Key Points
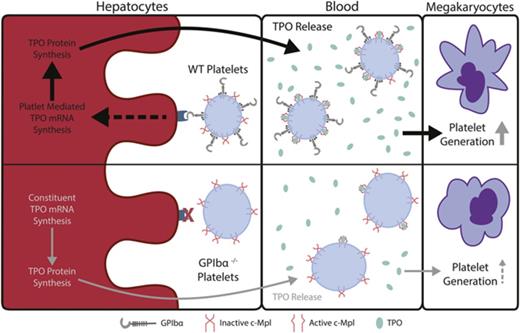
Platelet GPIbα induces hepatic TPO generation and maintains TPO levels in blood.
Antiextracellular GPIbα antibodies decrease TPO generation and may affect TPO levels in immune-mediated thrombocytopenias.
Abstract
Thrombopoietin (TPO), a hematopoietic growth factor produced predominantly by the liver, is essential for thrombopoiesis. Prevailing theory posits that circulating TPO levels are maintained through its clearance by platelets and megakaryocytes via surface c-Mpl receptor internalization. Interestingly, we found a two- to threefold decrease in circulating TPO in GPIbα−/− mice compared with wild-type (WT) controls, which was consistent in GPIbα-deficient human Bernard-Soulier syndrome (BSS) patients. We showed that lower TPO levels in GPIbα-deficient conditions were not due to increased TPO clearance by GPIbα−/− platelets but rather to decreased hepatic TPO mRNA transcription and production. We found that WT, but not GPIbα−/−, platelet transfusions rescued hepatic TPO mRNA and circulating TPO levels in GPIbα−/− mice. In vitro hepatocyte cocultures with platelets or GPIbα-coupled beads further confirm the disruption of platelet-mediated hepatic TPO generation in the absence of GPIbα. Treatment of GPIbα−/− platelets with neuraminidase caused significant desialylation; however, strikingly, desialylated GPIbα−/− platelets could not rescue impaired hepatic TPO production in vivo or in vitro, suggesting that GPIbα, independent of platelet desialylation, is a prerequisite for hepatic TPO generation. Additionally, impaired hepatic TPO production was recapitulated in interleukin-4/GPIbα–transgenic mice, as well as with antibodies targeting the extracellular portion of GPIbα, demonstrating that the N terminus of GPIbα is required for platelet-mediated hepatic TPO generation. These findings reveal a novel nonredundant regulatory role for platelets in hepatic TPO homeostasis, which improves our understanding of constitutive TPO regulation and has important implications in diseases related to GPIbα, such as BSS and auto- and alloimmune-mediated thrombocytopenias.
Introduction
Thrombopoietin (TPO) was first described in 1958 as a humoral factor regulating platelet production.1 However, it was not until its successful cloning in 1994,2 following the identification of its receptor, c-Mpl,3 that a greater understanding of TPO emerged. In thrombopoiesis, TPO binding to its cognate receptor c-Mpl expressed on megakaryocytes and its progenitors stimulates the expansion and differentiation of megakaryocyte precursors2,4 and maturation of megakaryocytes,5,6 but it is not required for platelet shedding or proplatelet production.7,8 TPO has been demonstrated to be critical in the stimulation of thrombopoiesis, as well as in maintaining hematopoiesis and the hematopoietic stem cell (HSC) niche.9,10
Given the important and nonredundant roles of TPO in thrombopoiesis and hematopoiesis, studies that elucidate regulatory mechanisms in the maintenance of steady-state circulatory levels are of great interest.11 Over the years, the prevailing theory posits that TPO is constitutively produced predominantly by the liver (∼65% to 100% of circulating TPO)12 and fine-tuned through uptake and clearance via c-Mpl expressed on platelets and megakaryocytes.13,14 Therefore, it follows that TPO levels are inversely proportional to platelets and megakaryocyte mass.15 Ancillary evidence suggests regulation of TPO levels does not occur at site of production in the liver. For example, mice with hemizygous deletion of the TPO gene exhibit a gene-dosage effect with no compensatory expression from the wild-type (WT) locus in the liver, suggesting constitutive TPO expression.4 Furthermore, induction of thrombocytopenia resulted in increased circulatory TPO, but not increased hepatic TPO, mRNA expression.16,17 In human aplastic anemia or chemotherapy-induced thrombocytopenic patients, TPO levels are significantly higher.16,18 However, conflicting data suggest that TPO levels cannot be exclusively adjusted through platelet/megakaryocyte mass, and regulation may occur at the site of production in the liver. For example, thrombocytosis, such as essential thrombocythemia, typically leads to unexpectedly elevated TPO levels;19 conversely, in immune thrombocytopenia (ITP), TPO levels are lower than expected.20 Additionally, it was recently reported that aged platelets can stimulate liver TPO production through the Ashwell-Morell receptor (AMR).21 Despite these reports, regulation of TPO at the level of production is an emerging concept, although the underlying mechanisms are not well understood.
Here, we identify that platelet GPIbα is responsible for the maintenance of steady-state hepatic TPO production. We found that GPIbα deficiency in mouse and human Bernard-Soulier syndrome (BSS) patients leads to chronically lower circulating TPO levels. Furthermore, transfusions of WT platelets into GPIbα-deficient mice ameliorate TPO levels through increased production in the liver. Importantly, these findings are recapitulated in interleukin-4 (IL4)/GPIbα-transgenic (tg) mice, localizing the functional domain on the extracellular portion of GPIbα as responsible in the induction of hepatic TPO generation. Interestingly, our data show that platelet desialylation cannot compensate for GPIbα deficiency and does not increase hepatic TPO generation. Therefore, GPIbα may act through AMR-dependent and -independent mechanisms to induce hepatic TPO generation. Lastly, we demonstrate that anti-GPIbα antibodies block platelet-mediated hepatic TPO generation, which may explain why TPO levels are not increased in ITP but occur in other thrombocytopenic patients. Our findings may have broad implications on the fundamental understanding of platelet-mediated hepatic TPO generation, as well as on antibody-mediated auto- and alloimmune thrombocytopenias and TPO mimetic-based therapies.
Methods
Mice
GPIbα−/− mice and IL4Rα/GPIbα-tg mice were described previously.22-24 β3 integrin–knockout (β3−/−) mice were originally provided by Dr. Richard O. Hynes (Massachusetts Institute of Technology, Boston, MA) and were further backcrossed to the BALB/c background.25-27 All BALB/c and C57BL/6J (6-8 weeks) mice were purchased from Charles River (Montreal, QC, Canada). GPIbα−/− and β3−/− mice were backcrossed to the BALB/c background 10 times and then bred to generate syngeneic gene-deficient mice. Mice between the ages of 8 and 12 weeks were used for experiments. All aforementioned mice were housed at St. Michael’s Hospital Research Vivarium, and all procedures were approved by the Animal Care Committee at St. Michael’s Hospital.
BSS patient samples
BSS patient plasma samples were provided by the National Hospital Organization Nagoya Medical Center. TPO levels in plasma were detected with a chemiluminescent enzyme immunoassay using an anti-TPO monoclonal antibody and an anti-TPO detection antibody on a chemiluminescence plate reader (SRL). The study was approved by the Ethics Review Committee at Nagoya Medical Center.
TPO quantification
Sera were collected from whole blood following clotting,28-30 and plasma was collected via centrifugation, as previously described.21,31-33 Murine livers were harvested following perfusion with phosphate-buffered saline (PBS), sliced into ∼500-mg sections on dry ice, placed in 2 mL of ice-cold PBS containing protease inhibitor, and homogenized through a 40-µm nylon tissue strainer to obtain single-cell suspensions; this was followed by red blood cell lysis with ACK lysis buffer (Thermo Fisher Scientific). The homogenate was centrifuged at 1500g for 15 minutes and lysed with NP-40 lysis buffer [150 mM NaCl, 1.0% NP-40, 50 mM tris(hydroxymethyl)aminomethane-HCl, pH 8.0] at 4°C overnight. After centrifugation, the clarified supernatant was apportioned into 1-mL aliquots and stored at −80°C until ready for use.34 All TPO levels in serum, plasma, and liver tissues were quantified using a Mouse Thrombopoietin Quantikine ELISA Kit (R&D Systems), according to the manufacturer’s instructions. The nonspecific liver background was measured with an enzyme-linked immunosorbent assay (ELISA) by replacing the rat monoclonal anti-mouse TPO coating antibody with nonspecific rat monoclonal IgG. The absorbance was measured and subtracted from the specific liver TPO-binding absorbance.
Platelet TPO clearance assay
A platelet TPO uptake assay was performed as previously described with modifications.35 Briefly, washed platelets (1 × 108/mL) were resuspended in a standard recombinant TPO solution (2 ng/mL) and incubated for 1 hour at 37°C. Ingested intracellular platelet TPO was obtained following platelet washing (1050g for 7 min) and NP-40 buffer lysis. Free noningested TPO was obtained from culture supernatant. Both were detected and quantified with a Quantikine ELISA Kit (R&D System), as described above.
Liver mRNA quantification with RT-qPCR
Mouse livers were homogenized as described above, and total RNA from liver cells was extracted with an RNeasy Mini Kit (QIAGEN), followed by cDNA preparation from 1 μg of total RNA with a SuperScript IV cDNA synthesis kit (Invitrogen). cDNA samples were quantified by real-time quantitative PCR (RT-qPCR) using SYBR Green PCR Master Mix (QIAGEN) on an Applied Biosystems StepOnePlus Real-Time PCR System, as previously described.36,37 Primers (5′ to 3′) used were TPO forward: CACAGCTGTCCCAAGCAGTA, TPO reverse: CATTCACAGGTCCGTGTGTC, cyclophilin A (cyclo A, housekeeping gene) forward: GCCGATGACGAGCCCTTG, and cyclo A reverse: TGCCGCCAGTGCCATTATG.
In vivo platelet-transfusion assay
Washed platelets were prepared as we previously described.38-40 A total of 2.5 × 108 of the indicated platelets was transfused intravenously to recipient mice. Mice were bled from the saphenous vein at indicated time points, and plasma was isolated as described above. Plasma TPO was measured with a Mouse Thrombopoietin Quantikine ELISA Kit. Livers were harvested at 24 hours posttransfusion, and RT-qPCR was performed to quantify TPO mRNA, as described above.
Hepatocyte platelet phagocytosis assay
FL83B cells (murine hepatocytes; American Type Culture Collection) were grown on coverslips in F-12K Medium (American Type Culture Collection) supplemented with 10% FBS and 1% Pen-Strep for 24 hours, with the addition of BALB/c or GPIbα−/− platelets, stained with 5-chloromethylfluorescein diacetate (CMFDA; 5 µM; Life Technologies), at a 1:20 ratio at 37°C in a 5% CO2 incubator. Following coculture, FL83B cells were fixed in 4% paraformaldehyde, permeabilized (Perm/Wash Buffer; BD Biosciences), and blocked with 3% bovine serum albumin (Sigma-Aldrich). Samples were incubated overnight at 4°C with an anti-albumin antibody (1:200; Thermo Fisher Scientific), followed by secondary Cy3 polyclonal rabbit anti-goat antibody (1:400; Invitrogen). Nuclei were visualized by inclusion of 4′,6-diamidino-2-phenylindole (Invitrogen) in mounting medium (VECTASHIELD; Vector Laboratories). Images were captured using an Olympus upright fluorescence microscope and analyzed with ImageJ.
In vitro platelet-mediated hepatocyte TPO-generation assay
FL83B cells were cultured as described above and incubated with the indicated platelets stained with CMFDA (5 µM) at a 1:10 ratio and cocultured for 24 hours. In some cases, platelets were desialylated with α2-3,6,8 neuraminidase from Clostridium perfringens (Sigma-Aldrich), desialylation was confirmed by lectin binding with fluorescein-labeled Ricinus communis agglutinin-I (RCA-1), succinylated wheat germ agglutinin, Erythrina crista-galli lectin, and peanut agglutinin (VECTOR Laboratories) using a flow cytometer (BD FACSCalibur), as we described previously.30,41,42 Polyclonal IgG was generated and purified as we previously described.26,37 Asialofetuin (Sigma-Aldrich) (100 µg/mL), monoclonal anti-GPIbα antibody (NIT G; 1 µg/mL),30 or polyclonal antibodies (anti-GPIbα or control anti-αIIb polyclonal IgG; 2 µg/mL) were also added, as indicated. Following coculture, FL83B cells were washed with PBS to remove nonadherent platelets, detached using 0.25% trypsin-EDTA, and lysed using buffer from an RNeasy Mini Kit (QIAGEN). Released TPO in culture media and cell lysates were quantified using a Thrombopoietin Quantikine ELISA Kit (R&D Systems), and TPO mRNA was quantified by RT-qPCR, as described above.21 Platelet-associated hepatocytes were also determined as CMFDA-positive events by flow cytometry, as we previously described.29
Preparation of recombinant GPIbα–coupled silica magnetic beads
Recombinant GPIbα ectodomain (R&D Systems) was covalently coupled to the surface of silica magnetic beads utilizing self-assembling monolayer (SAM) chemistry. SAMs allow for the covalent coupling of biomolecules to surfaces while providing resistance to nonspecific binding.43 The beads were first coated with (3-trimethoxysilylpropyl)diethylenetriamine using standard methods,44 followed by standard carbodiimide cross-coupling to recombinant GP1bα ectodomain.45 The coating efficacy was evaluated with anti-GPIbα antibodies. Silica magnetic beads coated with (3-trimethoxysilylpropyl)diethylenetriamine were used as controls.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software. Unless otherwise indicated, all data are presented as mean ± standard error of the mean, and significance was determined by 1-way analysis of variance or a Student t test. Flow cytometry and imaging data were analyzed using FlowJo and ImageJ, as indicated. Differences were considered statistically significant at P < .05.
Results
GPIbα deficiency leads to lower levels of circulating TPO in mice and humans
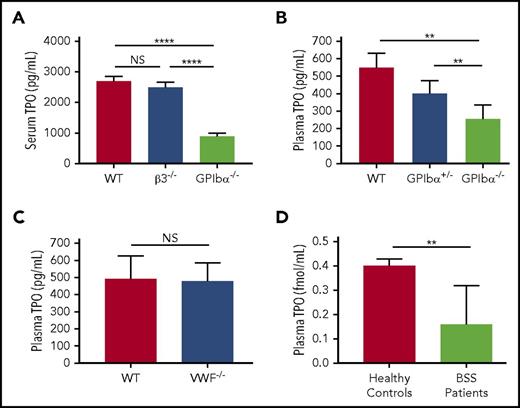
GPIbα−/− mice, like BSS patients, have larger platelets but are also thrombocytopenic (macrothrombocytopenia, ∼30% of WT levels).22 Because it has been shown that an inverse correlation exists between circulating TPO levels and platelet/megakaryocyte mass,46,47 we considered whether GPIbα−/− mice may have increased TPO levels. Unexpectedly, we observed an approximate twofold decrease in TPO levels in the sera and plasma of GPIbα−/− mice compared with syngeneic WT and control β3−/− mice (Figure 1A). Furthermore, GPIbα heterozygosity led to an ∼25% decrease in circulating TPO (Figure 1B). We did not observe significant differences in circulating TPO in von Willebrand factor (VWF)-deficient mice vs syngeneic WT mice (Figure 1C), indicating that lower circulating TPO in GPIbα−/− mice is not due to the absence of VWF bound to GPIbα.
Plasma/serum TPO levels are decreased in GPIbα-deficient mice and human BSS patients. (A) ELISA of serum TPO levels (1:10 dilution) in WT, GPIbα−/−, and β3−/− mice (all on BALB/c background; n = 6-10). ELISA of plasma TPO levels (1:2 dilution) in WT, GPIbα−/−, and heterozygous (GPIbα+/−) mice on BALB/c background (n = 6-10) (B) and WT and VWF−/− mice on C57BL/6J background (n = 3) (C). (D) ELISA-determined plasma TPO concentration in BSS patients (n = 7) and healthy donors (n = 99). **P < .01, ****P < .0001. NS, not significant.
Plasma/serum TPO levels are decreased in GPIbα-deficient mice and human BSS patients. (A) ELISA of serum TPO levels (1:10 dilution) in WT, GPIbα−/−, and β3−/− mice (all on BALB/c background; n = 6-10). ELISA of plasma TPO levels (1:2 dilution) in WT, GPIbα−/−, and heterozygous (GPIbα+/−) mice on BALB/c background (n = 6-10) (B) and WT and VWF−/− mice on C57BL/6J background (n = 3) (C). (D) ELISA-determined plasma TPO concentration in BSS patients (n = 7) and healthy donors (n = 99). **P < .01, ****P < .0001. NS, not significant.
To determine whether our findings from mouse studies are reproducible in humans, we obtained plasma samples from BSS patients. We found that plasma TPO levels were significantly decreased compared with healthy donor controls (Figure 1D). Therefore, our data revealed the surprising discovery that GPIbα deficiency leads to lower TPO levels in mice and humans.
Lower TPO levels in GPIbα−/− mice are not due to enhanced GPIbα−/− platelet-mediated TPO clearance
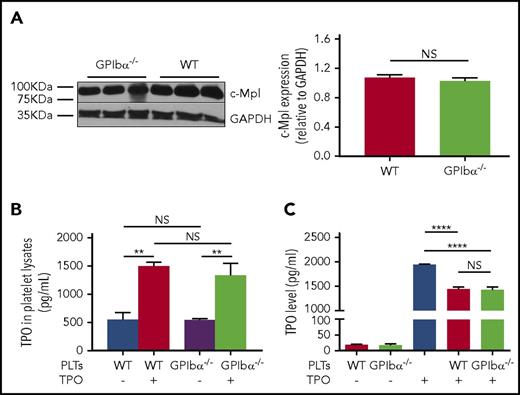
The cognate receptor of TPO, c-Mpl, mediates TPO internalization, as well as subsequent clearance, which has been considered the key mechanism in the regulation of TPO levels in blood circulation.48,49 Because GPIbα−/− platelets are larger,22 it is conceivable that decreased TPO levels may be attributed to increased c-Mpl numbers or function on GPIbα−/− platelets. We assessed whether the larger size of GPIbα−/− platelets reflects increased copy number of the c-Mpl receptor. As expected, we found that, per platelet, there is ∼50% more protein, including c-Mpl, in GPIbα−/− platelets, as shown in supplemental Figure 1, available on the Blood Web site. However, individual increased platelet mass is offset by a 70% decrease in platelet number in GPIbα−/− mice, netting an overall similar platelet mass to WT mice.50 Furthermore, western blot analysis revealed no significant difference in c-Mpl density between GPIbα−/− and WT platelets, with glyceraldehyde-3-phosphate dehydrogenase serving as an internal control (Figure 2A). Thus, quantitatively, per platelet volume, there is no significant increase in c-Mpl expression on GPIbα−/− platelets. To investigate whether, functionally, GPIbα−/− platelets could clear TPO more efficiently, we incubated the same number of GPIbα−/− or WT platelets in standard medium supplemented with TPO. We concurrently measured the increase in TPO in platelet lysates (effective platelet internalization) and the corresponding TPO decrease in the culture medium. We found no significant difference between the 2 groups (Figure 2B). Moreover, transfusion of GPIbα−/− or WT platelets into WT mice resulted in a comparable acute decrease in plasma TPO levels, suggesting similar platelet-mediated TPO uptake between the 2 strains (supplemental Figure 2). Altogether, these data indicate that lower TPO levels in GPIbα−/− mice are not due to enhanced TPO clearance by GPIbα−/− platelets.
GPIbα−/−platelets do not have increased c-Mpl density or enhanced TPO clearance function. (A) Representative immunoblots (left panel) and densitometry (right panel) of TPO receptor (c-Mpl) expression relative to glyceraldehyde-3-phosphate dehydrogenase (loading control) in GPIbα−/− and WT BALB/c platelets (n = 6). ELISA-determined TPO levels from platelet lysates (internalized TPO) of GPIbα−/− and WT BALB/c mice (B) and cultured supernatant supplemented with recombinant TPO following a 1-hour incubation of GPIbα−/− or WT platelets (C) (n = 6). **P < .01, ****P < .0001. NS, not significant.
GPIbα−/−platelets do not have increased c-Mpl density or enhanced TPO clearance function. (A) Representative immunoblots (left panel) and densitometry (right panel) of TPO receptor (c-Mpl) expression relative to glyceraldehyde-3-phosphate dehydrogenase (loading control) in GPIbα−/− and WT BALB/c platelets (n = 6). ELISA-determined TPO levels from platelet lysates (internalized TPO) of GPIbα−/− and WT BALB/c mice (B) and cultured supernatant supplemented with recombinant TPO following a 1-hour incubation of GPIbα−/− or WT platelets (C) (n = 6). **P < .01, ****P < .0001. NS, not significant.
Lower TPO levels in GPIbα−/− mice are due to impaired platelet-stimulated hepatic TPO production
Because the liver is the primary site of de novo TPO generation,12 we sought to determine whether the lower circulating TPO in GPIbα−/− mice was due to impaired hepatic TPO production. Measurement of TPO mRNA transcripts in the liver revealed an ∼50% decrease in GPIbα−/− mice compared with WT mice (Figure 3A; supplemental Figure 3), with corresponding lower TPO protein levels in liver tissue (Figure 3B). Furthermore, we did not observe any significant differences in TPO mRNA levels between GPIbα−/− and WT mice within other known organs of TPO generation,51 including spleen and kidneys (supplemental Figure 5). To determine whether GPIbα−/− mice possessed a lower constitutive hepatic TPO production or a disruption within platelet-mediated TPO generation, we assessed the ability of WT platelet transfusions to rescue lower TPO levels in GPIbα−/− mice. We found, indeed, that WT platelets, but not GPIbα−/− platelets, induced significantly increased (∼75% from baseline) TPO levels in the circulation of GPIbα−/− recipient mice (Figure 3C). Consistently, TPO mRNA within hepatic tissue of GPIbα−/− mice also increased following transfusion of WT, but not GPIbα−/−, platelets (Figure 3D), suggesting de novo platelet-mediated TPO production. In addition, assessment of production of IL-6 in GPIbα−/− mice, a proinflammatory cytokine previously identified to increase TPO generation in the liver,52 revealed no difference compared with WT mice (supplemental Figure 6). These findings confirm that impaired TPO generation in GPIbα−/− mice stems from lack of response to platelet-mediated TPO production, rather than an inherent hepatic defect.
GPIbα deficiency leads to a significant decrease in platelet-mediated hepatic TPO generation. TPO mRNA was measured via RT-qPCR (A) and TPO protein was measured by ELISA (B) (n = 6-10) in WT BALB/c and GPIbα−/− hepatic tissue lysates. (C) Plasma TPO levels were measured by ELISA at the indicated time points following transfusion of 2.5 × 108 WT BALB/c or GPIbα−/− platelets into GPIbα−/− recipient mice. Values are normalized to TPO levels on day 0 (100%) (n = 6-12). (D) TPO mRNA levels in liver of GPIbα−/− mice, as measured by RT-qPCR, 24 hours following transfusion of 2.5 × 108 platelets (n = 5). *P < .05, **P < .01. NS, not significant.
GPIbα deficiency leads to a significant decrease in platelet-mediated hepatic TPO generation. TPO mRNA was measured via RT-qPCR (A) and TPO protein was measured by ELISA (B) (n = 6-10) in WT BALB/c and GPIbα−/− hepatic tissue lysates. (C) Plasma TPO levels were measured by ELISA at the indicated time points following transfusion of 2.5 × 108 WT BALB/c or GPIbα−/− platelets into GPIbα−/− recipient mice. Values are normalized to TPO levels on day 0 (100%) (n = 6-12). (D) TPO mRNA levels in liver of GPIbα−/− mice, as measured by RT-qPCR, 24 hours following transfusion of 2.5 × 108 platelets (n = 5). *P < .05, **P < .01. NS, not significant.
Desialylation of GPIbα−/− platelets could not rescue hepatic TPO generation
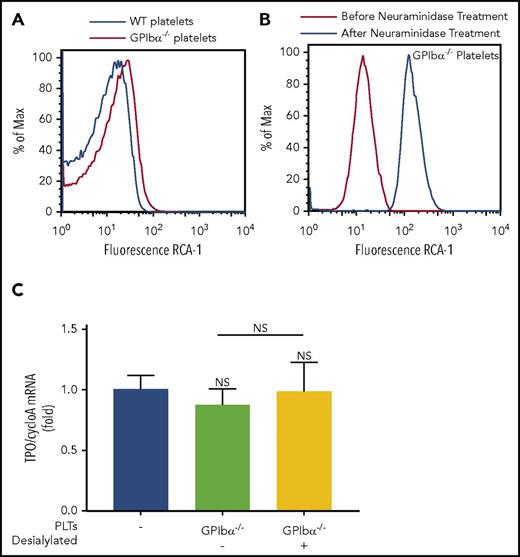
It was recently reported that platelet desialylation is an important mechanism underlying platelet-mediated hepatic TPO generation.21 Because GPIbα is the most sialylated platelet surface protein, we considered whether the inability of GPIbα−/− platelets to stimulate TPO generation is due to the paucity of available desialylated residues that would otherwise be present on GPIbα. Interestingly, assessment of baseline desialylation of GPIbα−/− platelets with RCA-1 binding revealed increased desialylation compared with WT platelets (Figure 4A; supplemental Figure 7). The increased desialylation profile was further confirmed with succinylated wheat germ agglutinin, peanut agglutinin, and E. crista-galli lectin binding (supplemental Figure 8). Treatment of GPIbα−/− platelets with neuraminidase demonstrates that, despite a >10-fold increase in desialylation (Figure 4B), transfusion of desialylated GPIbα−/− platelets did not rescue GPIbα−/− platelet–mediated hepatic TPO generation in vivo (Figure 4C). With the staining of platelet activation and apoptosis markers (P-selectin and annexin V), we showed that the alterations in GPIbα−/− platelets after sialidase treatment are not significantly different from WT platelets (supplemental Figure 9). These data suggest that GPIbα, independent of other platelet desialylated residues, is required for platelet-mediated hepatic TPO generation.
Desialylated GPIbα−/−platelets cannot rescue hepatic TPO generation in vivo. Representative line graphs of baseline desialylation levels of WT and GPIbα−/− platelets (A) and desialylation of GPIbα−/− platelets before and after neuraminidase treatment (B), as determined by fluorescein-conjugated RCA-1 binding. (C) WT mice were transfused with 2.5 × 108 desialylated or control GPIbα−/− platelets. Livers were harvested 24 hours posttransfusion, and TPO mRNA was quantified by RT-qPCR. n = 3. NS, not significant.
Desialylated GPIbα−/−platelets cannot rescue hepatic TPO generation in vivo. Representative line graphs of baseline desialylation levels of WT and GPIbα−/− platelets (A) and desialylation of GPIbα−/− platelets before and after neuraminidase treatment (B), as determined by fluorescein-conjugated RCA-1 binding. (C) WT mice were transfused with 2.5 × 108 desialylated or control GPIbα−/− platelets. Livers were harvested 24 hours posttransfusion, and TPO mRNA was quantified by RT-qPCR. n = 3. NS, not significant.
GPIbα−/− platelets exhibit impaired binding to hepatocytes and do not significantly stimulate hepatic TPO generation in vitro
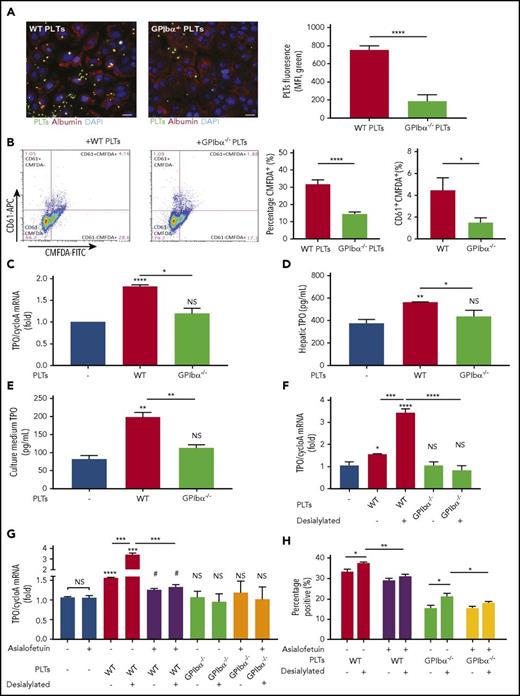
In vitro studies were performed to further elucidate the requirement of GPIbα in the interaction among platelets, hepatocytes, and subsequent hepatic TPO generation. We found that, under static conditions, hepatocytes cocultured with fluorescently labeled WT platelets had a significantly higher percentage of bound (Figure 5A-B) and internalized (Figure 5B) platelets compared with cocultures with fluorescently labeled GPIbα−/− platelets. The same assay was repeated under physiological low shear conditions of the liver vasculature (300 s−1); fluorescently labeled platelets perfused over adherent hepatocytes yielded similar results (supplemental Figure 10). Additionally, WT, but not GPIbα−/−, platelets stimulated hepatocytes to synthesize TPO mRNA (Figure 5C), as well as to produce and release significantly higher levels of TPO, as measured by cellular TPO concentrations (Figure 5D) and TPO concentration in culture medium (Figure 5E).
GPIbα−/−platelets exhibit impaired binding to hepatocytes and do not significantly induce hepatic TPO generation in vitro. FL83B cells (murine hepatocytes) were cocultured with CMFDA-labeled (A-B) or nonlabeled (C-E) WT or GPIbα−/− platelets (1:20 ratio) for 24 hours. (A) Representative immunofluorescence images of CMFDA-stained platelets bound to hepatocytes. Blue = 4′,6-diamidino-2-phenylindole (DAPI; nuclear counterstain), green = CMFDA-stained platelets, red = albumin (hepatocyte marker). Scale bars, 20 µm. Quantification of CMFDA-stained platelet–hepatocyte adherence presented as platelet mean fluorescence intensity (MFI) per field, analyzed from 3 individual experiments of 3 randomly selected fields. (B) Flow cytometry analysis of hepatocyte-associated CMFDA-stained platelets, quantified as CMFDA-stained platelet–positive hepatocytes. Alexa Fluor 647–conjugated anti-CD61 antibody was subsequently used to stain the hepatocyte surface-bound platelets (CD61+ population). TPO protein concentration was quantified by ELISA in hepatic cell lysates (C) and culture medium (D). (E) TPO mRNA expression in hepatocytes was measured by RT-qPCR (n = 3; 3 individual experiments in triplicate). (F-H) Platelets from the indicated strains (WT and GPIbα−/−) were incubated with FL83B cells for 24 hours. In some instances, platelets were desialylated with neuraminidase. (F-G) FL83B cellular TPO mRNA expression was measured by RT-qPCR. (G-H) Asialofetuin, a competitive blocker of AMR, was added as indicated. (H) The CMFDA-platelet–positive population was gated and quantified by flow cytometry to evaluate platelet binding or uptake by hepatocytes following desialylation. *P < .05, **P < .01, ***P < .001, ****P < .0001 vs platelet-negative control, #P < .05 vs asialofetuin-positive platelet-negative control. NS, not significant.
GPIbα−/−platelets exhibit impaired binding to hepatocytes and do not significantly induce hepatic TPO generation in vitro. FL83B cells (murine hepatocytes) were cocultured with CMFDA-labeled (A-B) or nonlabeled (C-E) WT or GPIbα−/− platelets (1:20 ratio) for 24 hours. (A) Representative immunofluorescence images of CMFDA-stained platelets bound to hepatocytes. Blue = 4′,6-diamidino-2-phenylindole (DAPI; nuclear counterstain), green = CMFDA-stained platelets, red = albumin (hepatocyte marker). Scale bars, 20 µm. Quantification of CMFDA-stained platelet–hepatocyte adherence presented as platelet mean fluorescence intensity (MFI) per field, analyzed from 3 individual experiments of 3 randomly selected fields. (B) Flow cytometry analysis of hepatocyte-associated CMFDA-stained platelets, quantified as CMFDA-stained platelet–positive hepatocytes. Alexa Fluor 647–conjugated anti-CD61 antibody was subsequently used to stain the hepatocyte surface-bound platelets (CD61+ population). TPO protein concentration was quantified by ELISA in hepatic cell lysates (C) and culture medium (D). (E) TPO mRNA expression in hepatocytes was measured by RT-qPCR (n = 3; 3 individual experiments in triplicate). (F-H) Platelets from the indicated strains (WT and GPIbα−/−) were incubated with FL83B cells for 24 hours. In some instances, platelets were desialylated with neuraminidase. (F-G) FL83B cellular TPO mRNA expression was measured by RT-qPCR. (G-H) Asialofetuin, a competitive blocker of AMR, was added as indicated. (H) The CMFDA-platelet–positive population was gated and quantified by flow cytometry to evaluate platelet binding or uptake by hepatocytes following desialylation. *P < .05, **P < .01, ***P < .001, ****P < .0001 vs platelet-negative control, #P < .05 vs asialofetuin-positive platelet-negative control. NS, not significant.
In vitro cocultures with desialylated platelets and hepatocytes confirm our in vivo data, whereby desialylation of GPIbα−/− platelets did not increase hepatic TPO generation (Figure 5F). These data again suggest that desialylation of non-GPIbα residues is not the key factor for platelet-mediated hepatic TPO production. To test whether GPIbα is acting through the AMR to induce hepatic TPO generation, we used asialofetuin to competitively block the AMR on hepatocytes. We found that asialofetuin effectively blunted increased desialylated WT platelet-mediated hepatic TPO production,21 but it had no significant effect in the presence of GPIbα−/− platelets (Figure 5G).
Interestingly, flow cytometry data in parallel, reproducibly showed that, following desialylation of GPIbα−/− platelets, they exhibited greater binding to hepatocytes (Figure 5H), without a concomitant increase in hepatic TPO mRNA (Figure 5F). Furthermore, increased desialylated GPIbα−/− platelet binding is decreased in the presence of asialofetuin (Figure 5H). This suggests that other desialylated platelet glycoproteins may bind the AMR but that the signal is insufficient to induce hepatic TPO generation in the absence of GPIbα. Alternatively, GPIbα may be acting through an AMR-independent pathway to induce hepatic TPO generation. Regardless, these data demonstrate that GPIbα is the essential link between the platelet and the hepatocyte in stimulating hepatic TPO generation.
The extracellular domain of GPIbα is required for hepatic TPO generation
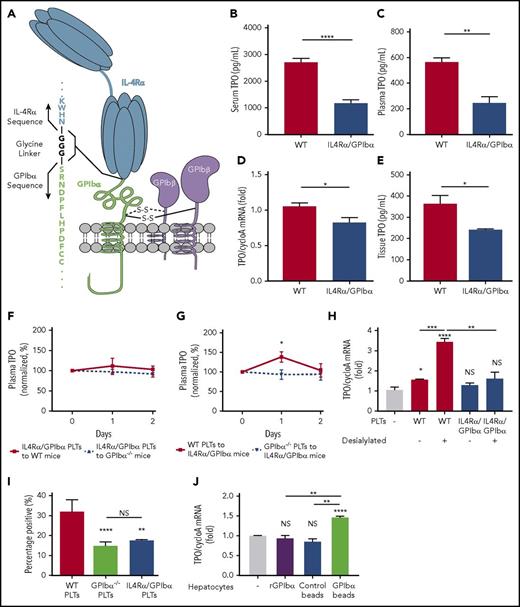
To determine whether it is the ligand-binding domain or the cytoplasmic portion of GPIbα that plays an active role in regulating TPO, we examined TPO levels in IL4Rα/GPIbα-tg mice. These mice have most of the extracytoplasmic region of GPIbα (residues 1-472) replaced with the α subunit of the human IL-4 receptor while retaining the intracellular and membrane-proximal region of GPIbα (Figure 6A).23,53 Consistent with GPIbα−/− mice, IL4Rα/GPIbα-tg mice were also found to have significantly lower plasma and sera TPO levels (Figure 6B-C), as a consequence of decreased hepatic TPO generation (Figure 6D-E). To further validate the importance of the extracellular region of GPIbα in platelet-induced TPO generation from liver, we transfused IL4Rα/GPIbα-tg platelets into WT or GPIbα−/− mice; as expected, similar to GPIbα−/− platelets, IL4Rα/GPIbα-tg platelets could not increase TPO production (Figure 6F-G). Importantly, desialylation of IL4Rα/GPIbα-tg platelets also had no significant effect on hepatic TPO mRNA expression (Figure 6H). IL4Rα/GPIbα-tg platelets exhibited similar decreased binding to hepatocytes as GPIbα−/− platelets (Figure 6I). Thus, the extracellular domain of GPIbα is a prerequisite and dictates the hepatic response to platelet-stimulated TPO generation.
The extracellular domain of GPIbα is required for hepatic TPO generation. (A) Representative diagram of the IL4Rα/GPIbα-tg receptor. ELISA-determined serum (B) and plasma (C) TPO levels (1:2 dilution) in WT and IL4Rα/GPIbα mice (C57BL/6J background, n = 5). TPO mRNA levels were measured via RT-qPCR (D) and TPO protein concentrations were measured by ELISA (E) (n = 6) in WT and IL4Rα/GPIbα-tg mice hepatic tissue. Plasma TPO levels were measured by ELISA at the indicated time points following transfusion of 2.5 × 108 IL4Rα/GPIbα platelets into WT or GPIbα−/− mice (F) or 2.5 × 108 WT or GPIbα−/− platelets into IL4Rα/GPIbα-tg mice (G). Values are normalized to TPO levels on day 0 (100%) (n = 3). (H) Platelets from IL4Rα/GPIbα mice were incubated with FL83B cells for 24 hours after which FL83B cellular TPO mRNA expression was measured by RT-qPCR. In some instances, platelets were desialylated with neuraminidase. (I) Flow cytometry analysis of hepatocyte-associated platelets (WT, GPIbα, and IL4Rα/GPIbα) quantified as CMFDA-stained platelet–positive hepatocytes. (J) TPO mRNA expression in hepatocytes was measured after incubation with recombinant GPIbα-coupled beads compared with control beads and recombinant GPIbα alone. *P < .05, **P < .01, ***P < .001, ****P < .0001. NS, not significant.
The extracellular domain of GPIbα is required for hepatic TPO generation. (A) Representative diagram of the IL4Rα/GPIbα-tg receptor. ELISA-determined serum (B) and plasma (C) TPO levels (1:2 dilution) in WT and IL4Rα/GPIbα mice (C57BL/6J background, n = 5). TPO mRNA levels were measured via RT-qPCR (D) and TPO protein concentrations were measured by ELISA (E) (n = 6) in WT and IL4Rα/GPIbα-tg mice hepatic tissue. Plasma TPO levels were measured by ELISA at the indicated time points following transfusion of 2.5 × 108 IL4Rα/GPIbα platelets into WT or GPIbα−/− mice (F) or 2.5 × 108 WT or GPIbα−/− platelets into IL4Rα/GPIbα-tg mice (G). Values are normalized to TPO levels on day 0 (100%) (n = 3). (H) Platelets from IL4Rα/GPIbα mice were incubated with FL83B cells for 24 hours after which FL83B cellular TPO mRNA expression was measured by RT-qPCR. In some instances, platelets were desialylated with neuraminidase. (I) Flow cytometry analysis of hepatocyte-associated platelets (WT, GPIbα, and IL4Rα/GPIbα) quantified as CMFDA-stained platelet–positive hepatocytes. (J) TPO mRNA expression in hepatocytes was measured after incubation with recombinant GPIbα-coupled beads compared with control beads and recombinant GPIbα alone. *P < .05, **P < .01, ***P < .001, ****P < .0001. NS, not significant.
To assess whether GPIbα ectodomain is sufficient to promote a hepatocyte response, we first tested soluble recombinant GPIbα and found that it did not stimulate TPO mRNA transcription in hepatocytes in vitro. To evaluate whether surface immobilized GPIbα can boost the hepatic effect, recombinant murine GPIbα was coupled onto platelet-sized silica beads coated with a SAM,54,55 which allows recombinant GPIbα to be oriented upward away from the bead surface (supplemental Figure 11). We found that the GPIbα-coupled beads stimulated hepatic TPO mRNA production similar to WT platelets. This suggests that platelet surface GPIbα alone, excluding other platelet factors, is sufficient to mediate hepatocyte TPO generation (Figure 6J).
Anti-GPIbα antibodies block hepatic TPO generation
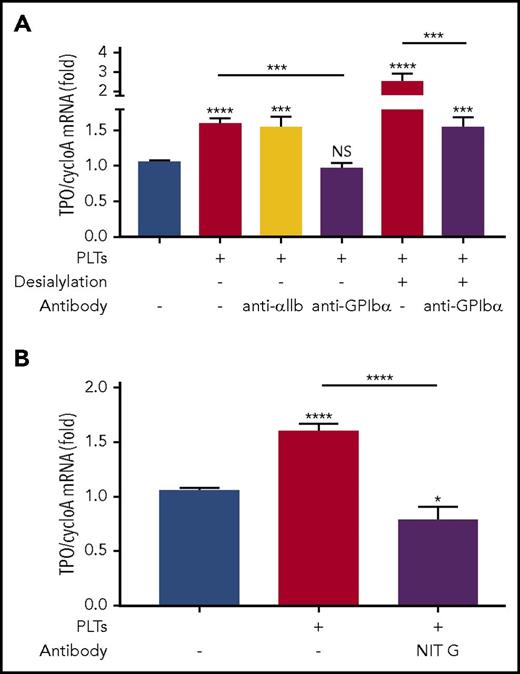
In immune-mediated thrombocytopenias, such as ITP, antibodies are frequently generated against platelet surface antigens, including GPIbα.56-62 To assess the clinical relevance of our study and whether anti-GPIbα antibodies could negatively affect platelet-mediated hepatic TPO generation, we used anti-GPIbα polyclonal antibodies generated in GPIbα−/− mice immunized with WT platelets.24,28,30 We found that WT platelets opsonized with anti-GPIbα antibodies could block hepatic TPO mRNA expression in vitro, mimicking the effect seen with GPIbα-deficient platelets (Figure 7A). However, when control anti–αIIb-antibodies were used, no significant inhibition was observed (Figure 7A). Additionally, as expected, anti-GPIbα antibodies had no significant effect on GPIbα−/− platelets (supplemental Figure 12). Interestingly, when we used a monoclonal antibody targeting the N terminus of GPIbα (NIT G),30 we observed similar levels of inhibition on hepatocyte TPO generation as on GPIbα−/− platelets (Figure 7B). Thus, our data suggest that the functional domain of GPIbα that mediates hepatic TPO generation is located on the N terminus. These data indicate that antibodies or antagonists targeting GPIbα (eg, in ITP), particularly those against the N terminus ligand-binding domain, may suppress TPO production from the liver.30,42,61
Anti-GPIbα antibodies block platelet-mediated hepatocyte TPO generation. (A-B) Platelets from BALB/c WT mice were incubated with FL83B cells for 24 hours, after which FL83B cellular TPO mRNA expression was measured by RT-qPCR. In some instances, as indicated, platelets were desialylated with neuraminidase. Purified anti-GPIbα (2 µg/mL) or control anti-αIIb polyclonal IgG (2 µg/mL) (A) or monoclonal anti-GPIbα antibody NIT G (1 µg/mL) (B) was added as indicated, n = 3 (in duplicate). *P < .05, ***P < .001, ****P < .0001. NS, not significant.
Anti-GPIbα antibodies block platelet-mediated hepatocyte TPO generation. (A-B) Platelets from BALB/c WT mice were incubated with FL83B cells for 24 hours, after which FL83B cellular TPO mRNA expression was measured by RT-qPCR. In some instances, as indicated, platelets were desialylated with neuraminidase. Purified anti-GPIbα (2 µg/mL) or control anti-αIIb polyclonal IgG (2 µg/mL) (A) or monoclonal anti-GPIbα antibody NIT G (1 µg/mL) (B) was added as indicated, n = 3 (in duplicate). *P < .05, ***P < .001, ****P < .0001. NS, not significant.
Discussion
In this study, we identified a previously unknown regulatory mechanism in the homeostatic maintenance of circulating TPO levels. Specifically, we found that the extracellular domain of GPIbα is required for de novo platelet-mediated TPO generation in the liver. The physiological importance of this mechanism is underscored in GPIbα-deficient mice and BSS patients, in whom the lack of extracellular expression of GPIbα on platelets led to an overall decrease in circulating TPO level (Figures 1 and 6). Furthermore, we demonstrated that desialylation of non-GPIbα platelet residues (ie, GPIb-deficient platelets) is insufficient to induce hepatic TPO generation (Figures 4-6). However, these data do not preclude that desialylation of GPIbα may be required. Thus, GPIbα–hepatic interaction may occur through AMR-dependent21 and -independent pathways. Moreover, IL4Rα/GPIbα-tg mice and our recombinant GPIbα-coupled beads further demonstrated that surface immobilized GPIbα alone is sufficient to stimulate TPO generation from hepatocytes (Figure 6). Lastly, we found that antibodies against GPIbα (eg, in immune-mediated thrombocytopenias) could recapitulate the effects of GPIbα deficiency, effectively blocking hepatic TPO generation (Figure 7). These findings clearly demonstrate the requirement of GPIbα for platelet-mediated hepatic TPO generation.
The regulation of circulating steady-state TPO levels has been widely debated.63 It is becoming more evident that both mechanisms of regulation, including TPO clearance via c-Mpl on platelets/megakaryocytes and production in the liver, are not mutually exclusive events. Our data show that, despite similar c-Mpl abundance and function in GPIbα−/− mice (Figure 2), circulating TPO levels are chronically lower. This suggests that platelets, through GPIbα, contribute significantly to the constitutive steady-state production of TPO from the liver. These findings unveil novel perspectives in our understanding of TPO levels in thrombocytopenic disorders, such as ITP. It is not well understood why ITP patients often exhibit TPO levels lower than expected. This study offers plausible scenarios in which lower TPO levels could be attributed to diversion of platelets away from the liver due to antibody-mediated clearance in the spleen leading to loss of platelet stimulated hepatic TPO generation. Alternatively, as we have shown previously, anti-GPIbα antibodies may cause platelet desialylation and clearance in the liver. However, platelets opsonized with anti-GPIbα antibodies, despite targeting to the liver, may still be unable to stimulate hepatic TPO generation, as we demonstrated (Figure 6). In addition, we cannot completely exclude that antibodies targeting GPIbα may cause signaling events and subsequent indirect blocking of GPIbα–hepatocyte interactions. These differential antibody effects on platelets may impact the response to therapy, such as TPO mimetics, and deserve further investigation. Earlier studies observed abnormal megakaryocyte morphology in GPIbα−/− bone marrow, including disordered membrane demarcation in megakaryocytes.22,64 There are also reports that anti-GPIbα antibodies suppress in vitro human megakaryocyte production65,66 and platelet release from megakaryocytes.67 Whether the reduced TPO from GPIbα deficiency or blockage contributes to this process is worthwhile for future exploration.
It is well known that c-Mpl expression is not limited to platelets and megakaryocytes but is also present on HSCs.9,68,69 Importantly, TPO has been implicated as a nonredundant factor in maintaining the stem cell niche and quiescence.70 TPO−/− and c-Mpl−/− mice experience an age-progressive loss of their HSC pool,70 leading to a permanent decrease in multilineage progenitors in the bone marrow.68,71 A similar phenotype is observed in human patients with amegakaryocytic thrombocytopenia caused by loss-of-function mutations in c-Mpl.72 These patients progress to bone marrow failure as early as 2 months of age.73 These suggest the critical importance of maintaining threshold levels of TPO on hematopoiesis. The long-term sequelae on the HSC pool in chronically lower TPO levels in our GPIbα-deficient mice or, more importantly, in human BSS patients, have not been explored and may be worthwhile for future studies.
A recent report identified the role of the platelet in hepatic TPO generation.21 This elegant study demonstrated that desialylated platelets drive hepatic TPO generation through the AMR. Interestingly, we observed that desialylation and asialofetuin did not significantly alter the inability of GPIbα−/− platelets to induce hepatic TPO generation (Figure 5). One possible explanation to reconcile our data with the previous report is that desialylated GPIbα on platelets is the binding partner of the AMR, which would not be unreasonable because GPIbα is the most heavily glycosylated platelet surface antigen.74 However, we do find increased binding to hepatocytes when GPIbα−/− platelets are desialylated, which is reduced in the presence of asialofetuin. These findings suggest that the AMR does not exclusively bind GPIbα. Furthermore, our data showed that blocking of the N-terminal ligand binding domain with monoclonal antibody NIT G can effectively inhibit platelet-mediated hepatic TPO generation (Figure 7B), suggesting that this is the site of the functional epitope that drives hepatic TPO generation. Given that there are no glycosylation sites at the N terminus of murine GPIbα, it is unlikely that this epitope binds the AMR. As well, it was recently shown that desialylated epitopes on GPIbα do not significantly bind the AMR.75 This evidence supports the idea of an AMR-independent pathway in GPIbα-mediated hepatic TPO generation. This may be also the reason why Aspgr1−/− (AMR-deficient) mice do not exhibit a significant decrease in plasma TPO.21 We propose that the AMR and GPIbα work synergistically, whereby the AMR attracts and binds desialylated platelets, which then brings GPIbα at a proximal advantage to induce TPO generation or vice versa. However, GPIbα is required, whereas desialylation enhances this process. Future investigations may open new therapeutic avenues for potential receptor agonists (eg, GPIbα-anchored liposomes) that could potentially serve as alternatives to TPO mimetics.
Although murine GPIbα does not have an NXS/T sequence motif for N-glycosylation, which contains the AMR high-affinity galactose residues,76 we cannot exclude the possibility that the AMR can recognize murine GPIbα O-glycan–linked galactose residues within the mucin-like region. As previous studies demonstrated, neuraminidase-treated murine GPIbα plays an important role in AMR-dependent clearance,30,77 suggesting the presence of potential AMR-recognition sites on murine GPIbα.76,78 We also showed that recombinant murine GPIbα–coupled beads stimulated hepatic TPO mRNA production, indicating that the GPIbα ectodomain is sufficient for the hepatocyte response (Figure 6J). However, it is still unclear whether the N terminus alone can mediate the AMR response or whether the mucin-like region is also required. Future studies are required to further elucidate the recognition pattern(s) of hepatic receptor(s).
In conclusion, our study demonstrates that de novo platelet-mediated synthesis of TPO in the liver requires GPIbα. These findings should have significant impact on our understating of TPO regulation and TPO therapies. These will also have broad implications in disease conditions (eg, BSS and immune-mediated auto- and alloimmune thrombocytopenias) and therapeutics that target GPIbα.79-84
Some of the data from this manuscript were presented at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5 December 2015; the Platelets 2016 International Symposium, Boston, MA, 20 September 2016; and the 2017 Congress of the International Society of Thrombosis and Haemostasis, Berlin, Germany, 10 July 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Richard O. Hynes (Massachusetts Institute of Technology, Boston, MA) for the generous gift of β3−/− mice and Reid C. Gallant and Niyati Malkani (Keenan Research Center, St. Michael’s Hospital) for editing the manuscript.
This work was supported by the Canadian Institutes of Health Research (MOP #97918 and MOP #119540), the Heart and Stroke Foundation of Canada (Ontario), and the Canadian Foundation for Innovation. M.X. is a recipient of a State Scholarship from the China Scholarship Council, an Ontario Trillium Scholarship, and a Graduate Fellowship from Canadian Blood Services. J.L. is a recipient of a Graduate Fellowship from Canadian Blood Services in Cardiovascular Studies. N.C. is a recipient of a Canadian Blood Services Postdoctoral Fellowship.
Authorship
Contribution: M.X. planned and carried out most of the experiments, analyzed data, and wrote the manuscript. J.L. carried out experiments, analyzed data, and wrote the manuscript. M.A.D.N., G.Z., N.C., R.Y., and S.G. carried out experiments and analyzed data. S.K. carried out experiments on and provided patient samples. J.W. and Z.M.R. provided the key GPIbα−/− and IL4Rα/GPIbα-tg mice. J.M., O.R., M.H., J.P., A.H.L., and D.R.B. provided valuable suggestions and comments during the research and manuscript preparation. J.F. provided key equipment and analyzed data. H.N. (principal investigator) supervised the research, analyzed data, and prepared the manuscript.
Conflict-of-interest disclosure: H.N. and G.Z. hold patents (United States patent application number 12/082686; Canadian patent application number 2628900; European patent application number 08153880.3) for the mouse anti-mouse monoclonal antibodies used in this study. A.H.L. receives research funding from CSL Behring and Rigel Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Heyu Ni, Department of Laboratory Medicine and Pathobiology, Department of Medicine and Department of Physiology, University of Toronto; Canadian Blood Services Centre for Innovation; Platform Director for Hematology, Cancer and Immunological Diseases, St. Michael’s Hospital, Room 421, LKSKI - Keenan Research Centre, 209 Victoria Street, Toronto, ON M5B 1W8, Canada; e-mail: nih@smh.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal