Key Points
Gadolinium contrast enhancement on brain magnetic resonance imaging resolves with donor myeloid engraftment after HCT.
Graft failure is associated with retention of contrast enhancement in cALD.
Abstract
Adrenoleukodystrophy (ALD) is caused by mutations within the X-linked ABCD1 gene, resulting in the inability to transport acylated very long chain fatty acids (VLCFAs) into the peroxisome for degradation. VLCFAs subsequently accumulate in tissues, including the central nervous system. Up to 40% of boys develop a severe progressive demyelinating form of ALD, cerebral ALD, resulting in regions of demyelination observed on brain magnetic resonance imaging that are associated with a “garland ring” of gadolinium contrast enhancement. Gadolinium enhancement indicates blood-brain barrier (BBB) disruption and an active inflammatory disease process. Only hematopoietic cell transplant (HCT) has been shown to halt neurologic progression, although the mechanism of disease arrest is unknown. We evaluated imaging- and transplant-related biomarkers in 66 males who underwent HCT. In 77% of patients, gadolinium contrast resolved by 60 days post-HCT. We determined that time to neutrophil recovery and extent of donor chimerism correlated significantly with time to contrast resolution post-HCT. Graft failure was associated with a significantly slower rate of contrast resolution (P < .0001). Time to neutrophil recovery remained significant in multivariate analysis with other biomarkers (P = .03). Our data suggest that robust donor myeloid recovery is necessary for timely repair of the BBB.
Introduction
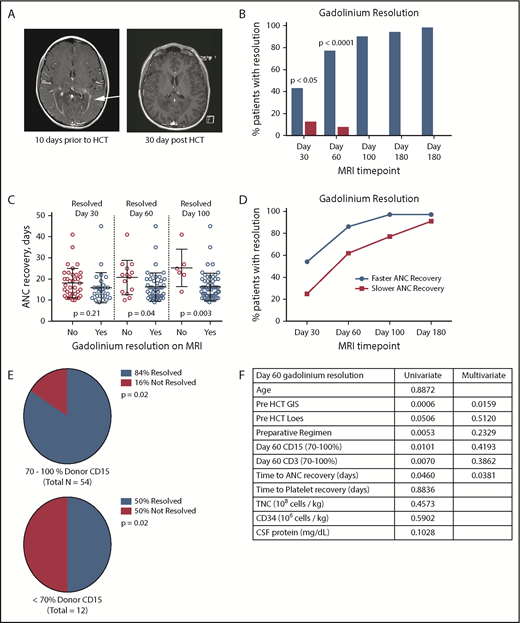
Adrenoleukodystrophy (ALD) is a rare X-linked peroxisomal disorder caused by mutations within the ABCD1 gene resulting in the accumulation of very long chain fatty within tissues, particularly the adrenal glands, testes, and the central nervous system (CNS).1 Most boys develop adrenal dysfunction at ages 4 to 7 years, and up to 40% of boys develop a severe demyelinating form of ALD, cerebral ALD (cALD), between ages 4 and 10 years (median 7 years).1 cALD is progressive, neurologically devastating, and fatal without intervention. The hallmarks of cALD disease manifestation are regions of demyelination observed on brain magnetic resonance imaging (MRI) that are associated with a “garland ring” of gadolinium contrast enhancement, often localized to the occipital–parietal region. Gadolinium enhancement indicates active blood-brain barrier (BBB) disruption.2 Only hematopoietic cell transplant (HCT) has been shown to halt neurologic progression, with the best outcomes when HCT is performed early in the disease process, although the mechanism of disease arrest by donor hematopoietic cells is unknown.3 Following HCT, the gadolinium enhancement resolves as soon as 28 days after transplant in some patients (Figure 1A), and it is thought that most patients will show gadolinium resolution by 100 days after HCT, indicating elimination of active neuroinflammation. The factors associated with gadolinium resolution are not known. Our goal was to evaluate gadolinium resolution in boys undergoing HCT for cALD and determine whether there were other biomarkers of disease or transplant characteristics that were associated with resolution.
Gadolinium resolution on brain MRI correlates with donor neutrophil recovery in cALD patients. (A) Example of a T1-weighted MRI showing a classic ring of gadolinium contrast (arrow; left panel). An MRI exhibiting complete gadolinium contrast resolution 30 days after HCT (right panel). (B) Percentage of patients having complete gadolinium resolution at the indicated time points after HCT. The blue columns represent patients with neutrophil recovery and single HCT, and the red columns represent patients who failed to engraft. (C) Day of neutrophil recovery for patients with contrast resolution at the indicated cumulative time points post-HCT. (D) Frequency of contrast resolution between patients who had ANC recovery ≤ 16 days (faster) vs >16 days (slower). (E) Frequency of contrast resolution in cALD patients with higher CD15 chimerism (70-100%) vs lower CD15 chimerism (<70%) at 60 days post-HCT. (F) Table of pre-HCT and HCT parameters with univariate analysis P values. Variables used in the multivariate analysis model are in bold type. All P values were derived using Fisher’s exact test.
Gadolinium resolution on brain MRI correlates with donor neutrophil recovery in cALD patients. (A) Example of a T1-weighted MRI showing a classic ring of gadolinium contrast (arrow; left panel). An MRI exhibiting complete gadolinium contrast resolution 30 days after HCT (right panel). (B) Percentage of patients having complete gadolinium resolution at the indicated time points after HCT. The blue columns represent patients with neutrophil recovery and single HCT, and the red columns represent patients who failed to engraft. (C) Day of neutrophil recovery for patients with contrast resolution at the indicated cumulative time points post-HCT. (D) Frequency of contrast resolution between patients who had ANC recovery ≤ 16 days (faster) vs >16 days (slower). (E) Frequency of contrast resolution in cALD patients with higher CD15 chimerism (70-100%) vs lower CD15 chimerism (<70%) at 60 days post-HCT. (F) Table of pre-HCT and HCT parameters with univariate analysis P values. Variables used in the multivariate analysis model are in bold type. All P values were derived using Fisher’s exact test.
Study design
In all cases, consent was obtained on Institutional Review Board–approved protocols at the University of Minnesota. We evaluated 66 males (median age, 8.3 years, Table 1; supplemental Methods, available on the Blood Web site) who underwent a single successful HCT characterized by long-term neutrophil recovery with >10% donor chimerism (treated from December of 2002 to April of 2017). A portion of these patients was reported previously.4 Each patient had a pre-HCT MRI with gadolinium to assess disease status, as well as lumbar puncture to include cerebral spinal fluid (CSF) biomarker measurement, as previously described.5 Post-HCT MRIs were scheduled at 30, 60, 100, 180, and 365 days post-HCT. The day of neutrophil recovery was defined when the absolute neutrophil count (ANC) was ≥500 cells per microliter for 3 consecutive days. Time to platelet engraftment was defined as the first of 3 consecutive days with a count > 50 000 platelets per microliter without platelet transfusion support for 7 days. Donor chimerism was determined per standard methods using short tandem repeat analyses. We also performed a 2-way analysis of day-60 gadolinium resolution between the engrafted cohort and a cohort of patients who experienced graft failure and whose characteristics are given in supplemental Methods.
cALD patient and transplant characteristics (N = 66)
| . | Data . |
|---|---|
| Age, y | 8.3 (4.4-47.1) |
| Baseline Loes score | 9 (1-19.5) |
| Baseline gadolinium intensity score | 2 (1-3) |
| CSF total protein, mg/dL | 37 (11-186) |
| Preparative regimen, n | |
| Nonmyeloablative | 14 |
| Myeloablative | 52 |
| Stem cell source, n | |
| Umbilical cord blood | 34 |
| Unrelated donor | 11 |
| Sibling marrow donor | 19 |
| Parent donor | 2 |
| Total nucleated cell dose, 108 cells/kg | 0.90 (0.24-8.25) |
| CD34 dose, 106 cells/kg | 1.43 (0.11-15.97) |
| ANC recovery, d | 16 (9-45) |
| Platelet recovery, d | 34 (13-244) |
| Day-60 CD15 chimerism, % | 100 (14.1-100) |
| Day-60 CD3 chimerism, % | 93.5 (0-100) |
| . | Data . |
|---|---|
| Age, y | 8.3 (4.4-47.1) |
| Baseline Loes score | 9 (1-19.5) |
| Baseline gadolinium intensity score | 2 (1-3) |
| CSF total protein, mg/dL | 37 (11-186) |
| Preparative regimen, n | |
| Nonmyeloablative | 14 |
| Myeloablative | 52 |
| Stem cell source, n | |
| Umbilical cord blood | 34 |
| Unrelated donor | 11 |
| Sibling marrow donor | 19 |
| Parent donor | 2 |
| Total nucleated cell dose, 108 cells/kg | 0.90 (0.24-8.25) |
| CD34 dose, 106 cells/kg | 1.43 (0.11-15.97) |
| ANC recovery, d | 16 (9-45) |
| Platelet recovery, d | 34 (13-244) |
| Day-60 CD15 chimerism, % | 100 (14.1-100) |
| Day-60 CD3 chimerism, % | 93.5 (0-100) |
All data are median (range), unless noted otherwise.
Statistics
A Student t test was used to compare the means of continuous variables. Logistic regression analyses were performed on continuous variables for effect on day-60 gadolinium resolution. The Pearson’s χ2 test or Fisher’s exact test was applied to sets of categorical data. Nominal logistical fit was performed for multivariate analysis. Analyses were performed using Prism (version 7.0a) and JMP Pro (version 14.0.0).
Results and discussion
We found that 43% of patients had resolution of their prior gadolinium enhancement on MRI by 30 days following HCT. Gadolinium resolved in 77%, 90%, 94%, and 98% of patients at the 60-, 100-, 180-, and 365-day MRI post HCT, respectively (Figure 1B). In no case was gadolinium positivity observed following initial resolution. We also noted a difference in time to ANC recovery at days 60, 100, and 180 with respect to gadolinium resolution (Figure 1C). The median time to neutrophil recovery after HCT was 16 days. Patients showing faster count recovery (≤16 days) were more likely to show gadolinium resolution on MRI than those engrafting more slowly (Figure 1D). We were also able to demonstrate an association between ANC recovery and total nucleated cell dose and CD34 cell dose (logistic regression P < .0001 and P = .0002, respectively, data not shown) consistent with prior studies evaluating cell dose and immune reconstitution.6,7 The CD15 donor chimerism also correlated with day-60 gadolinium resolution because those with higher (70-100%) chimerism were more likely to show resolution of enhancement compared with those with <70% donor chimerism (Figure 1E, P = .01). Similar results were also observed with CD3 chimerism (Figure 1F, P = .007). It is notable that many of the patients with lower chimerism (12/14) had undergone a nonmyeloablative HCT. We did not find a correlation between donor source and day-60 gadolinium resolution. Interestingly, in a separate cohort of males with cALD who experienced primary or secondary graft failure (n = 20), we observed a significant decrease in the fraction of patients showing gadolinium resolution at 30 and 60 days after HCT (12.5% and 7.7% with resolution, respectively) compared with engrafted patients (n = 66).
We have previously described biomarkers associated with severity of cerebral disease in cALD, including gadolinium intensity score (GIS),4 MRI severity score (Loes score),3 and CSF total protein concentration.5 In univariate analysis, higher GIS and higher Loes scores were inversely associated with day-60 gadolinium resolution (Figure 1F), although CSF protein concentration was not. Multivariate analysis indicated that day of ANC recovery and pre-HCT GIS remained significantly associated with gadolinium resolution by 60 days post-HCT (Figure 1F).
These results demonstrate, for the first time, a correlation between donor cell recovery and recovery of the BBB, as indicated by the resolution of gadolinium enhancement on MRI. Gadolinium resolution was observed in nearly all patients by 100 days following HCT, with the majority showing resolution by 60 days. Our data confirm that graft failure prolongs BBB disruption, suggesting that donor myeloid recovery is necessary for stabilization. Mixed chimerism is known to be associated with nonmyeloablative conditioning regimens, especially in nonmalignant disease diagnoses8,9 and was associated with contrast retention in our cohort. Importantly, there was no difference in ANC recovery between the intensity of preparative regimens (both had a mean ANC recovery time of 17 days, P = .93). The cellular mechanisms of cALD cerebral disease stabilization by HCT remain unknown. It has been postulated that healthy donor mononuclear cells or microglia precursors cross the BBB after HCT, differentiate into microglia, and assist in attenuating inflammation, allowing BBB repair. Alternatively, we have previously shown that CSF levels of chitotriosidase are a marker of CNS inflammation, and Weinhofer et al have shown the ALD macrophages retain a proinflammatory phenotype.10,11 Perhaps donor monocytes engrafting in the CNS suppress inflammation or differentiate to noninflammatory macrophages, which could be a potential explanation for why engrafting donor cells decrease gadolinium enhancement. The timing of “brain” engraftment is unknown but, in various murine models of metabolic disease, it has been demonstrated to occur within 90 days of HCT,12,13 which is within the time frame of our observations. Collectively, our results support busulfan-containing regimens with an ablative intent.
Although further studies are needed to evaluate the long-term clinical effects, if any, of early vs late gadolinium resolution, these data have important implications for novel drug therapies and conditioning regimens under development that treat cALD. In addition, there are efforts underway utilizing gene therapy to stabilize cALD,14,15 and comparing the kinetics of active cerebral disease mitigation between therapies will be important to understand the relative effectiveness of emerging therapies and approaches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.J.O. and A.G. edited the manuscript; D.R.N. interpreted the brain MRIs and scored gadolinium intensity; W.P.M. and D.K.-J. evaluated patients and edited the manuscript; and T.C.L. wrote the manuscript.
Conflict-of-interest disclosure: P.J.O. and T.C.L. are investigators for bluebird bio, Inc.’s sponsored gene therapy study for cALD (Starbeam). The remaining authors declare no competing financial interests.
Correspondence: Troy C. Lund, University of Minnesota, Pediatric Blood and Marrow Transplant Division, Metabolic Program, Stem Cell Institute, Global Pediatrics, MMC 366, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: lundx072@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal