In this issue of Blood, Orchard et al demonstrate the association of rapid absolute neutrophil count (ANC) recovery with resolution of posttransplant cerebral inflammation in 66 boys with X-linked adrenoleukodystrophy (ALD).1
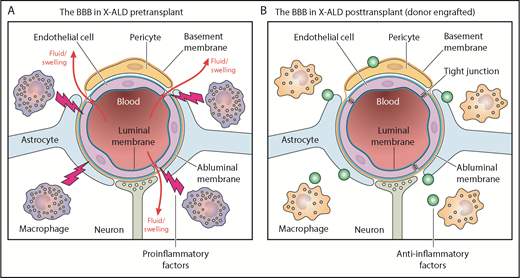
The BBB in X-linked ALD patients before (A) and after (B) hematopoietic cell transplantation. Professional illustration by Patrick Lane, ScEYEnce Studios.
The BBB in X-linked ALD patients before (A) and after (B) hematopoietic cell transplantation. Professional illustration by Patrick Lane, ScEYEnce Studios.
By using gadolinium enhancement on magnetic resonance imaging (MRI) as a marker of blood-brain barrier (BBB) disruption, Orchard et al show that patients with ANC recovery by day 16 after hematopoietic cell transplantation (HCT) had better resolution of the BBB injury. Other factors that played a role in the resolution of gadolinium enhancement included high-level donor myeloid chimerism and higher nucleated and CD34+ cell doses. In many patients, this resolution was noted by day +30 post-HCT.
ALD results from mutations in the ABCD1 gene, which encodes a peroxisomal membrane transporter responsible for transporting very long chain fatty acids (VLCFAs) into the peroxisomes for degradation. VCLFA build-up disrupts the BBB, a highly selective semipermeable border that separates the cerebrospinal fluid from circulating blood, controlled by a complex of endothelial cells, astrocytes, and pericytes. In their early school years, a percentage of boys with ALD develop cerebral inflammation that manifests as regions of demyelination on MRI, and this progressive loss of vision and neurologic function is fatal. It is known that preemptive allogeneic HCT in pre- or early-symptomatic individuals can prevent neurologic progression,2 and rapidly moving to HCT as soon as the patient begins to demonstrate MRI changes improves outcomes.3 However, there is an incomplete understanding of the mechanisms underpinning this correction by HCT.
A previous study of the outcomes of HCT for boys with cerebral ALD noted that the degree of donor myeloid chimerism (>80%) at day +100 correlated significantly with survival.4 The article by Orchard et al extends this finding by demonstrating the significance of prompt donor myeloid engraftment in repair of the BBB in ALD patients. Patients with graft failure did not experience BBB recovery, which suggests a minimal role for the immunosuppressive conditioning and graft-versus-host disease (GVHD) prophylaxis regimens on dampening cerebral inflammation. Instead, the major goal for HCT for patients with ALD should be to engraft donor myeloid cells as quickly as possible, because these cells seem to be actively suppressing further inflammation. A minimal level of donor myeloid chimerism for complete ALD stabilization was not identified, but >70% was associated with faster resolution of the BBB disruption.
Moreover, one wonders whether it is the neutrophils themselves that are responsible for repair of the BBB, or if are they simply a biomarker. After HCT, monocytes typically increase simultaneously with neutrophils. They become tissue macrophages, and defects in them have been shown to be at least partially responsible for the excessive inflammation in ALD.5 However, data from the study by Orchard et al suggest that the benefit of HCT goes beyond simply removing proinflammatory macrophages and that engrafting healthy donor macrophages may be actively suppressing neuroinflammatory responses either directly and/or by altering the VLCFA levels in the central nervous system (see figure).
These findings may inform transplant practices and could help determine the optimal stem cell source for patients with ALD. Given the slower ANC recovery and higher rejection rates associated with umbilical cord blood (UCB) transplants, it might be hypothesized that UCB recipients would have slower resolution of BBB disruption. This was not seen in the cohort reported by Orchard et al, although another cohort demonstrated that HLA matching is important for predicting survival post-HCT.2 In addition, allele-level matching seems to be associated with better outcomes in UCB transplantation for metabolic disorders.6 Until more patients have been analyzed, it seems for now that any well-matched stem cell source is a reasonable choice as long as the graft contains high numbers of nucleated and CD34+ cells. The caveat is that some reports suggest an increased incidence of GVHD with higher cell doses, so this potential risk must be balanced with the benefit of faster ANC recovery.
The question of which conditioning regimen is optimal for patients with ALD remains to be answered. Certain conditioning regimens seem to have good penetration of the BBB (eg, busulfan, fludarabine, thiotepa), whereas others (eg, melphalan, clofarabine) have lower penetration. But it is still unclear whether highly penetrating agents exacerbate cerebral inflammation or if they are preferred because they make space in the brain for donor macrophages to engraft and become microglial cells. The article by Orchard et al does not settle this question, although it does make clear that the primary goal of the conditioning regimen is to establish high-level donor myeloid chimerism without which repair of BBB is slowed. Improved chimerism was noted with a busulfan-based, rather than melphalan-based, regimen. Future efforts might further refine the optimal dosing of the concomitant agents, such as pharmacokinetically targeted fludarabine,7 in hopes of minimizing the risk of immunologic rejection.
There is also a question regarding the optimal GVHD prophylaxis regimen for patients with ALD. The cohort reported in Orchard et al did not receive methotrexate in favor of mycophenolate mofetil or methylprednisolone. Given the known association of methotrexate with slower ANC recovery, this seems like a wise choice for all donor types, with the caveat that development of GVHD is associated with lower survival in ALD and should therefore be strongly avoided.2
Does granulocyte-colony stimulating factor (G-CSF) play a role in the resolution of BBB injury? Although administration of G-CSF post-HCT is associated with lower risk of infections, it has not been demonstrated to improve mortality, and thus it is not universally administered. All patients except those receiving grafts from matched siblings in the cohort of Orchard et al received G-CSF. Careful analysis of the impact of G-CSF administration in larger data sets might inform future trials in the peri-transplant period to expedite ANC recovery (such as G-CSF, granulocyte macrophage-CSF, or plerixafor).8
Finally, an early report suggests that autologous cells corrected by a lentiviral vector may result in ALD stabilization.9 This trial included the use of G-CSF for most patients, and the article by Orchard et al implies that this practice should continue. Furthermore, attempts to increase infused cell doses may help improve outcomes after gene therapy for ALD, just as it seems to do that for ALD patients undergoing allogeneic HCT.
Conflict-of-interest disclosure: C.C.D. served as a consultant for Jazz Pharmaceuticals and Alexion, Inc. S.K. declares no competing financial interests.