumulative incidence of death was noted in both age groups (P = .38 and P = .98; respectively). In those patients proceeding to maintenance therapy after alloHCT (n = 75) and after HiDAC (n = 22), CIR was significantly lower after alloHCT, with 13.3% (95% CI, 5,1%-21.6%), compared with HiDAC, with 43.5% (95% CI, 21.3%-65.9%), at 2 years (P = .02). In a landmark analysis in patients proceeding to alloHCT in first CR/CRi according to the protocol (n = 134) who were event-free at day 100 after transplant (n = 116), those starting maintenance therapy within 100 days after transplant (n = 72) had a significantly better EFS (P = .01, univariable; P = .004, multivariable) and OS (P = .02, univariable; P = .01, multivariable) compared with those who did not (supplemental Figure 2).
Kaplan-Meier plot illustrating the influence of age on EFS and OS. (A) EFS rates at 2 years were 39.3% (95% CI, 32.7%-47.3%) and 33.6% (95% CI, 24.0%-47.1%) for younger and older patients, respectively. (B) OS rates at 2 years were 53.2% (95% CI, 46.2%-61.3%) and 45.6% (95% CI, 35.3%-58.8%) for younger and older patients, respectively.
Kaplan-Meier plot illustrating the influence of age on EFS and OS. (A) EFS rates at 2 years were 39.3% (95% CI, 32.7%-47.3%) and 33.6% (95% CI, 24.0%-47.1%) for younger and older patients, respectively. (B) OS rates at 2 years were 53.2% (95% CI, 46.2%-61.3%) and 45.6% (95% CI, 35.3%-58.8%) for younger and older patients, respectively.
Propensity-score-based comparative analysis to historical controls
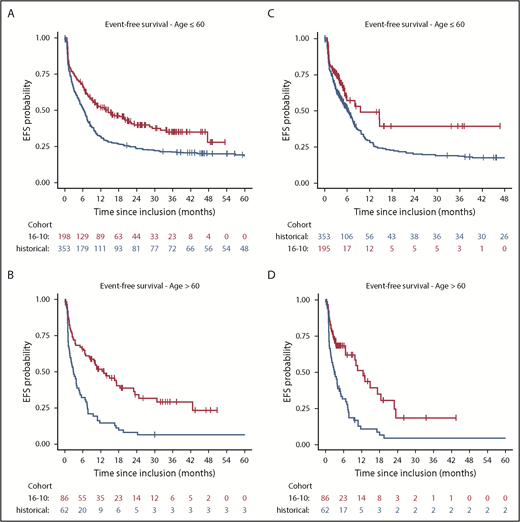
Overall, the comparison of EFS in the AMLSG 16-10 study and the historical controls revealed a significant risk reduction for an event by midostaurin (hazard ratio [HR], 0.58; 95% CI, 0.48-0.70; P < .001). The subgroup analysis according to age group revealed significant differences in younger (HR, 0.61; 95% CI, 0.49-0.76; P < .001) and older (HR, 0.42; 95% CI, 0.29-0.61; P < .001) patients. Evaluating treatment effect modification by age group (older vs younger) resulted in an estimated effect modifier of 0.67 (95% CI, 0.44-1.02; P = .06). In a sensitivity analysis, we performed a Cox regression analysis for EFS by censoring patients proceeding to alloHCT in first CR/CRi at the date of transplant. Still, there was a benefit for midostaurin overall (HR, 0.59; 95% CI, 0.47-0.74; P < .001), as well as in the age subgroups of younger (HR, 0.63; 95% CI, 0.47-0.85; P = .002) and older (HR, 0.41; 95% CI, 0.27-0.62; P = .00002) patients (Figure 3).
Kaplan-Meier plots illustrating the comparison of the AMLSG 16-10 study (16-10, red) to historical controls (historical, blue). Analyses included younger (A) and older (B) patients, as well as a sensitivity analysis with censoring of alloHCT in CR1 in younger (C) and older (D) patients.
Kaplan-Meier plots illustrating the comparison of the AMLSG 16-10 study (16-10, red) to historical controls (historical, blue). Analyses included younger (A) and older (B) patients, as well as a sensitivity analysis with censoring of alloHCT in CR1 in younger (C) and older (D) patients.
Discussion
After the approval by the US Food and Drug Administration and the European Medicines Agency of midostaurin for patients with FLT3-mutated AML, our hypothesis-generating trial is the first to show efficacy and safety data of midostaurin in older patients (age 61-70 years) with FLT3-ITD, given in combination with intensive chemotherapy and as single-agent maintenance after alloHCT. In addition, the treatment schedule of midostaurin was adapted in our trial, in that patients were kept receiving midostaurin beyond day 21 until 2 days before start of the subsequent treatment cycle to achieve sustained FLT3 signaling inhibition. Furthermore, antifungals were allowed as comedication, but triggered dose adaptation after the first amendment. Of note, efficacy of midostaurin was comparable in older and younger patients. The CR/CRi-rate in older patients was 77.9%, which compares favorably to data from previous studies.13,30 The toxicity rates in our study were comparable to those observed in the RATIFY study.11 However, in older patients, cardiac toxicities (22%) were significantly more frequent compared with previously reported cardiac event rates,14 including arrhythmias with an observed rate of 10%, highlighting the necessity of close electrocardiogram and electrolyte monitoring in this patient population. Furthermore, we observed a trend toward a higher frequency (14%) of pulmonary adverse events, mainly pneumonia, in older compared with younger patients. This higher vulnerability of older patients was also reflected in the higher induction death rate of 10.5%, which, however, is still low compared with previous reports.14,31 The induction death rate in older patients was lower in the second cohort (2.4%) compared with the first cohort (15.7%). This may be an observation by chance, or possibly attributable to the implemented dose reduction of midostaurin in case of comedication with CYP3A4 inhibitors in the second cohort and a learning curve of staffs dealing with midostaurin in combination with intensive induction therapy in older patients.
In contrast to the RATIFY study, we intended alloHCT in all patients in CR/CRi, which was reached in 72.4% of our patients. Together with the addition of midostaurin, the high rate of alloHCT in first CR/CRi may have contributed to the good overall survival compared with previous reports,7 especially in older patients.13,14 The direct comparison with the results of the RATIFY study is somewhat difficult, as outcome data in this study were not given for the FLT3-ITD-positive subgroup, including rates of alloHCT.11 However, in line with the results from the RATIFY study,11 and a recent exploratory analysis of the trial,32 there seems to be a particular good efficacy if induction therapy with midostaurin is followed by alloHCT in first CR, although exposure to the inhibitor was shorter compared with patients receiving conventional consolidation. In our study, CIR after HiDAC was, for both younger and older patients (supplemental Figure 1), similar to what has been reported for midostaurin.32 In contrast, CIR was low in patients receiving alloHCT in first remission irrespective of age, and remarkably lower compared with previously reported data.33 Thus, this approach may be of benefit similarly in older and younger patients.
In contrast to the RATIFY study, in which midostaurin maintenance therapy was only applied after HiDAC consolidation, in our study, midostaurin maintenance therapy was also administered after alloHCT; 56% and 55% of patients started maintenance therapy after alloHCT and HiDAC, according to the protocol. The median time on maintenance therapy was 9 months after alloHCT and 10.5 months after HiDAC, which was less than reported in the RATIFY study.11,34 Early discontinuation resulting from nonrelapse causes was common after alloHCT (86%), whereas relapse and nonrelapse causes were nearly equally distributed after HiDAC. Thus, midostaurin maintenance therapy added some degree of toxicity, especially after alloHCT, with anticipated interactions between midostaurin and immunosuppressants and anti-infectives. However, the landmark analysis in our study at day 100 after transplant favors maintenance therapy after alloHCT with better EFS and OS in patients starting maintenance therapy within 100 days after transplant. This underlines the need of data from randomized trials to establish the concept of maintenance with targeted agents after consolidation therapy to prevent AML recurrence. Trials evaluating maintenance with gilteritinib, a second generating FLT3-inhibitor, after conventional chemotherapy and after alloHCT, have been initiated (ClinicalTrials.gov identifiers: NCT02927262, NCT02997202).
Our study was planned to use historical controls from 5 previous AMLSG trials13-17 to increase external validity. Such an approach has several limitations, including, among others, the observational nature of the comparison and the possibility of unmeasured confounders that could not be adjusted in multivariable analysis, and thus may heavily influence the observed results. We used a propensity score to reduce the bias because of confounding variables. This analysis revealed for the primary end point, EFS, that the treatment with midostaurin significantly improved EFS overall, with a HR of 0.58 (95% CI, 0.48-0.70), and in both age subgroups, whereby the improvement of EFS in older patients with a HR of 0.42 (95% CI, 0.29-0.61) was even better than in younger patients, with a HR of 0.61 (95% CI, 0.49-0.76). On the basis of the different transplant rates in the historical cohorts and the AMLSG 16-10 study, and to obtain reliable results of efficacy of midostaurin independent from alloHCT, we performed a sensitivity analysis with censoring of patients proceeding to alloHCT at the date of transplant. This analysis revealed very consistent results with an improvement of EFS by midostaurin overall, with a HR of 0.59 (95% CI, 0.47-0.74), as well as in both age subgroups, and underlines that the improvement of EFS in our study compared with historical controls was not triggered by the higher transplant rate.
In summary, we show that midostaurin plus intensive chemotherapy also can be safely administered in older patients. Compared with historical controls, midostaurin significantly improved EFS. AlloHCT in first remission after midostaurin and chemotherapy is feasible and highly effective, irrespective of age. Maintenance with midostaurin can only be administered to a fraction of patients after completion of intensive chemo consolidation or alloHCT. Therefore, its role needs to be further explored, preferably in a randomized setting with better-tolerated schedules and doses of midostaurin, as well as better-tolerable second-generation FLT3 inhibitors.
Presented in part at the 58th Congress of the American Society of Hematology, San Diego, CA, 4 December 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all members of the German-Austrian AML Study Group for providing leukemia specimens and clinical data.
This work was supported by an unrestricted grant from Novartis.
Authorship
Contribution: R.F.S. and H.D. provided conception and design; R.F.S., D. Weber, W.F., H.R.S., G.W., H.S., T.S., T.K., M.L., D. Wolf, J.W., D.K., K.S.G., H.-A.H., J.K., M. Girschikofsky., M.R., T.S., G.H., H.-G.D., R.S., R.G., M. Grießhammer, E.L., A. Burchardt, U.M., B.H., L.M., M.H., F.T., V.I.G., W.H., A. Benner, K.D., A.G., P.P., and H.D. provided provision of study materials or patients; R.F.S. and D. Weber provided collection and assembly of data; R.F.S., H.D., J.K., and A. Benner provided data analysis and interpretation; R.F.S. and H.D. provided manuscript writing; and R.F.S., D. Weber, W.F., H.R.S., G.W., H.S., T.S., T.K., M.L., D. Wolf, J.W., D.K., K.S.G., H.-A.H., J.K., M. Girschikofsky, M.R., T.S., G.H., H.-G.D., R.S., R.G., M. Grießhammer, E.L., A. Burchardt, U.M., B.H., L.M., M.H., F.T., V.I.G., W.H., J.K., A. Benner, K.D., A.G., P.P., and H.D. provided final approval of manuscript.
Conflict-of-interest disclosure: R.F.S., K.D., and H.D. received research funding from Novartis; R.F.S., K.D., H.D., K.S.G., and D. Wolf received speakers honoraria from Novartis; R.F.S., K.D., P.P., and H.D. participated in Novartis Advisory Boards. The remaining authors declare no competing financial interests.
The current affiliation for J.K. is Department Hematology and Oncology, Braunschweig Municipal Hospital, Germany.
The current affiliation for G.H. is Department of Internal Medicine I, Westpfalzklinikum, Kaiserslautern, Germany.
The current affiliation for W.H. is Department of Internal Medicine III, University Hospital of Regensburg, Germany.
A list of German-Austrian AML Study Group institutions and investigators that participated in this study appears in the supplemental Appendix.
Correspondence: Richard F. Schlenk, National Center of Tumor Diseases Trial Center, National Center for Tumor Diseases, Im Neuenheimer Feld 130.3, 69120 Heidelberg, Germany; e-mail: richard.schlenk@nct-heidelberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal