Key Points
Ponatinib continued to provide deep, durable responses in heavily pretreated patients with CP-CML.
Tolerability was acceptable in this heavily pretreated population with 5 years of follow-up.
Abstract
Ponatinib has potent activity against native and mutant BCR-ABL1, including BCR-ABL1T315I. The pivotal phase 2 Ponatinib Ph+ ALL and CML Evaluation (PACE) trial evaluated efficacy and safety of ponatinib at a starting dose of 45 mg once daily in 449 patients with chronic myeloid leukemia (CML) or Philadelphia chromosome–positive acute lymphoblastic leukemia (ALL) resistant/intolerant to dasatinib or nilotinib, or with BCR-ABL1T315I. This analysis focuses on chronic-phase CML (CP-CML) patients (n = 270) with 56.8-month median follow-up. Among 267 evaluable patients, 60%, 40%, and 24% achieved major cytogenetic response (MCyR), major molecular response (MMR), and 4.5-log molecular response, respectively. The probability of maintaining MCyR for 5 years was 82% among responders. Dose reductions were implemented in October 2013 to decrease the risk of arterial occlusive events (AOEs); ≥90% of CP-CML patients who had achieved MCyR or MMR maintained response 40 months after elective dose reductions. Estimated 5-year overall survival was 73%. In CP-CML patients, the most common treatment-emergent adverse events were rash (47%), abdominal pain (46%), thrombocytopenia (46%), headache (43%), dry skin (42%), and constipation (41%). The cumulative incidence of AOEs in CP-CML patients increased over time to 31%, while the exposure-adjusted incidence of new AOEs (15.8 and 4.9 per 100 patient-years in years 1 and 5, respectively) did not increase over time. These final PACE results demonstrate ponatinib provides durable and clinically meaningful responses, irrespective of dose reductions, in this population of heavily pretreated CP-CML patients. This trial was registered at www.clinicaltrials.gov as #NCT01207440.
Introduction
Ponatinib is a third-generation tyrosine kinase inhibitor (TKI) with potent activity against native BCR-ABL1 and clinically relevant resistant mutants, including the BCR-ABL1T315I gatekeeper mutant, which confers a high degree of resistance to all other currently available TKIs.1,2 Initially licensed in the United States in 2012 and the European Union in 2013, ponatinib is currently approved in >30 countries for use in adults with refractory chronic myeloid leukemia (CML) or Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) and those harboring the resistant BCR-ABL1T315I mutant.3,4 The pivotal phase 2 Ponatinib Ph+ ALL and CML Evaluation (PACE) trial evaluated the efficacy and safety of ponatinib at a starting dose of 45 mg once daily in CML or Ph+ ALL patients with resistance or intolerance to dasatinib or nilotinib, or with the BCR-ABL1T315I mutation.5 The primary results of the PACE trial demonstrated substantial responses to ponatinib in this heavily pretreated patient population with a median follow-up of 15 months.5 In the initial report, among 267 chronic-phase CML (CP-CML) patients evaluable for response, of whom >90% had received at least 2 prior approved TKIs (ie, at least 2 of the following: imatinib, dasatinib, nilotinib, and bosutinib), responses occurred rapidly and 56% met the primary end point of major cytogenetic response (MCyR) by 12 months. Twelve-month estimates of progression-free survival (PFS) and overall survival (OS) in CP-CML patients were 80% and 94%, respectively. Among patients with advanced disease, 55% of accelerated-phase CML (AP-CML), 31% of blast-phase CML (BP-CML), and 41% of Ph+ ALL patients achieved a major hematologic response (MaHR) by 6 months (the primary end point for these disease states). These CP-CML response rates appeared to be higher than those reported in patients who had received second-generation TKIs (nilotinib, dasatinib, and bosutinib) after resistance and/or intolerance to 2 prior TKIs.6-8
Parallel to the high response rates to ponatinib in patients with refractory CML or Ph+ ALL, with continued follow-up of the study an accumulation of arterial occlusive events (AOEs) was observed. Elective dose reductions were recommended for patients remaining in the study. Here, we report final data on the efficacy and safety of ponatinib in the PACE trial, ∼5 years after enrollment was completed, with a focus on the maintenance and depth of responses in patients with CP-CML and the impact of these responses on long-term outcomes, as well as the occurrence and clinical characteristics of AOEs over time in this population.
Patients and methods
Study design and patient eligibility
PACE was a phase 2 trial in adult patients with CML or Ph+ ALL who were resistant or intolerant to dasatinib or nilotinib, or who had the BCR-ABL1T315I mutation regardless of prior TKI use (N = 449; n = 270 CP-CML). The trial was recently completed and data as of the final database lock (6 February 2017) are reported. At the time of study closure, patients who continued to derive clinical benefit from ponatinib had the option to continue therapy outside the clinical trial. Details of the study design and eligibility criteria have been previously published.5 The starting dose for ponatinib was 45 mg once daily, and dose reductions to 30 mg or 15 mg once daily were applied to manage adverse events (AEs), per protocol, or implemented proactively following recommendations from the sponsor in October 2013 in response to concerns regarding an accumulation of AOEs with continued follow-up. Unless benefit-risk analysis justified treatment with a higher dose, the following dose reductions were recommended: 15 mg once daily for CP-CML patients with MCyR, and 30 mg once daily for CP-CML patients without MCyR, AP-CML patients, and BP-CML patients. Treatment was continued until disease progression (per protocol), intolerance, or the patient/investigator decision to stop treatment. This study was approved by local ethics committees and was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization guidelines for good clinical practice. All patients provided written informed consent.
Evaluations and analyses
Complete details of the initial efficacy and safety assessments have been previously published.5 The primary end point was MCyR by 12 months for patients with CP-CML and MaHR by 6 months for patients with AP-CML, BP-CML, or Ph+ ALL. Secondary end points included major molecular response (MMR), time to and duration of response, PFS, OS, and safety. The safety population included all patients who received at least 1 dose of ponatinib (N = 449); the efficacy population (n = 444), the basis for prespecified efficacy analyses, included all treated patients assigned to a cohort, excluding 5 treated patients who had not received dasatinib or nilotinib, and in whom the BCR-ABL1T315I mutation was not centrally confirmed at baseline.
Bone marrow aspirates and cytogenetic assessments were performed every 3 months in patients with CP-CML through the end of cycle 27 (cycles were 28 days), and at the end of cycles 1 and 2, every 2 months through cycle 24, and at cycle 27 in patients with AP-CML, BP-CML, or Ph+ ALL. After 27 cycles, CP-CML patients who were not in complete cytogenetic response (CCyR) continued to require a bone marrow aspirate and cytogenetic assessment every 6 cycles through cycle 39, and at least yearly thereafter; AP-CML, BP-CML, and Ph+ ALL patients who were not in CCyR continued to require a bone marrow aspirate and cytogenetic assessment every 3 cycles through cycle 39, and at least yearly thereafter. Patients in documented CCyR after cycle 27 were not required to have any further bone marrow aspirates, unless there was at least a 10-fold increase in BCR-ABL1 transcripts from nadir and the patient was not in MMR. Patients were followed for PFS and OS during ponatinib treatment and after discontinuation of ponatinib. For PFS, patients without progression were censored at the last response assessment, and for OS, patients who remained alive were censored at last contact.
AEs were monitored continuously and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. AOEs and venous thromboembolic events (VTEs) were categorized based on a broad collection of >400 Medical Dictionary for Regulatory Activities (MedDRA) preferred terms related to vascular ischemia or thrombosis. Exposure-adjusted AOE rates were calculated as: (number of first events in interval)/(total exposure for interval in patient-years) ×100.
Analyses were conducted to assess the relative risk of serious AOEs by baseline risk category in patients from the safety population for whom all baseline risk categories were available (N = 449). Risk categories analyzed included intrinsic factors (age and sex), commonly recognized cardiovascular risk factors on which data were collected (hypertension, hypercholesterolemia, diabetes, and obesity), and history of heart disease (ischemic or nonischemic). The association between serious AOEs and the indicated risk category was described with a risk ratio and associated 95% confidence interval (CI).
Results
Patient disposition
Between September 2010 and October 2011, 449 patients were enrolled, including 270 CP-CML, 85 AP-CML, 62 BP-CML, and 32 Ph+ ALL patients; baseline characteristics have been reported previously (Table 1).5 Median age at baseline was 59 years (range, 18–94 years), and 47% were female. Most (93%) patients had previously received at least 2 approved TKIs and 56% of patients had previously received at least 3 approved TKIs. Nearly one-third (29%) of patients had the BCR-ABL1T315I mutation at study entry. The results presented here reflect data analysis as of 6 February 2017, with median follow-up of 37.3 months for all patients and 56.8 months (range, 0.1-73.1 months) for CP-CML patients. Time from the last enrolled patient’s first visit to date of analysis was 64.1 months. Patients with more advanced disease experienced higher rates of discontinuation due to progressive disease and death (Table 1).
Baseline and end-of-study characteristics
| . | CP-CML, n = 270 . | AP-CML, n = 85 . | BP-CML, n = 62 . | Ph+ ALL, n = 32 . | Total,* N = 449 . |
|---|---|---|---|---|---|
| Characteristic at baseline | |||||
| Median age (range), y | 60 (18-94) | 60 (23-82) | 53 (18-74) | 62 (20-80) | 59 (18-94) |
| Female, n (%) | 126 (47) | 48 (56) | 25 (40) | 12 (38) | 211 (47) |
| Previous use of approved TKIs, n (%)† | |||||
| ≥2 drugs | 251 (93) | 80 (94) | 60 (97) | 26 (81) | 417 (93) |
| ≥3 drugs | 154 (57) | 47 (55) | 37 (60) | 12 (38) | 250 (56) |
| Median duration of previous treatment with approved TKIs (range), y† | 5.4 (0.4-13.3) | 5.1 (0.3-12.1) | 2.0 (0.1-11.6) | 1.2 (0.1-8.2) | 4.6 (0.1-13.3) |
| Resistant or intolerant to dasatinib or nilotinib, n (%) | |||||
| Resistant | 215 (80) | 74 (87) | 59 (95) | 27 (84) | 375 (84) |
| Intolerant only | 39 (14) | 6 (7) | 2 (3) | 2 (6) | 49 (11) |
| Both resistant and intolerant | 52 (19) | 11 (13) | 13 (21) | 5 (16) | 81 (18) |
| Mutation status, n (%)‡ | |||||
| No mutation detected | 138 (51) | 40 (47) | 17 (27) | 3 (9) | 198 (44) |
| BCR-ABL1T315I | 64 (24) | 18 (21) | 24 (39) | 22 (69) | 128 (29) |
| Best response to most recent regimen containing dasatinib or nilotinib, n (%)§ | |||||
| MaHR or better‖ | ND | 17 (21) | 9 (15) | 13 (43) | ND |
| MCyR or better¶ | 66 (26) | 12 (15) | 7 (11) | 8 (27) | ND |
| MMR | 8 (3) | 2 (3) | 1 (2) | 5 (17) | ND |
| Patient disposition at end of study | |||||
| Median duration of treatment, mo (range) | 32.1 (0.1-73.0) | 19.4 (0.5-71.3) | 2.9 (0.03-59.1) | 2.7 (0.1-39.3) | 16.7 (0.03-73.0) |
| Median follow-up, mo (range) | 56.8 (0.1-73.1) | 32.3 (3.6-71.8) | 6.2 (0.1-66.4) | 5.4 (0.1-59.6) | 37.3 (0.1-73.1) |
| Median dose intensity, mg/d (range) | 27.2 (5-45) | 33.1 (6-45) | ND | 0 | ND |
| Primary reason for discontinuation, n (%) | |||||
| Disease progression | 29 (11) | 26 (31) | 32 (52) | 18 (56) | 105 (23) |
| Adverse event | 57 (21) | 10 (12) | 9 (15) | 3 (9) | 79 (18) |
| Patient request | 31 (11) | 7 (8) | 3 (5) | 1 (3) | 42 (9) |
| Lack of efficacy | 15 (6) | 6 (7) | 1 (2) | 4 (13) | 26 (6) |
| Death# | 9 (3) | 5 (6) | 7 (11) | 5 (16) | 26 (6) |
| Investigator decision | 11 (4) | 5 (6) | 1 (2) | 0 | 17 (4) |
| Lost to follow-up | 0 | 3 (4) | 0 | 0 | 3 (<1) |
| Noncompliance | 3 (1) | 1 (1) | 0 | 0 | 4 (<1) |
| Protocol violation | 2 (<1) | 0 | 0 | 0 | 2 (<1) |
| Study closure** | 90 (33) | 14 (16) | 3 (5) | 0 | 107 (24) |
| Other**,†† | 14 (5) | 7 (8) | 6 (10) | 1 (3) | 28 (6) |
| . | CP-CML, n = 270 . | AP-CML, n = 85 . | BP-CML, n = 62 . | Ph+ ALL, n = 32 . | Total,* N = 449 . |
|---|---|---|---|---|---|
| Characteristic at baseline | |||||
| Median age (range), y | 60 (18-94) | 60 (23-82) | 53 (18-74) | 62 (20-80) | 59 (18-94) |
| Female, n (%) | 126 (47) | 48 (56) | 25 (40) | 12 (38) | 211 (47) |
| Previous use of approved TKIs, n (%)† | |||||
| ≥2 drugs | 251 (93) | 80 (94) | 60 (97) | 26 (81) | 417 (93) |
| ≥3 drugs | 154 (57) | 47 (55) | 37 (60) | 12 (38) | 250 (56) |
| Median duration of previous treatment with approved TKIs (range), y† | 5.4 (0.4-13.3) | 5.1 (0.3-12.1) | 2.0 (0.1-11.6) | 1.2 (0.1-8.2) | 4.6 (0.1-13.3) |
| Resistant or intolerant to dasatinib or nilotinib, n (%) | |||||
| Resistant | 215 (80) | 74 (87) | 59 (95) | 27 (84) | 375 (84) |
| Intolerant only | 39 (14) | 6 (7) | 2 (3) | 2 (6) | 49 (11) |
| Both resistant and intolerant | 52 (19) | 11 (13) | 13 (21) | 5 (16) | 81 (18) |
| Mutation status, n (%)‡ | |||||
| No mutation detected | 138 (51) | 40 (47) | 17 (27) | 3 (9) | 198 (44) |
| BCR-ABL1T315I | 64 (24) | 18 (21) | 24 (39) | 22 (69) | 128 (29) |
| Best response to most recent regimen containing dasatinib or nilotinib, n (%)§ | |||||
| MaHR or better‖ | ND | 17 (21) | 9 (15) | 13 (43) | ND |
| MCyR or better¶ | 66 (26) | 12 (15) | 7 (11) | 8 (27) | ND |
| MMR | 8 (3) | 2 (3) | 1 (2) | 5 (17) | ND |
| Patient disposition at end of study | |||||
| Median duration of treatment, mo (range) | 32.1 (0.1-73.0) | 19.4 (0.5-71.3) | 2.9 (0.03-59.1) | 2.7 (0.1-39.3) | 16.7 (0.03-73.0) |
| Median follow-up, mo (range) | 56.8 (0.1-73.1) | 32.3 (3.6-71.8) | 6.2 (0.1-66.4) | 5.4 (0.1-59.6) | 37.3 (0.1-73.1) |
| Median dose intensity, mg/d (range) | 27.2 (5-45) | 33.1 (6-45) | ND | 0 | ND |
| Primary reason for discontinuation, n (%) | |||||
| Disease progression | 29 (11) | 26 (31) | 32 (52) | 18 (56) | 105 (23) |
| Adverse event | 57 (21) | 10 (12) | 9 (15) | 3 (9) | 79 (18) |
| Patient request | 31 (11) | 7 (8) | 3 (5) | 1 (3) | 42 (9) |
| Lack of efficacy | 15 (6) | 6 (7) | 1 (2) | 4 (13) | 26 (6) |
| Death# | 9 (3) | 5 (6) | 7 (11) | 5 (16) | 26 (6) |
| Investigator decision | 11 (4) | 5 (6) | 1 (2) | 0 | 17 (4) |
| Lost to follow-up | 0 | 3 (4) | 0 | 0 | 3 (<1) |
| Noncompliance | 3 (1) | 1 (1) | 0 | 0 | 4 (<1) |
| Protocol violation | 2 (<1) | 0 | 0 | 0 | 2 (<1) |
| Study closure** | 90 (33) | 14 (16) | 3 (5) | 0 | 107 (24) |
| Other**,†† | 14 (5) | 7 (8) | 6 (10) | 1 (3) | 28 (6) |
ALL, acute lymphoblastic leukemia; AP, accelerated phase; BP, blast phase; CCyR, complete cytogenetic response; CML, chronic myeloid leukemia; CP, chronic phase; MaHR, major hematologic response; MCyR, major cytogenetic response; MMR, major molecular response; ND, not determined; PCyR, partial cytogenetic response; Ph, Philadelphia chromosome; TKI, tyrosine kinase inhibitor.
Includes 5 patients (3 CP-CML, 2 AP-CML) who were not assigned to a cohort (postimatinib, non-T315I at baseline) but were treated.
Approved TKIs were imatinib, nilotinib, dasatinib, and bosutinib. Previous investigational TKIs received by at least 1% of patients included radotinib (received by 2% of patients), bafetinib (2%), rebastinib (2%), and XL-228 (2%).
Assessed by conventional Sanger sequencing at baseline.
Percentages were calculated according to the number of patients who received previous dasatinib or nilotinib: 256 patients with CP-CML, 80 patients with AP-CML, 61 patients with BP-CML, and 30 patients with Ph+ ALL.
This category includes MaHR, PCyR, CCyR, and MMR.
This category includes PCyR, CCyR, and MMR.
Seven deaths were assessed by investigators as possibly or probably related to ponatinib (CP-CML: pneumonia, acute myocardial infarction; AP-CML: fungal pneumonia, gastrointestinal hemorrhage; BP-CML: hemorrhagic gastritis; Ph+ ALL: cardiac arrest, mesenteric arterial occlusion).
Patients who continued to derive clinical benefit from their treatment had the option to receive ponatinib through alternative mechanisms.
This category includes stem cell transplantation (in 11 patients with CP-CML, 5 with AP-CML, 6 with BP-CML, and 1 with Ph+ ALL). The 9 CP-CML patients and 1 AP-CML patient who remained on study at the time of last response assessment are not included in this category.
In the overall safety population, 304 patients (68%) had at least 1 dose reduction and 320 patients (71%) had a dose interruption of at least 3 days at any time; among CP-CML patients, these rates were 82% (221 of 270) and 82% (221 of 270), respectively. Most patients with dose reductions already had their dose reduced prior to October 2013; as of 3 September 2013, 59% of patients (265 of 449), including 71% of CP-CML patients (192 of 270), had at least 1 dose reduction.
Efficacy in patients with CP-CML
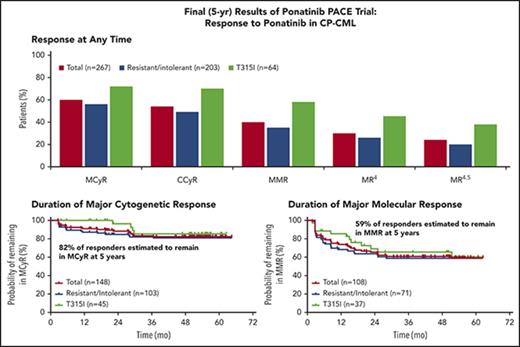
The rates of response at any time among the 267 evaluable patients with CP-CML are shown in Figure 1A (three CP-CML patients who had not received prior dasatinib or nilotinib did not have T315I confirmed at baseline and, per protocol, were not evaluable for efficacy). A total of 159 CP-CML patients (60%) achieved MCyR at any time, of whom 144 (54% of evaluable CP-CML patients) achieved CCyR. Additionally, 108 patients (40%) achieved MMR and 64 (24%) achieved 4.5-log molecular response (MR4.5; ie, ≤0.0032% BCR-ABL1IS or undetectable disease in complementary DNA [cDNA] with ≥32 000 ABL1 transcripts). Molecular and cytogenetic responses were achieved rapidly, with median times to MCyR, CCyR, and MMR among those who achieved response of 2.8 months (range, 1.6-58.0 months), 2.9 months (range, 1.6-58.0 months), and 5.5 months (range, 1.8-55.4 months), respectively. Supplemental Figure 1 (available on the Blood Web site) reports the time to MCyR, CCyR, and MMR for all evaluable patients.
Efficacy of ponatinib in patients with CP-CML, overall, and among patients resistant or intolerant to previous treatment with dasatinib or nilotinib or with the BCR-ABL1T315I mutation. (B-C) Results are shown in CP-CML patients remaining on study as of the last response assessment. (B-E) The 95% confidence intervals are shown. (A) Response at any time. MR4 is the 4-log molecular response (≤0.01% BCR-ABL1IS or undetectable disease in cDNA with ≥10 000 ABL1 transcripts); MR4.5 is the 4.5-log molecular response (≤0.0032% BCR-ABL1IS or undetectable disease in cDNA with ≥32 000 ABL1 transcripts). MCyR and MMR rates in patients who were resistant to dasatinib or nilotinib were 54% and 41%, respectively. (B) Duration of MCyR. Patients who achieved MCyR by 12 months (n = 148) are shown. Because of a data correction between the original PACE publication and the current report, 148 (55%) rather than 149 (56%) of 267 CP-CML patients achieved MCyR by 12 months. Of 267 CP-CML patients evaluated for efficacy, 148 achieved MCyR, and 21 of these patients lost MCyR, leaving 127 (48%) of 267 CP-CML patients with continuous MCyR as of the last response assessment. *Failed to meet criteria for MCyR in 2 consecutive assessments ≥28 days apart, or discontinued after a single assessment in which the criteria for MCyR were not met. †Kaplan-Meier estimate. (C) Duration of MMR. Patients who achieved MMR at any time are shown. Of 267 CP-CML patients evaluated for efficacy, 108 achieved MMR, and 28 of these patients lost MMR, leaving 80 (30%) of 267 CP-CML patients with continuous MMR as of last response assessment. *Failed to meet criteria for MMR at any single time point after initial response. †Kaplan-Meier estimate. (D) PFS. Progression from CP was defined as death, development of AP or BP, loss of complete hematologic response (in absence of cytogenetic response), loss of MCyR, or increasing white blood cell count without complete hematologic response. (E) OS.
Efficacy of ponatinib in patients with CP-CML, overall, and among patients resistant or intolerant to previous treatment with dasatinib or nilotinib or with the BCR-ABL1T315I mutation. (B-C) Results are shown in CP-CML patients remaining on study as of the last response assessment. (B-E) The 95% confidence intervals are shown. (A) Response at any time. MR4 is the 4-log molecular response (≤0.01% BCR-ABL1IS or undetectable disease in cDNA with ≥10 000 ABL1 transcripts); MR4.5 is the 4.5-log molecular response (≤0.0032% BCR-ABL1IS or undetectable disease in cDNA with ≥32 000 ABL1 transcripts). MCyR and MMR rates in patients who were resistant to dasatinib or nilotinib were 54% and 41%, respectively. (B) Duration of MCyR. Patients who achieved MCyR by 12 months (n = 148) are shown. Because of a data correction between the original PACE publication and the current report, 148 (55%) rather than 149 (56%) of 267 CP-CML patients achieved MCyR by 12 months. Of 267 CP-CML patients evaluated for efficacy, 148 achieved MCyR, and 21 of these patients lost MCyR, leaving 127 (48%) of 267 CP-CML patients with continuous MCyR as of the last response assessment. *Failed to meet criteria for MCyR in 2 consecutive assessments ≥28 days apart, or discontinued after a single assessment in which the criteria for MCyR were not met. †Kaplan-Meier estimate. (C) Duration of MMR. Patients who achieved MMR at any time are shown. Of 267 CP-CML patients evaluated for efficacy, 108 achieved MMR, and 28 of these patients lost MMR, leaving 80 (30%) of 267 CP-CML patients with continuous MMR as of last response assessment. *Failed to meet criteria for MMR at any single time point after initial response. †Kaplan-Meier estimate. (D) PFS. Progression from CP was defined as death, development of AP or BP, loss of complete hematologic response (in absence of cytogenetic response), loss of MCyR, or increasing white blood cell count without complete hematologic response. (E) OS.
Among CP-CML patients, responses were durable, with 82% and 59% of those who achieved MCyR by 12 months and MMR at any time, respectively, estimated to maintain these responses at 5 years, based on Kaplan-Meier methods (Figure 1B-C). At the time of last response assessment, the median durations of MCyR and MMR had not been reached. Kaplan-Meier–estimated PFS and OS at 5 years were 53% and 73%, respectively (Figure 1D-E). Of the 267 evaluable patients with CP-CML at study entry, 9 (3%) transformed to AP-CML (n = 5) or BP-CML (n = 4), with a median time to transformation of 6.4 months (range, 0.2-30.3 months). Of these 9 patients, 6 had not achieved MCyR.
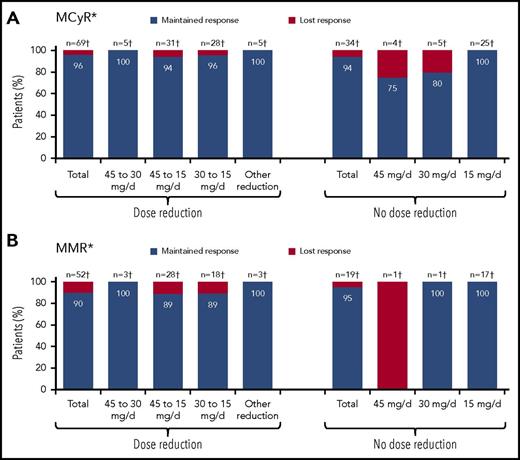
A post hoc analysis was performed to assess the effect of prospective dose reductions on maintenance of response. As of 10 October 2013, 145 CP-CML patients remained on ponatinib. Dose reductions were implemented in 86 of these patients in response to prospective recommendations. Of the other 59 CP-CML patients, most (88%; 52 of 59) were already receiving a reduced dose. Rates of MCyR and MMR maintenance were high, regardless of preemptive dose reduction (Figure 2). Of 69 CP-CML patients who were in MCyR as of 10 October 2013 and had their dose reduced, 66 (96%) maintained MCyR after dose reduction. Similarly, 90% of CP-CML patients in MMR (47 of 52) as of October 2013 who had a dose reduction maintained MMR following the dose reduction. Among CP-CML patients without preemptive dose reduction (per investigator decision), 94% of patients (32 of 34) and 95% of patients (18 of 19) maintained MCyR and MMR, respectively, after October 2013.
Maintenance of response following prospective dose reduction in October 2013 in CP-CML patients. (A) MCyR maintenance. (B) MMR maintenance. Of the 42 CP-CML patients without MCyR who remained on study as of 10 October 2013, 17 had a dose reduction (45 to 30 mg per day, n = 7; 45 to 15 mg per day, n = 2; 30 to 15 mg per day, n = 6; other reduction, n = 2), whereas 3, 6, and 16 patients continued to receive 45, 30, and 15 mg per day, respectively. *Response maintained as of last response assessment. †Number of patients with response as of 10 October 2013.
Maintenance of response following prospective dose reduction in October 2013 in CP-CML patients. (A) MCyR maintenance. (B) MMR maintenance. Of the 42 CP-CML patients without MCyR who remained on study as of 10 October 2013, 17 had a dose reduction (45 to 30 mg per day, n = 7; 45 to 15 mg per day, n = 2; 30 to 15 mg per day, n = 6; other reduction, n = 2), whereas 3, 6, and 16 patients continued to receive 45, 30, and 15 mg per day, respectively. *Response maintained as of last response assessment. †Number of patients with response as of 10 October 2013.
Efficacy in patients with advanced disease
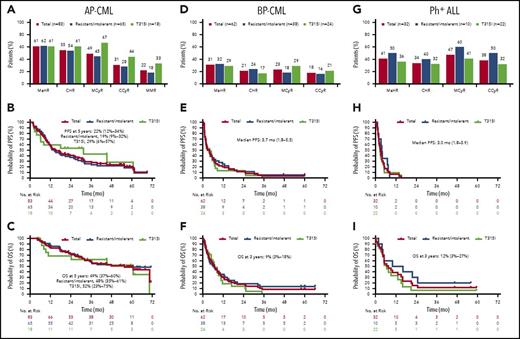
Among patients with AP-CML (median follow-up, 32.3 months), MaHR was achieved by 61% of patients (51 of 83) (Figure 3A), with a median time to response of 0.7 (range, 0.4-17.2) months in these patients. Among the 47 patients (57%) who achieved MaHR within the first 6 months (primary end point), the median duration of response was 12.9 months (range, 1.1-68.3 months). MCyR, CCyR, and MMR were achieved by 49% (41 of 83), 31% (26 of 83), and 22% (18 of 83) of patients, respectively (Figure 3A). Kaplan-Meier–estimated PFS and OS at 5 years were 22% and 49%, respectively (Figure 3B-C).
Efficacy in patients with advanced disease, overall, and among patients resistant or intolerant to previous treatment with dasatinib or nilotinib or with the BCR-ABL1T315Imutation. (A-C) AP-CML, (D-F) BP-CML, and (G-I) Ph+ ALL. (B-C,E-F,H-I) The 95% confidence intervals are shown. BP-CML includes myeloid BP (n = 52) and lymphoid BP (n = 10). (A) AP-CML: Response at any time. (B) AP-CML: PFS. Progression from AP was defined as death, development of confirmed BP, loss of previous major or minor hematologic response over a 2-week period, or no decrease from baseline levels in percentage of blasts in peripheral blood or bone marrow on all assessments over a 4-week period, or increasing blasts over a 4-week period. (C) AP-CML: OS. (D) BP-CML: Response at any time. (E) BP-CML: PFS. Progression from BP was defined as death, or increasing blasts in peripheral blood or bone marrow over a 4-week period. (F) BP-CML: OS. (G) Ph+ ALL: Response at any time. (H) Ph+ ALL: PFS. (I) Ph+ ALL: OS. NE, not evaluable.
Efficacy in patients with advanced disease, overall, and among patients resistant or intolerant to previous treatment with dasatinib or nilotinib or with the BCR-ABL1T315Imutation. (A-C) AP-CML, (D-F) BP-CML, and (G-I) Ph+ ALL. (B-C,E-F,H-I) The 95% confidence intervals are shown. BP-CML includes myeloid BP (n = 52) and lymphoid BP (n = 10). (A) AP-CML: Response at any time. (B) AP-CML: PFS. Progression from AP was defined as death, development of confirmed BP, loss of previous major or minor hematologic response over a 2-week period, or no decrease from baseline levels in percentage of blasts in peripheral blood or bone marrow on all assessments over a 4-week period, or increasing blasts over a 4-week period. (C) AP-CML: OS. (D) BP-CML: Response at any time. (E) BP-CML: PFS. Progression from BP was defined as death, or increasing blasts in peripheral blood or bone marrow over a 4-week period. (F) BP-CML: OS. (G) Ph+ ALL: Response at any time. (H) Ph+ ALL: PFS. (I) Ph+ ALL: OS. NE, not evaluable.
Among patients with BP-CML (median follow-up, 6.2 months), 31% of patients (19 of 62) achieved MaHR (Figure 3D); median time to MaHR was 1.0 month (range, 0.4-3.7 months) and the median duration of MaHR was 6.0 months (range, 1.8-59.5 months) in these patients. MMR, MCyR, and CCyR were achieved by 13% of patients (8 of 62), 23% of patients (14 of 62), and 18% of patients (11 of 62), respectively. Median PFS was 3.7 months, and Kaplan-Meier–estimated OS at 3 years was 9% (Figure 3E-F).
Among patients with Ph+ ALL (median follow-up, 5.4 months; Figure 3F), the MaHR rate was 41% (13 of 32). Median time to MaHR among responders was 0.7 months (range, 0.4-5.5 months). The median duration of MaHR in responders with Ph+ ALL was 3.2 months (range, 1.8-12.8 months). MCyR and CCyR were achieved by 47% (15 of 32) and 38% (12 of 32) of patients, respectively (Figure 3G). Median PFS was 3.0 months, and OS in patients with Ph+ ALL at 3 years was 12% (Figure 3H-I).
Safety
Treatment-emergent AEs (TEAEs) occurring in ≥20% of all patients are listed in Table 2 by disease state. The most common any-grade TEAEs observed in CP-CML patients (with frequency ≥40%) were rash (47%), abdominal pain (46%), thrombocytopenia (46%), headache (43%), dry skin (42%), and constipation (41%). The most common grade 3/4 TEAEs in CP-CML patients (with frequency ≥10% [n ≥ 27]) were thrombocytopenia (35%), neutropenia (17%), hypertension (14%), increased lipase (13%), abdominal pain (10%), and anemia (10%). The type and incidence of the most common TEAEs were generally consistent across all disease states. Individual serious AEs reported in ≥5% of CP-CML patients were pancreatitis (7%), atrial fibrillation (6%), pneumonia (6%), and angina pectoris (5%). In the overall population, serious AEs reported in ≥5% of patients (excluding disease progression) were pneumonia (7%) and pancreatitis (6%); atrial fibrillation and angina pectoris were reported in 4% and 3% of patients, respectively.
Treatment-emergent AEs
| . | CP-CML, n = 270 . | AP-CML, n = 85 . | BP-CML, n = 62 . | Ph+ ALL, n = 32 . | Total, N = 449 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Nonhematologic AEs, n (%) | ||||||||||
| Abdominal pain | 125 (46) | 28 (10) | 36 (42) | 7 (8) | 21 (34) | 5 (8) | 10 (31) | 2 (6) | 192 (43) | 42 (9) |
| Rash* | 127 (47) | 10 (4) | 32 (38) | 4 (5) | 22 (35) | 3 (5) | 7 (22) | 1 (3) | 188 (42) | 18 (4) |
| Constipation | 112 (41) | 7 (3) | 25 (29) | 2 (2) | 17 (27) | 0 | 17 (53) | 1 (3) | 171 (38) | 10 (2) |
| Headache | 116 (43) | 9 (3) | 26 (31) | 1 (1) | 19 (31) | 2 (3) | 8 (25) | 0 | 169 (38) | 12 (3) |
| Dry skin | 114 (42) | 9 (3) | 27 (32) | 1 (1) | 16 (26) | 1 (2) | 8 (25) | 0 | 165 (37) | 11 (2) |
| Fatigue | 81 (30) | 6 (2) | 32 (38) | 4 (5) | 16 (26) | 3 (5) | 9 (28) | 0 | 138 (31) | 13 (3) |
| Hypertension† | 99 (37) | 37 (14) | 22 (26) | 9 (11) | 13 (21) | 5 (8) | 8 (25) | 3 (9) | 142 (32) | 54 (12) |
| Pyrexia | 70 (26) | 3 (1) | 34 (40) | 6 (7) | 23 (37) | 2 (3) | 8 (25) | 0 | 135 (30) | 11 (2) |
| Arthralgia | 90 (33) | 8 (3) | 29 (34) | 2 (2) | 12 (19) | 0 | 4 (13) | 0 | 135 (30) | 10 (2) |
| Nausea | 79 (29) | 2 (<1) | 27 (32) | 0 | 21 (34) | 1 (2) | 7 (22) | 0 | 134 (30) | 3 (<1) |
| Diarrhea | 54 (20) | 2 (<1) | 25 (29) | 2 (2) | 15 (24) | 2 (3) | 4 (13) | 1 (3) | 98 (22) | 7 (2) |
| Increased lipase | 73 (27) | 34 (13) | 13 (15) | 11 (13) | 9 (15) | 8 (13) | 3 (9) | 2 (6) | 98 (22) | 55 (12) |
| Vomiting | 50 (19) | 4 (1) | 23 (27) | 0 | 17 (27) | 1 (2) | 8 (25) | 0 | 98 (22) | 5 (1) |
| Myalgia | 65 (24) | 3 (1) | 18 (21) | 0 | 11 (18) | 0 | 2 (6) | 0 | 96 (21) | 3 (<1) |
| Pain in extremity | 65 (24) | 8 (3) | 17 (20) | 0 | 8 (13) | 0 | 4 (13) | 0 | 94 (21) | 8 (2) |
| Hematologic AEs, n (%) | ||||||||||
| Thrombocytopenia | 123 (46) | 95 (35) | 45 (53) | 37 (44) | 23 (37) | 22 (35) | 7 (22) | 6 (19) | 198 (44) | 160 (36) |
| Neutropenia | 53 (20) | 45 (17) | 31 (37) | 31 (37) | 22 (35) | 18 (29) | 8 (25) | 7 (22) | 114 (25) | 101 (22) |
| Anemia | 53 (20) | 28 (10) | 31 (37) | 19 (22) | 21 (34) | 20 (32) | 8 (25) | 6 (19) | 113 (25) | 73 (16) |
| . | CP-CML, n = 270 . | AP-CML, n = 85 . | BP-CML, n = 62 . | Ph+ ALL, n = 32 . | Total, N = 449 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Nonhematologic AEs, n (%) | ||||||||||
| Abdominal pain | 125 (46) | 28 (10) | 36 (42) | 7 (8) | 21 (34) | 5 (8) | 10 (31) | 2 (6) | 192 (43) | 42 (9) |
| Rash* | 127 (47) | 10 (4) | 32 (38) | 4 (5) | 22 (35) | 3 (5) | 7 (22) | 1 (3) | 188 (42) | 18 (4) |
| Constipation | 112 (41) | 7 (3) | 25 (29) | 2 (2) | 17 (27) | 0 | 17 (53) | 1 (3) | 171 (38) | 10 (2) |
| Headache | 116 (43) | 9 (3) | 26 (31) | 1 (1) | 19 (31) | 2 (3) | 8 (25) | 0 | 169 (38) | 12 (3) |
| Dry skin | 114 (42) | 9 (3) | 27 (32) | 1 (1) | 16 (26) | 1 (2) | 8 (25) | 0 | 165 (37) | 11 (2) |
| Fatigue | 81 (30) | 6 (2) | 32 (38) | 4 (5) | 16 (26) | 3 (5) | 9 (28) | 0 | 138 (31) | 13 (3) |
| Hypertension† | 99 (37) | 37 (14) | 22 (26) | 9 (11) | 13 (21) | 5 (8) | 8 (25) | 3 (9) | 142 (32) | 54 (12) |
| Pyrexia | 70 (26) | 3 (1) | 34 (40) | 6 (7) | 23 (37) | 2 (3) | 8 (25) | 0 | 135 (30) | 11 (2) |
| Arthralgia | 90 (33) | 8 (3) | 29 (34) | 2 (2) | 12 (19) | 0 | 4 (13) | 0 | 135 (30) | 10 (2) |
| Nausea | 79 (29) | 2 (<1) | 27 (32) | 0 | 21 (34) | 1 (2) | 7 (22) | 0 | 134 (30) | 3 (<1) |
| Diarrhea | 54 (20) | 2 (<1) | 25 (29) | 2 (2) | 15 (24) | 2 (3) | 4 (13) | 1 (3) | 98 (22) | 7 (2) |
| Increased lipase | 73 (27) | 34 (13) | 13 (15) | 11 (13) | 9 (15) | 8 (13) | 3 (9) | 2 (6) | 98 (22) | 55 (12) |
| Vomiting | 50 (19) | 4 (1) | 23 (27) | 0 | 17 (27) | 1 (2) | 8 (25) | 0 | 98 (22) | 5 (1) |
| Myalgia | 65 (24) | 3 (1) | 18 (21) | 0 | 11 (18) | 0 | 2 (6) | 0 | 96 (21) | 3 (<1) |
| Pain in extremity | 65 (24) | 8 (3) | 17 (20) | 0 | 8 (13) | 0 | 4 (13) | 0 | 94 (21) | 8 (2) |
| Hematologic AEs, n (%) | ||||||||||
| Thrombocytopenia | 123 (46) | 95 (35) | 45 (53) | 37 (44) | 23 (37) | 22 (35) | 7 (22) | 6 (19) | 198 (44) | 160 (36) |
| Neutropenia | 53 (20) | 45 (17) | 31 (37) | 31 (37) | 22 (35) | 18 (29) | 8 (25) | 7 (22) | 114 (25) | 101 (22) |
| Anemia | 53 (20) | 28 (10) | 31 (37) | 19 (22) | 21 (34) | 20 (32) | 8 (25) | 6 (19) | 113 (25) | 73 (16) |
Treatment-emergent AEs of any grade occurring in ≥20% of the total population are listed.
AE, adverse event. Other abbreviations are explained in Table 1.
Combines the terms erythematous, macular, and papular rash.
At baseline, 379 of 449 patients (84%) had elevated blood pressure (212 of 449 [47%] had blood pressure ≥140/90 mm Hg); 307 of 449 patients (68%) experienced any increase from baseline in blood pressure on study.
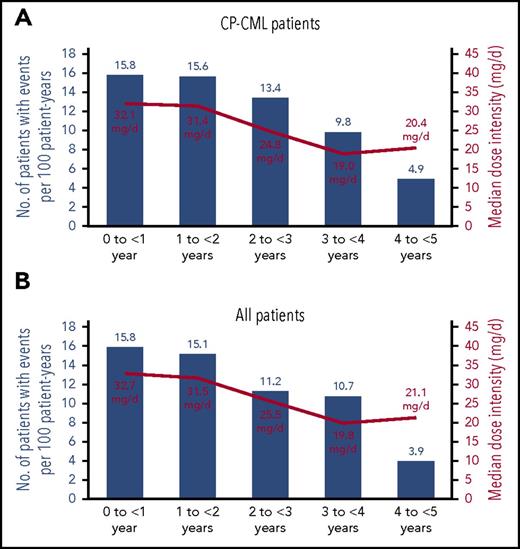
Table 3 summarizes cumulative incidences of treatment-emergent AOEs, including cardiovascular, cerebrovascular, and peripheral vascular events, and treatment-emergent VTEs, as well as the incidence of AOEs and VTEs on an exposure-adjusted basis. AOEs included a collection of MedDRA preferred terms in which no individual term occurred in >10% of patients (supplemental Table 1). The cumulative incidence of AOEs continued to increase over time, but the exposure-adjusted incidence of newly occurring AOEs remained relatively constant throughout the study period in both CP-CML patients and all patients (Figure 4). The lack of an increase in exposure-adjusted incidence of newly occurring AOEs was accompanied by a decrease in median dose intensity in years 1 to 4 (Figure 4). Among 111 total patients who had at least 1 AOE, the median time to initial onset of an AOE was 13.4 months (range, 0.1-59.7 months); by subtype of AOE, the median times to initial onset of cardiovascular, cerebrovascular, and peripheral AOEs were 11.5 months (range, 0.3-59.7 months), 20.1 months (range, 0.2–53.5 months), and 19.9 months (range, 0.1–58.5 months), respectively. Dose at initial onset was 45 mg in 42% of patients, 30 mg in 24%, and 15 mg in 26%; initial onset of first AOE occurred after study discontinuation in 7% of patients (in these patients, time of onset ranged from 1 to 56 days from last dose). In the total population, 5 patients had grade 5 AOEs: 3 patients with CP-CML had an acute myocardial infarction (n = 1), a cerebrovascular accident (n = 1), and a hemorrhagic cerebral infarction (n = 1), and 2 patients with Ph+ ALL had a mesenteric arterial occlusion (n = 1) and cerebral ischemia and peripheral ischemia (n = 1, both events in the same patient). There were no reported grade 5 VTEs.
Cumulative and exposure-adjusted incidences of treatment-emergent AOEs and VTEs
| . | CP-CML, n = 270 . | Total, N = 449 . | ||
|---|---|---|---|---|
| AE . | SAE . | AE . | SAE . | |
| AOEs, n (%) | 84 (31)* | 69 (26)† | 111 (25)‡ | 90 (20)§ |
| Cardiovascular | 42 (16) | 33 (12) | 59 (13) | 44 (10) |
| Cerebrovascular | 35 (13) | 28 (10) | 41 (9) | 33 (7) |
| Peripheral vascular | 38 (14) | 31 (11) | 48 (11) | 38 (8) |
| Exposure-adjusted AOEs, no. of patients with events per 100 patient-years | 14.1 | 10.9 | 13.8 | 10.6 |
| VTEs, n (%) | 15 (6) | 13 (5) | 27 (6) | 23 (5) |
| Exposure-adjusted VTEs, no. of patients with events per 100 patient-years | 2.1 | 1.8 | 2.8 | 2.4 |
| . | CP-CML, n = 270 . | Total, N = 449 . | ||
|---|---|---|---|---|
| AE . | SAE . | AE . | SAE . | |
| AOEs, n (%) | 84 (31)* | 69 (26)† | 111 (25)‡ | 90 (20)§ |
| Cardiovascular | 42 (16) | 33 (12) | 59 (13) | 44 (10) |
| Cerebrovascular | 35 (13) | 28 (10) | 41 (9) | 33 (7) |
| Peripheral vascular | 38 (14) | 31 (11) | 48 (11) | 38 (8) |
| Exposure-adjusted AOEs, no. of patients with events per 100 patient-years | 14.1 | 10.9 | 13.8 | 10.6 |
| VTEs, n (%) | 15 (6) | 13 (5) | 27 (6) | 23 (5) |
| Exposure-adjusted VTEs, no. of patients with events per 100 patient-years | 2.1 | 1.8 | 2.8 | 2.4 |
Categorization of AOEs and VTEs is based on a broad collection of >400 MedDRA preferred terms related to vascular ischemia or thrombosis.
AE, total adverse event (including SAEs); AOE, arterial occlusive event; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event only (designated as serious by the investigator, in accordance with standard regulatory criteria); VTE, venous thromboembolic event. Other abbreviations are explained in Table 1.
Forty-six patients had >1 AOE.
Thirty-one patients had >1 serious AOE.
Fifty-seven patients had >1 AOE.
Thirty-eight patients had >1 serious AOE.
Exposure-adjusted yearly incidence rates for newly occurring arterial occlusive events and median dose intensity by year. (A) CP-CML patients. For CP-CML patients, in the 5 intervals shown (0 to <1 year, 1 to <2 years, 2 to <3 years, 3 to <4 years, and 4 to <5 years): 32, 21, 14, 8, and 3 patients had events, respectively, of 270, 152, 121, 91, and 73 patients in each interval, respectively. (B) All patients. For all patients, in the 5 intervals shown (0 to <1 year, 1 to <2 years, 2 to <3 years, 3 to <4 years, and 4 to <5 years): 47, 27, 15, 11, and 3 patients had events, respectively, of 449, 212, 158, 115, and 93 patients in each interval, respectively. Median follow-up was 56.8 months for CP-CML patients and 37.3 months for all patients.
Exposure-adjusted yearly incidence rates for newly occurring arterial occlusive events and median dose intensity by year. (A) CP-CML patients. For CP-CML patients, in the 5 intervals shown (0 to <1 year, 1 to <2 years, 2 to <3 years, 3 to <4 years, and 4 to <5 years): 32, 21, 14, 8, and 3 patients had events, respectively, of 270, 152, 121, 91, and 73 patients in each interval, respectively. (B) All patients. For all patients, in the 5 intervals shown (0 to <1 year, 1 to <2 years, 2 to <3 years, 3 to <4 years, and 4 to <5 years): 47, 27, 15, 11, and 3 patients had events, respectively, of 449, 212, 158, 115, and 93 patients in each interval, respectively. Median follow-up was 56.8 months for CP-CML patients and 37.3 months for all patients.
When considering risk factors for the development of serious AOEs (including commonly recognized cardiovascular risk factors on which data were collected [hypertension, hypercholesterolemia, diabetes, and obesity] or a history of ischemic disease, nonischemic cardiac disease, or venous thromboembolism), patients without any of the indicated risk factors had a lower relative risk of developing serious AOEs (0.4 [95% CI, 0.2-0.7]); patients with 1 risk factor had an intermediate relative risk (0.8 [95% CI, 0.5-1.2]); and patients with ≥2 risk factors had the highest relative risk (2.2 [95% CI, 1.5-3.3]). Relative risk based on each risk factor is shown in supplemental Figure 2.
Previous analyses of the PACE study suggested that AOEs are dose-related, with a 15-mg reduction in average daily dose intensity predicted to lead to ∼33% reduction in the risk of AOEs.9-11 At the time of this analysis, a total of 48 of 449 patients (11%) had ponatinib dose modifications (reductions and/or interruptions) due to AOEs: 37 dose interruption only, 6 dose reduction only, and 5 both; 17 of 449 patients (4%) discontinued ponatinib because of an AOE. Of 270 CP-CML patients, 35 (13%) had dose modifications due to AOEs: 26 dose interruption only, 4 dose reduction only, and 5 both; 16 of 270 patients (6%) discontinued ponatinib because of an AOE. When VTEs were considered, 3 additional patients in the overall population interrupted treatment (2 with CP-CML) and 4 additional patients in the overall population discontinued treatment (all CP-CML patients). A post hoc analysis was performed to assess the effect of prospective dose reductions on AOEs. Rates of AOEs occurring after the prospective dose reductions in October 2013 are shown in Table 4. Among CP-CML patients with no prior AOEs, the percentage of patients who had a first AOE occurring after October 2013 was 19% (12 of 63) for those who had preemptive dose reduction and 18% (8 of 44) for those who maintained treatment at the same dose; a summary of AOEs in patients with preemptive dose reduction to 15 mg is presented in supplemental Table 2.
Occurrence of AOEs following prospective dose reduction in October 2013 in CP-CML patients with no prior AOEs as of 10 October 2013
| . | Occurrence of AOEs in patients with dose reduction after October 2013 . | Occurrence of AOEs in patients with no dose reduction after October 2013 . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | 45 to 30 mg/d . | 45 to 15 mg/d . | 30 to 15 mg/d . | Other reduction . | Total . | 45 mg/d . | 30 mg/d . | 15 mg/d . | |
| Denominator* | 63 | 10 | 26 | 24 | 3 | 44 | 5 | 10 | 29 |
| AOE, n (%) | 12 (19) | 1 (10) | 4 (15)† | 6 (25)† | 1 (33) | 8 (18) | 1 (20) | 1 (10) | 6 (21) |
| No AOE, n (%) | 51 (81) | 9 (90) | 22 (85) | 18 (75) | 2 (67) | 36 (82) | 4 (80) | 9 (90) | 23 (79) |
| . | Occurrence of AOEs in patients with dose reduction after October 2013 . | Occurrence of AOEs in patients with no dose reduction after October 2013 . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | 45 to 30 mg/d . | 45 to 15 mg/d . | 30 to 15 mg/d . | Other reduction . | Total . | 45 mg/d . | 30 mg/d . | 15 mg/d . | |
| Denominator* | 63 | 10 | 26 | 24 | 3 | 44 | 5 | 10 | 29 |
| AOE, n (%) | 12 (19) | 1 (10) | 4 (15)† | 6 (25)† | 1 (33) | 8 (18) | 1 (20) | 1 (10) | 6 (21) |
| No AOE, n (%) | 51 (81) | 9 (90) | 22 (85) | 18 (75) | 2 (67) | 36 (82) | 4 (80) | 9 (90) | 23 (79) |
Patients are classified according to the lowest dose received after October 2013.
Number of patients with no AOEs occurring before 10 October 2013.
See supplemental Table 2 for additional information.
A total of 56 deaths (in 12% of all patients) occurred on study or within 30 days of the end of study treatment. Twelve occurred in CP-CML patients (4% of all CP-CML patients), including 4 deaths due to disease progression; 10 of these deaths occurred in AP-CML patients and 34 occurred in BP-CML or Ph+ ALL patients. The most common reasons for death (in >2 patients overall) were disease progression (n = 26), sepsis/septic shock (n = 5), and cardiac arrest (n = 3). Seven of the deaths occurring within 30 days of the last dose of ponatinib were assessed by investigators as possibly or probably related to ponatinib (n = 1 for each: CP-CML: pneumonia, acute myocardial infarction; AP-CML: fungal pneumonia, gastrointestinal hemorrhage; BP-CML: hemorrhagic gastritis; Ph+ ALL: cardiac arrest, mesenteric arterial occlusion).
Discussion
After 5 years of follow-up of the PACE trial, ponatinib provided clinical benefit for patients with heavily pretreated CML or Ph+ ALL. In the initial report, 56% of CP-CML patients achieved the primary end point of MCyR by 12 months,5 and in this report the probability of maintaining MCyR at 5 years was 82%. Considering the extent of prior exposure to multiple TKIs in this patient population, these results compare favorably with those of second-generation TKIs in both the second-line setting and later lines.8,12-18 In studies of CP-CML patients who received their second TKI after resistance or intolerance to imatinib only: 57% achieved MCyR while receiving dasatinib (100 mg once daily), with 87% of these patients maintaining MCyR at 2 years13 ; 51% achieved MCyR while receiving nilotinib, with 62% of these patients having MCyR lasting >18 months16 ; and 59% achieved or maintained baseline MCyR while receiving bosutinib, with a 77% probability of maintaining MCyR at 2 years.17 In the only other prospective evaluation of a TKI for patients treated with 2 or (rarely) 3 prior TKIs, 32% of CP-CML patients achieved MCyR with bosutinib, and the probability of maintaining MCyR at 2 years was 59%.8 Responses in CP-CML patients in PACE have deepened over time, with 24% of CP-CML patients having achieved MR4.5 at any time. Maintenance of response, including deep response, was high among CP-CML patients irrespective of dose reductions; 40 months after prospective dose reductions, there was no detectable difference in the rates of MCyR or MMR in CP-CML patients with or without dose reductions. It should be noted that dose reductions were more profound (to 15 mg daily) in patients with deeper responses (at least MCyR). This may influence the durability of response, particularly for those with a lesser response at the time of dose reduction. This analysis does not address the effect of initiating therapy with a lower dose, an important issue that is being addressed by an ongoing randomized clinical trial (NCT02467270). For patients with advanced disease, initial responses were rapid,5 and durability of response was similar to that observed with second-generation TKI therapy for advanced disease after imatinib therapy (65% and 77% maintenance of MCyR at 12 months with bosutinib and nilotinib, respectively, in AP-CML; median duration of MCyR of ∼4-10 months with dasatinib and ∼7 months with bosutinib in BP-CML).19-21
Responses achieved by CP-CML patients in PACE correlated with favorable long-term outcomes, with estimated PFS/OS rates at 5 years of 53%/73% overall, and with comparable rates in the resistant/intolerant and BCR-ABL1T315I cohorts. Long-term estimated OS rates among CP-CML patients with the BCR-ABL1T315I mutation, in particular, were in contrast to those that have been reported for any other approved TKI.22 Previous multivariate analyses conducted to evaluate the impact of clinical factors on the response rates observed in the PACE study have shown that the presence of a T315I mutation is not an independent predictor of better response.23 Rather, patients with T315I tended to have characteristics that are associated with higher response rates (ie, T315I patients were generally younger, more recently diagnosed, and treated with fewer prior TKIs, and they received higher doses of ponatinib).5 In a separate analysis, no single or compound mutation was identified that consistently conferred primary and/or secondary resistance to ponatinib in CP-CML patients.24 Therefore, ponatinib is effective in CP-CML patients irrespective of baseline mutation status.
The types of AEs reported with ponatinib in this 5-year follow-up were generally similar to those reported previously.5,25,26 A previous analysis of CP-CML patients in the PACE trial revealed that treatment-related AEs were associated with a distinct temporal profile, with most events (eg, thrombocytopenia, rash, and pancreatitis) occurring early in therapy, typically within the first 3 months.27 Consistent with this profile, with continued follow-up the number of discontinuations due to an AE reflects that the majority of these discontinuations occurred early, within the first 15 months of therapy.5
The continued follow-up of patients in the PACE trial has elucidated the vascular occlusive event profile in this population. In the initial report of the PACE trial, the cumulative incidence of AOEs across all disease states was 17.1% (serious AOEs, 11.8%), with 2 years of follow-up.5 In this 5-year follow-up, the cumulative incidence was 25% in the overall population (serious AOEs, 20%) and 31% in the CP-CML population (serious AOEs, 26%); the higher cumulative incidence in CP-CML correlates with the longer duration of treatment in this report. No individual AOE in the broad collection of MedDRA preferred terms used in this study occurred in >10% of patients (supplemental Table 1). Although the cumulative incidence of AOEs continued to increase over time, the exposure-adjusted incidence of newly occurring AOEs did not increase with longer duration of ponatinib treatment. The lack of increase in exposure-adjusted AOE incidence in later years could be due to the natural history or etiology of AOEs, dose reductions, or change in the patient population (ie, enrichment of patients who have not had vascular events and may be inherently at lower risk of experiencing such events). It is thus difficult to know whether lower doses might carry lower risks in patients with risk factors. A previously published time-to-event modeling analysis suggested a lag of up to 6 months between dose reduction and the development of some AOEs.11 In this study, of 10 patients who had a first AOE following preemptive dose reduction to 15 mg after October 2013, 4 had the event within 6 months (supplemental Table 2). Patients receiving ponatinib should be monitored for high blood pressure, evidence of arterial occlusive or thromboembolic events, and reduced cardiac function.3,4 These conditions should be managed as clinically indicated, and ponatinib dosing should be reduced, interrupted, or discontinued as needed.3,4,28-30 Further research on the mechanisms of vascular events with TKIs is ongoing, and may help determine more specific interventions to mitigate or eliminate risk.31
Although the mechanistic basis for ponatinib-associated AOEs is unknown, this vascular toxicity appears to be dose-related9-11 and modified by preexisting cardiovascular disease and other risk factors. Interestingly, of the mechanisms known to contribute to the development of cardiovascular disease that have been examined thus far, ponatinib therapy does not appear to modify platelets32 or the plasma lipid profile.33 Ponatinib inhibits vascular endothelial growth factor receptors and other targets known to regulate vascular homeostasis (TIE2, platelet-derived growth factor receptors, fibroblast growth factor receptors, and ephrin receptor family members), but the effect on AOEs is unknown. In contrast to AOEs, VTEs do not appear to be dose-related,9-11 and the frequency of VTEs in this study was within the range observed in the general cancer population.34,35
Of note, vascular occlusive events have been reported with other BCR-ABL1 TKIs.29,36 A recent meta-analysis37 highlighted the variability in the clinical definitions and adjudication of these events, as well as differences in patient selection criteria and patient populations, which make the actual incidence and nature of vascular events difficult to compare across studies. In our analysis, we used a very broad definition of these events with more than 400 MedDRA terms included. The inclusiveness of the analysis is not clear in all other reports, making assessments of the comparative incidence of vascular occlusive events across reports with different drugs impossible based on the available literature. Still, in a trial of nilotinib in newly diagnosed CP-CML patients, the rate of cardiovascular events was 9.3% with nilotinib 300 mg twice daily and 15.2% with nilotinib 400 mg twice daily after a median of 5 years.16 With dasatinib in newly diagnosed CP-CML patients, cardiac ischemic events (3.9%), cardiac-related fluid retention (8.5%), conduction system abnormalities (7.0%), and transient ischemic attacks (0.8%) occurred with 5 years of follow-up.38 With a median treatment duration of 11.1 months, vascular AEs, including hypertension, have been reported in patients treated with bosutinib, although the incidence was not different from that associated with imatinib.39 Although the pathophysiologies underlying the vascular toxicities associated with TKIs are unclear, preexisting comorbidities, particularly cardiovascular risk factors, are associated with an increased risk of such events.29 The finding that vascular occlusive events are observed in patients treated with different BCR-ABL1 TKIs suggests that the drugs may share a common mechanism of vascular toxicity.
In summary, these final results of the PACE study support ponatinib as an effective treatment of patients with CML who have received prior therapies. The decision to initiate ponatinib therapy should be guided by carefully weighing the risk-to-benefit ratio for each patient, particularly in those who may be at increased risk of AOEs. Appropriate dose reduction/interruption, and active monitoring and management of preexisting conditions, are important to mitigate risk in patients receiving ponatinib treatment. A prospective dose-ranging trial (NCT02467270, the Optimizing Ponatinib Treatment in CML [OPTIC] trial) is under way to evaluate the impact of 3 starting doses (45, 30, and 15 mg per day), as well as dose reductions for patients with response at predetermined time points, on the safety and efficacy of ponatinib in patients with refractory CP-CML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, their families, and their caregivers; the PACE investigators and their team members at each study site; and colleagues from ARIAD Pharmaceuticals, Inc (a wholly owned subsidiary of Takeda Pharmaceutical Company Limited [ARIAD]), MolecularMD, and the CML community. M.B. acknowledges his colleague, Fausto Castagnetti, for overseeing the treatment of PACE patients in Bologna, Italy. Professional medical writing assistance was provided by Lela Creutz of Peloton Advantage, LLC (Parsippany, NJ), and editorial assistance was provided by Jessica Tyler (ARIAD) and Blythe Thomson (formerly of ARIAD).
J.F.A. acknowledges the support of the Imperial College National Institute for Health Research Biomedical Research Centre. This work was supported by research funding from ARIAD to the institutions of J.E.C., D.-W.K., J.P.-I., P.D.l.C., R.P., C.C., F.E.N., J.F.A., H.J.K., M.T., D.J.D., E.A., D.R., M.B., M.C.M., C.G.-P., F.G., M.W.D., A.H., and T.P.H. Professional medical writing assistance was funded by ARIAD.
Authorship
Contribution: J.E.C., M.T., M.B., F.G., M.W.D., A.H., T.P.H., and N.P.S. were members of the PACE Steering Committee; J.E.C., D.-W.K., J.P.-I., P.D.l.C., R.P., C.C., F.E.N., J.F.A., H.J.K., M.T., D.J.D., E.A., D.R., M.B., M.C.M., C.G.-P., F.G., M.W.D., A.H., and T.P.H. enrolled patients and/or collected data; and all authors analyzed or interpreted data, wrote or critically reviewed the manuscript, and agreed upon the final version.
Conflict-of-interest disclosure: J.E.C. has received research funding from ARIAD, Bristol-Myers Squibb, Novartis, Pfizer, and Teva, and has served as a consultant for ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer. D.-W.K. has received research funding from ARIAD. J.P.-I. has received honoraria from Bristol-Myers Squibb; has received research funding from ARIAD and Novartis; and has served as a consultant or speaker for ARIAD, Bristol-Myers Squibb, Novartis, Pfizer, and Teva. P.D.l.C. has received honoraria from ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer, and research funding from ARIAD. R.P. has received honoraria from, and has served as a speaker for, ARIAD, Bristol-Myers Squibb, Incyte, and Novartis; has served in a consulting or advisory role for AstraZeneca; and has received research funding from ARIAD. C.C. has received research funding from ARIAD. F.E.N. has received honoraria from ARIAD, Bristol-Myers Squibb, and Novartis; has served in a consulting or advisory role for Bristol-Myers Squibb and Novartis; has served on speakers’ bureaus for ARIAD, Bristol-Myers Squibb, and Novartis; has received research funding from ARIAD and Novartis; and has received travel, accommodations, or other expense reimbursements from Bristol-Myers Squibb and Novartis. J.F.A. has received honoraria from ARIAD, Bristol-Myers Squibb, MSD, Novartis, and Pfizer, and has received research funding from ARIAD and Novartis. H.J.K. received honoraria and research funding from ARIAD. M.T. has served in a consulting or advisory role for ARIAD, Novartis, and Pfizer; has received research funding from ARIAD, Incyte, Novartis, Pfizer, and Sanofi; and has received travel, accommodations, or other expense reimbursements from ARIAD, Incyte, Novartis, and Pfizer. D.J.D. has served in a consulting or advisory role for Amgen, ARIAD, Bristol-Myers Squibb, Incyte, Novartis, and Pfizer, and has received research funding from ARIAD. E.A. has served in a consulting or advisory role for ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer, and has received research funding from ARIAD. D.R. has received honoraria for nonpromotional oral presentations from, and has served on advisory boards for, ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer, and has received research funding from ARIAD. M.B. has served as a consultant and speaker for, and received honoraria from, ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer, and has received research funding from ARIAD. M.C.M. has received honoraria for consulting or advisory roles and research funding from ARIAD, Bristol-Myers Squibb, and Novartis. C.G.-P. has served in an advisory role to Bristol-Myers Squibb and Pfizer and has received research funding from ARIAD. S.L. is an employee of, and may own stock/stock options in, ARIAD. V.M.R. is an employee of, and may own stock/stock options in, ARIAD. F.G.H. was an employee of, and may have owned stock/stock options in, ARIAD at the time the work was conducted. F.G. has received honoraria from Novartis and Pfizer; has served in a consulting or advisory role for Celgene; has received travel, accommodations, or other expense reimbursements from Novartis; and has received research funding from ARIAD. M.W.D. has served in a consulting or advisory role for ARIAD, Bristol-Myers Squibb, Incyte, Novartis, and Pfizer; has received travel, accommodations, or other expense reimbursements from ARIAD, Bristol-Myers Squibb, CTI BioPharma, Novartis, and Pfizer; has provided expert testimony for Bristol-Myers Squibb; has received honoraria from ARIAD, Bristol-Myers Squibb, CTI BioPharma, Incyte, Novartis, and Pfizer; and has received research funding from ARIAD, Bristol-Myers Squibb, Celgene, Incyte, and Novartis. A.H. has received research funding from ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer. T.P.H. has received honoraria from, has served in a consulting or advisory role for, and has received research funding from ARIAD, Bristol-Myers Squibb, and Novartis, and has received travel, accommodations, or other expense reimbursements from Bristol-Myers Squibb and Novartis. N.P.S. has received research funding from ARIAD, Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer, and Plexxikon. H.M.K. has received research funding from Amgen, Bristol-Myers Squibb, Novartis, and Pfizer.
The current affiliation for M.C.M. is Institute for Hematology and Oncology (IHO GmbH), Mannheim, Germany.
H. Jean Khoury died on 22 May 2017.
Correspondence: Jorge E. Cortes, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030-4008; e-mail: jcortes@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal