Key Points
Risk-adapted therapy allowed achieving remarkable cure rates in an international trial on childhood APL.
Reduction of the anthracycline cumulative dose coupled with ATRA extended use does not compromise the outcome of children with APL.
Abstract
Pediatric acute promyelocytic leukemia (APL) can be cured with all-trans retinoic acid (ATRA) and anthracycline. However, most published trials have employed high cumulative doses of anthracyclines. Here, we report the outcome of newly diagnosed APL patients enrolled in the International Consortium for Childhood APL (ICC-APL-01) trial, which reduced anthracycline exposure but extended that of ATRA. The study recruited 258 children/adolescents with molecularly/cytogenetically proven APL. Patients were stratified into standard-risk (SR) and high-risk (HR) groups according to baseline white blood cell counts (<10 × 109/L or ≥10 × 109/L); both groups received identical induction treatment with ATRA and 3 doses of idarubicin. Two or 3 blocks of consolidation therapy were administered to SR and HR patients, respectively, while maintenance therapy with low-dose chemotherapy and ATRA cycles was given to all patients for 2 years. The cumulative dose of daunorubicin equivalent anthracyclines in SR and HR patients was lower than that of previous studies (355 mg/m2 and 405 mg/m2, respectively). Hematologic remission was obtained in 97% of patients; 8 children died of intracranial hemorrhage in the first 2 weeks following diagnosis. Five-year overall and event-free survival for the whole cohort were 94.6% and 79.9%, respectively; they were 98.4% and 89.4% in SR patients and 84.3% and 74.2% in HR patients (P = .002 and P = .043, respectively). These data demonstrate that extended use of ATRA coupled to a risk-adapted consolidation can achieve high cure rates in childhood APL and limit anthracycline exposure. The trial was registered at www.clinicaltrials.gov as EudractCT 2008-002311-40.
Introduction
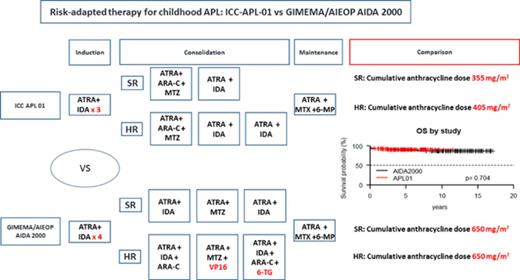
Acute promyelocytic leukemia (APL) is rare in children, although its incidence differs by geographical area. In the United States, as in Central and Northern Europe, APL accounts for 5% to 7% of all pediatric acute myeloid leukemias (AMLs), while a higher frequency (15% to 20%) is reported in children of Latino/Hispanic descent.1-6 Because of the disease’s rarity, a single national pediatric hematology group annually recruits a small number of patients, and, thus, childhood APL has historically been treated on adult protocols. Since 1993, Italian patients <18 years of age have been enrolled into 2 consecutive multicenter APL trials (AIDA0493 designed by GIMEMA [Gruppo Italiano Malattie Ematologiche dell’Adulto] and subsequently AIDA2000 in collaboration with AIEOP [Associazione Italiana di Ematologia e Oncologia Pediatrica]) enrolling adults and children with newly diagnosed APL.1,7 Both treatment protocols combined all-trans retinoic acid (ATRA) and idarubicin in induction and delivered anthracycline-based consolidation.1,7-9 In AIDA2000, consolidation was based on the Sanz risk score,10 and cytarabine (ARA-C), etoposide, and 6-thioguanine were omitted for non–high-risk (non-HR) patients. The anthracycline cumulative dosage was high (650 mg/m2 of anthracycline daunorubicin equivalence) and was identical in standard-risk (SR) and HR patients (details in supplemental Figure 1, available on the Blood Web site). The results, analyzed separately for adults and children, showed overall survival (OS) ≥80% in both age groups.1,7-9 However, high anthracycline doses are associated with a significant risk of both acute and long-term sequelae (eg, cardiomyopathy and second neoplasms), a significant obstacle for achieving long-term favorable outcome, especially in children with a long life expectancy.
Based on these considerations, we conducted a prospective study (ICC-APL-01 trial) in the framework of the International Consortium for Childhood APL for treatment of children/adolescents with newly diagnosed APL. The ICC-APL-01 trial was designed with the aim of reducing the cumulative anthracyclines doses while maintaining an excellent likelihood of cure and of investigating the effects on patients’ outcome of a risk-adapted consolidation therapy based on the initial white blood cell (WBC) count. Moreover, we evaluated the efficacy of an extended use of ATRA during consolidation.
We hereby report the results of this international study compared with those achieved by children in the previous risk-adapted Italian AIDA2000 trial. The AIDA2000 results in children have been updated for the present analysis.
Materials and methods
The ICC-APL-01 trial for children/adolescents with newly diagnosed APL started in January 2008 and involved 8 pediatric cooperative groups/countries (AIEOP, the Children’s Cancer and Leukaemia Group [United Kingdom], Grupo Argentino de Tratamiento de la Leucemia Aguda, French Group for Childhood ALL, the Dutch Childhood Oncology Group, the Nordic Society of Pediatric Haematology and Oncology [NOPHO], and the Belgian Society of Pediatric Hematology and Oncology, with a single center in both Brazil and Israel). Patient data were collected using a Web-based system developed by CINECA (an Italian nonprofit interuniversity consortium) and housed in Bologna (Italy).
Eligibility
Patients aged 1 to 21 years (18 years was the upper age limit in Italy, the Netherlands, and NOPHO) with newly diagnosed APL, subsequently confirmed as positive for PML-RARα, NPM1-RARα, or NUMA-RARα fusion transcripts, were eligible for enrollment. Since the disease represents a hematologic emergency requiring that ATRA be started as soon as the diagnosis is suspected, patients could have commenced ATRA before the diagnosis was molecularly and/or cytogenetically confirmed. However, molecular and cytogenetic confirmation was a mandatory requirement for eligibility. Other eligibility criteria were no cardiac contraindications to anthracycline-based chemotherapy, written informed consent from parents/relatives, and negative pregnancy test result (for females of childbearing age). The protocol was approved by the national and local ethical committees at each treatment center, according to national requirements, prior to patients’ enrollment. ICC-APL-01 was conducted in accordance with the EU Directive for Good Clinical Practice in Clinical Trials.
Study design and treatment
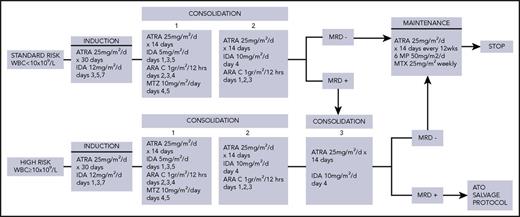
Details of the ICC-APL-01 protocol are shown in Figure 1. Patients were stratified into 2 risk categories according to the baseline WBC count; those with a WBC <10 × 109 /L were classified as SR and, those with an WBC count ≥10 × 109 /L were classified as HR.
ICC-APL-01 study protocol design. 6-MP, 6-mercaptopurine; IDA, idarubicin; MTX, methotrexate; MTZ, mitoxantrone.
ICC-APL-01 study protocol design. 6-MP, 6-mercaptopurine; IDA, idarubicin; MTX, methotrexate; MTZ, mitoxantrone.
ATRA was included in all phases (induction, consolidation, and maintenance). For both SR and HR, induction consisted of oral ATRA (pediatric dose of 25 mg/m2 per day), administered for 30 consecutive days and idarubicin (3 doses). Following induction, SR and HR patients received 2 or 3 consolidation blocks, respectively. Each consolidation course was started after peripheral blood count recovery (neutrophil count ≥1.0 × 109/L and platelet count ≥100 × 109/L). ATRA was administered, at the same induction dose, for 14 consecutive days. It was associated in the first block with intermediate-dose (ID) ARA-C and mitoxantrone, in the second block with idarubicin and in the third block with ID-ARA-C and idarubicin. Intrathecal ARA-C (dose adjusted according to age) was given at the start of each consolidation block. SR patients, still positive for the PML-RARα transcript at the end of the second consolidation block, received a third block identical to that of HR. All patients who tested polymerase chain reaction (PCR) negative for PML/RARα at the end of consolidation were given maintenance with low-dose chemotherapy (oral 6-mercaptopurine daily and methotrexate weekly) and ATRA (this drug given for the first 14 days every 12 weeks). Total maintenance duration was 2 years.
Patients who proved PCR positive after third consolidation block completion were considered to have resistant disease and thus eligible for refractory/relapsed salvage therapy, including arsenic trioxide (ATO) ± gemtuzumab-ozogamicin (GO) ± ATRA. Refractory/relapsed patients who were PML-RARα positive after salvage therapy were candidates for an allogeneic hematopoietic stem cell transplant (HSCT), while for molecularly negative patients, an individualized treatment with minimal residual disease (MRD) monitoring was suggested. Patients with isolated central nervous system relapse received systemic and intrathecal chemotherapy (HD-ARA-C + GO + ATRA and triple intrathecal therapy) and cranial radiotherapy (2400 cGy), unless previous radiation had been delivered as part of a transplant procedure.
Supportive measures
It was recommended that platelets and fresh frozen plasma be transfused to maintain the platelet count >50 × 109/L and the fibrinogen level >1.5g/dL, respectively, during the first 10 days of induction or longer in the presence of a coagulopathy. Factor VIIa infusion was permitted only in cases of severe bleeding refractory to conventional treatment.
For HR patients, dexamethasone (5 mg/m2 per dose; maximum single dose 10 mg every 12 hours) was recommended during the first 5 induction days as ATRA-differentiation syndrome (DS; defined as unexplained fever, weight gain, respiratory distress, interstitial pulmonary infiltrates, and pleural/pericardial effusion, with or without an elevated WBC count) prophylaxis.11,12 At the earliest sign of DS and prior to the development of the full-blown syndrome, ATRA was temporarily discontinued and dexamethasone was administered IV (5 mg/m2 every 12 hours) until resolution of signs and symptoms, and for a minimum of 3 days. Furosemide was added if clinically required. Pseudotumor cerebri (PC; defined as the presence of severe headache with nausea, vomiting, papilledema, and visual disorders) was treated with analgesics, diuretics, and transitory ATRA discontinuation. In case of ATRA-related hepatotoxicity (increase in serum bilirubin, hepatic transaminases, or alkaline phosphatase >5 times the normal upper level), temporary ATRA suspension was recommended. The protocol specified necessary dose modifications during treatment (renal or liver impairment). A lumbar puncture was not performed at diagnosis to avoid the risk of hemorrhage but was delayed until beginning of the first consolidation course.
Response evaluation during and after treatment
At diagnosis, bone marrow (BM) samples were sent to the designated national reference laboratory for molecular analysis in order to define the underlying molecular subtype (including definition of breakpoint location) and as a baseline for subsequent MRD monitoring. In addition to PML-RARα transcript analysis, patients were investigated for the presence of FLT3 abnormalities in laboratories of participating centers where this analysis could be performed. Central morphological BM samples review was organized by each participating national group where this was part of standard practice. BM samples for immunophenotype and cytogenetics, including fluorescence in situ hybridization, were obtained at diagnosis; these investigations were carried out locally and/or nationally according to the national policy. At hematopoietic recovery after induction and each consolidation course, a BM sample was tested by quantitative reverse-transcriptase PCR (RT-PCR) for the PML-RARα fusion transcript. Thereafter, BM was monitored every 3 months during maintenance and for one year after treatment completion. Samples were taken before ATRA therapy during each 12-weekly cycle and sent to the national laboratory. In case of a doubtful or positive PCR result in a previously negative patient, a further BM was tested within 2/4 weeks to confirm the results. Immunophenotype and cytogenetics were systematically performed in case of relapse.
Outcome definition and statistical analysis
Hematologic complete remission (HCR) was defined as a normally regenerating BM with <5% blasts by morphology, together with the achievement of an absolute neutrophil count > 1.0 × 109/L and a platelet count >100 × 109/L. Cytogenetic remission was defined as the disappearance of the diagnostic clonal translocation, and molecular remission (mCR) as the absence of PML-RARα transcript by BM RT-PCR or quantitative RT-PCR, with an assay sensitivity of at least 10−4. Treatment failures were defined as follows: early death (ED), any death occurring before HCR; death in CR, any death occurring in HCR; resistant/refractory disease, the persistent detection of morphologic, cytogenetic, and/or molecular evidence of APL after consolidation. A relapse was defined hematologically as >5% abnormal BM promyelocytes, cytogenetically as the reappearance of the t(15;17) by karyotype and/or fluorescence in situ hybridization in patients previously in cytogenetic remission, and molecularly as the reappearance of the PML-RARα transcript in 2 successive samples in patients previously in mCR.
A descriptive analysis of the demographic and clinical characteristics of patients was performed, including median and range for continuous variables and absolute and relative frequencies for categorical variables. Nonparametric tests were used to evaluate differences among groups (Fisher exact test and Wilcoxon test for categorical and continuous variables, respectively).
OS was defined as the time from diagnosis to death due to any cause or date of last follow-up for patients alive; event-free survival (EFS) was calculated from diagnosis to relapse (either hematologic or molecular), persistence of PCR positivity at consolidation completion, or death from any cause or date of the last follow-up for patients in mCR. Survival curves (OS and EFS) were estimated according to the Kaplan-Meier product-limit method and tested for significant differences between groups using the log-rank test. The cumulative incidence of relapse (CIR) was calculated from the time of HCR to relapse (hematologic or molecular) and it estimated using the cumulative incidence method considering death in HCR as the competing risk; the Gray test was applied to test differences between subgroups. In all analyses, 95% confidence intervals (CIs) were reported for the main summary statistics, and all statistical comparisons were based on 2-tailed tests, accepting P ≤ .05 as significant. All analyses were performed using SAS software (version 9.4) and R (http://www.R-project.org).
Results
Two hundred and fifty-eight children and adolescents with molecularly/cytogenetically proven APL (all PML-RARα positive) were registered. The present analysis was performed in August 2017 with a median follow-up period of 4.4 years (range, 0.1-9.5). The distribution of patients according to different groups/countries was as follows: AIEOP, 102; Children’s Cancer and Leukaemia Group, 46; Brazil, 37; GATLA, 29; French Group for Childhood ALL, 24; Israel, 7; NOPHO, 6; Dutch Childhood Oncology Group, 5; and Belgian Society of Pediatric Hematology and Oncology, 2 patients. Patient characteristics, shown in Table 1, were compared with those of the 127 patients enrolled in the Italian AIDA2000 trial. The 2 cohorts had similar clinical and biological characteristics; no significant differences in sex and initial WBC count were found, although ICC-APL-01 patients had a significantly higher WBC count than AIDA2000 patients (6.3 vs 3.6 × 109/L, respectively; P = .13); consequently, the percentage of HR patients was slightly higher in the former protocol (42% vs 33%; P = .09). The incidence of microgranular variant (M3v) was identical in the 2 cohorts (17%) and similar to that observed in adults. M3v was more frequently associated with HR (35.5% vs 4.1% in SR). FLT3 gene mutations were investigated in 133 out of 258 patients (52%) registered in the ICC-APL-01 trial, and a FLT3-ITD was found in 33 (25%). The presence of FLT3-ITD was associated with a higher WBC count (P = .005) and a higher frequency of the BCR3 short isoform of the PML/RARα gene (P = .04); no significant correlation was found between FLT3-ITD and the M3v subtype (P = .6), platelet count (P = .22), age (P = .14), sex (P = .31), or countries participating in the trial.
Baseline features of children enrolled in ICC-APL-01 and GIMEMA/AIEOP AIDA2000 trials
| Characteristics . | ICC-APL-01 (n = 258) . | AIDA2000 (n = 127) . | P . |
|---|---|---|---|
| Sex, male/female | 121/137 | 77/50 | .01 |
| Age, y | .26 | ||
| Median | 10.3 | 11.9 | |
| Minimum-maximum | 1.1-20.7 | 1.1-18.0 | |
| WBC count, ×109/L | .13 | ||
| Median | 6.3 | 3.6 | |
| Minimum-maximum | 0.08-339 | 0.2-187.0 | |
| Platelet count, ×109/L | .78 | ||
| Median | 23.0 | 27.56 | |
| Minimum-maximum | 2.0-262.0 | 7.0-250.0 | |
| FAB type, M3/M3v/NA | 210/44/4 | 105/22 | 1.0 |
| PML/RARα isoform | |||
| BCR, 1/2/3/NA | 110/7/102/39 | 50/6/37/34 | .28 |
| SR/HR, % | 149 (58)/109 (42) | 85 (67)/42 (33) | .09 |
| Characteristics . | ICC-APL-01 (n = 258) . | AIDA2000 (n = 127) . | P . |
|---|---|---|---|
| Sex, male/female | 121/137 | 77/50 | .01 |
| Age, y | .26 | ||
| Median | 10.3 | 11.9 | |
| Minimum-maximum | 1.1-20.7 | 1.1-18.0 | |
| WBC count, ×109/L | .13 | ||
| Median | 6.3 | 3.6 | |
| Minimum-maximum | 0.08-339 | 0.2-187.0 | |
| Platelet count, ×109/L | .78 | ||
| Median | 23.0 | 27.56 | |
| Minimum-maximum | 2.0-262.0 | 7.0-250.0 | |
| FAB type, M3/M3v/NA | 210/44/4 | 105/22 | 1.0 |
| PML/RARα isoform | |||
| BCR, 1/2/3/NA | 110/7/102/39 | 50/6/37/34 | .28 |
| SR/HR, % | 149 (58)/109 (42) | 85 (67)/42 (33) | .09 |
NA, not available.
All 258 eligible patients were evaluable for induction response; 250 (97%) achieved HCR (Table 2); 8 died of intracranial hemorrhage (ICH) at a median of 4 days after diagnosis (range, 1-13). These patients presented with initial laboratory/clinical coagulopathy signs. The median WBC count was 73.3 × 109/L (range, 1.8-257.0); only 1 belonged to the SR group. No difference between the 2 protocols in terms of induction response (HCR 97% vs 96%) and death (3% vs 4%, P = .76) was recorded.
ICC-APL-01 and GIMEMA/AIEOP AIDA2000 trials: induction results
| Trial . | ICC-APL-01 . | AIDA2000 . |
|---|---|---|
| Evaluable patients | 258 | 126 |
| HCR, n (%) | 250 (97) | 121 (96) |
| Induction death, n (%) | 8 (3) | 5 (4) |
| ED | 8 | 5 |
| Risk category, SR/HR | 1/7 | 0/5 |
| Cause of death | ||
| ICH | 8 | 4 |
| Sepsis | — | 1 |
| Trial . | ICC-APL-01 . | AIDA2000 . |
|---|---|---|
| Evaluable patients | 258 | 126 |
| HCR, n (%) | 250 (97) | 121 (96) |
| Induction death, n (%) | 8 (3) | 5 (4) |
| ED | 8 | 5 |
| Risk category, SR/HR | 1/7 | 0/5 |
| Cause of death | ||
| ICH | 8 | 4 |
| Sepsis | — | 1 |
Of the 250 HCR patients (148 SR; 102 HR) who proceeded to consolidation, 3 received treatment that differed from that prescribed in the protocol (1 did not receive ATRA because of previous severe PC, 1 had no anthracycline because of cardiotoxicity, and 1 had ATO instead of chemotherapy based on the physician’s decision); 29 were not evaluable because of incomplete data, and 218 were evaluable for molecular response after consolidation. One hundred and seventeen (94%) of the 125 SR patients achieved mCR after the second consolidation cycle; 8 (still PML/RARα positive) received the third course, and 5 achieved mCR. Of the 93 HR patients, 91 (98%) tested PCR negative after the third consolidation course. Molecular persistence of the PML/RARα rearrangement was detected in 5 patients (3 SR; 2 HR) (2.3%). All 5 patients were treated with ATO salvage therapy followed by HSCT in 2; all achieved an mCR and are alive and leukemia-free at a median of 43 months (range, 30-57 months). In the AIDA2000 trial, RT-PCR for the PML/RARα fusion gene was performed in 121 children at consolidation completion. Molecular remission was documented in 118 cases (97.5%); 3 patients (2 SR and 1 HR; 2.5%) remained PCR positive. No difference in molecular response was detected between the 2 trials (P = 1).
Postconsolidation events included 10 hematologic relapses, 16 molecular relapses, 1 isolated extramedullary relapse, 1 death in mCR (occurring 5 months from diagnosis) in a patient with left cavernous sinus thrombosis at onset triggered by a Staphylococcus infection, 1 therapy-related acute myeloid leukemia (t-AML), and 1 thyroid carcinoma. Hematologic relapses occurred in 5 HR and 5 SR patients after a median of 15 months (range, 8-63 months) from diagnosis. All children received salvage ATO therapy. One patient died of ICH, and the other 9 achieved a second mCR. Three were consolidated with allogeneic HSCT, and 1 subsequently developed a central nervous system relapse treated with high-dose chemotherapy. All are alive in mCR (median, 45 months; range, 12-74 months from relapse). A molecular relapse was detected in 16 patients (8 HR; 8 SR) at a median of 25 months (range, 7-46 months) from diagnosis. All received ATO salvage therapy according the protocol indications (3 courses) and achieved second mCR. Two patients subsequently presented a hematologic relapse (12 and 16 months from first relapse, respectively) and were transplanted (1 allotransplant-related death; 1 mCR); 12 patients are alive and leukemia-free at a median of 36 months (range, 17-67 months) and 2 received allogeneic HSCT (both alive at 70 and 71 months). The SR patient with extramedullary skin relapse (43 months from diagnosis) is still on treatment. Two patients in mCR developed a therapy-related second neoplasms: a thyroid carcinoma (74 months from diagnosis) successfully treated with surgery and local radiotherapy and a t-AML (18 months from diagnosis) who failed treatment and died.
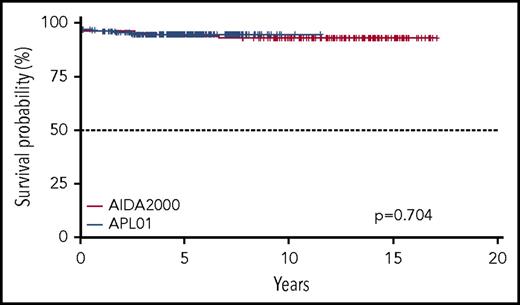
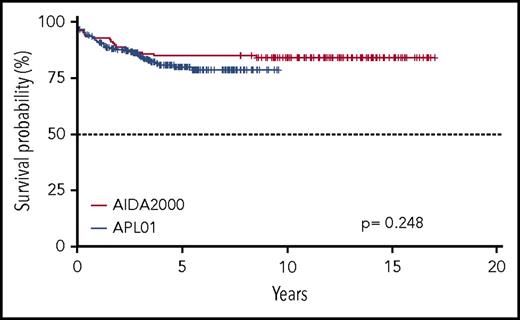
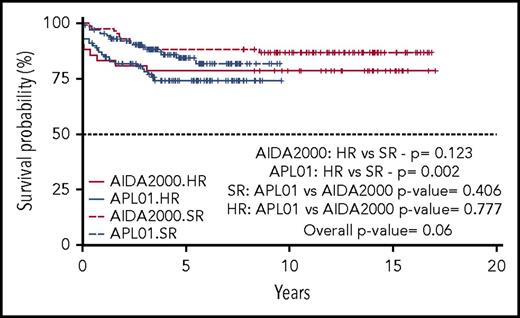
Five-year OS and EFS for the entire cohort of patients enrolled in the ICC-APL-01 protocol are 94.6% (95% confidence interval [CI], 91.7% to 97.5%) and 79.9% (95% CI, 74.4% to 85.7%), respectively. These results are comparable to those obtained in the AIDA2000 protocol (OS and EFS, 93.7% and 84.9%; P = .704 and P = .248, respectively) (Figures 2 and 3). SR patients had a better OS and EFS than HR patients in the ICC-APL-01 study. The OS was 98.4% (95% CI, 96.3% to 100%) and 89.4% (95% CI, 83.6% to 95.5%), and the EFS was 84.3% (95% CI, 77.5% to 91.7%) and 74.2% (95% CI, 65.7% to 83.8%), respectively, for SR and HR patients (P = .002 and P = .043). The outcome of SR and HR patients treated with the ICC-APL-01 protocol did not differ significantly from that of patients enrolled in the AIDA2000 trial (SR P = .40 and P = .37; HR P = .77 and P = .68 for OS and EFS, respectively) (Figures 4 and 5).
Five-year OS probability according to the protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01).
Five-year OS probability according to the protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01).
Five-year EFS probability according to protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01).
Five-year EFS probability according to protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01).
Five-year OS probability according to the protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01) and risk group (SR or HR).
Five-year OS probability according to the protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01) and risk group (SR or HR).
Five-year EFS probability according to the protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01) and risk group (SR or HR).
Five-year EFS probability according to the protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01) and risk group (SR or HR).
The 5-year CIR was 14.3% (95% CI, 14.2% to 14.5%) and 8.5% (95% CI, 8.3% to 8.6%) for the whole cohort in ICC-APL-01 and AIDA2000, respectively (P = .116; Figure 6). For SR patients, the CIR was 12.1% (95% CI, 11.9% to 12.3%) and 8.5% (95% CI, 8.3% to 8.7%) for patients enrolled in the ICC-APL-01 and the previous AIDA2000 trial (P = .369), respectively. Finally, the CIR for HR patients was 17.4% (95% CI, 17.0% to 17.8%) in the ICC-APL-01 and 8.3% (95% CI, 7.9% to 8.8%) in the AIDA2000 trials, respectively (P = .282) (supplemental Figure 2).
CIR according to protocol employed (GIMEMA/AIEOP AIDA2000 or ICC-APL-01).
In the ICC-APL-01 study, the effect on outcome of diagnostic features, such as age, sex, BCR3 short isoform of the PML/RARα gene, and country where patients were treated (Europe vs Brazil, Argentina, and Israel) was also analyzed. Patients were divided into 3 subgroups according to age: 0-5 years, 6-12 years, and >12 years. The 5-year OS, EFS, and CIR were not statistically different in very young children compared with the 2 other age groups. No statistical difference in OS, EFS, and CIR was observed for sex and BCR3 isoform (Table 3). Patients treated in Europe or non-European countries had similar baseline characteristics (supplemental Table 1) and outcomes (P = .87 and P = .76 for OS and EFS, respectively). The probability of OS and EFS of the 133 patients analyzed for FLT3-ITD was 97.3% (95% CI, 94.4% to 100%) and 73.1% (95% CI, 66.7% to 85.4%), respectively; they were 92.3% (95% CI, 82.6% to 100%) and 73.1% (95% CI, 57.9% to 92.3%) in the 33 who carried the molecular aberration (P = .09 and P = .29, respectively).
ICC-APL-01 protocol: OS, EFS, and CIR estimates
| . | 5-y OS estimate (95% CI) . | P . | 5-y EFS estimate (95% CI) . | P . | 5-y CIR estimate (95% CI) . | P . |
|---|---|---|---|---|---|---|
| HR | 89.4 (83.6-95.5) | .002 | 74.2 (65.7-83.8) | .043 | 17.5 (17.2-17.9) | .276 |
| SR | 98.4 (96.3-100) | 84.3 (77.2-91.7) | 12.1 (11.9-12.4) | |||
| 0-5 y | 100 (100-100) | .548 | 78.0 (63.7-95.5) | .865 | 13.3 (12.2-14.4) | .968 |
| 6-12 y | 94.3 (90.0-98.9) | 80.9 (72.9-89.8) | 14.7 (14.4-15.0) | |||
| >12 y | 93.0 (88.1-98.2) | 79.4 (71.2-88.5) | 14.0 (13.7-14.4) | |||
| Female | 92.2 (87.6-97.0) | .072 | 82.3 (75.3-90) | .56 | 10.6 (10.4-10.9) | .203 |
| Male | 97.2 (94.1-100) | 77.2 (69.0-86.3) | 18.4 (18.1-18.8) | |||
| PML/RARα: bcr1+bcr2 | 96 (92.3-99.9) | .072 | 80.4 (72.5-89.2) | .581 | 15.3 (14.9-15.6) | .736 |
| bcr3 | 91.9 (86.5-97.5) | 79.7 (71.2-89.2) | 12.0 (11.7-12.3) |
| . | 5-y OS estimate (95% CI) . | P . | 5-y EFS estimate (95% CI) . | P . | 5-y CIR estimate (95% CI) . | P . |
|---|---|---|---|---|---|---|
| HR | 89.4 (83.6-95.5) | .002 | 74.2 (65.7-83.8) | .043 | 17.5 (17.2-17.9) | .276 |
| SR | 98.4 (96.3-100) | 84.3 (77.2-91.7) | 12.1 (11.9-12.4) | |||
| 0-5 y | 100 (100-100) | .548 | 78.0 (63.7-95.5) | .865 | 13.3 (12.2-14.4) | .968 |
| 6-12 y | 94.3 (90.0-98.9) | 80.9 (72.9-89.8) | 14.7 (14.4-15.0) | |||
| >12 y | 93.0 (88.1-98.2) | 79.4 (71.2-88.5) | 14.0 (13.7-14.4) | |||
| Female | 92.2 (87.6-97.0) | .072 | 82.3 (75.3-90) | .56 | 10.6 (10.4-10.9) | .203 |
| Male | 97.2 (94.1-100) | 77.2 (69.0-86.3) | 18.4 (18.1-18.8) | |||
| PML/RARα: bcr1+bcr2 | 96 (92.3-99.9) | .072 | 80.4 (72.5-89.2) | .581 | 15.3 (14.9-15.6) | .736 |
| bcr3 | 91.9 (86.5-97.5) | 79.7 (71.2-89.2) | 12.0 (11.7-12.3) |
Toxicity
The percentage of adverse events varied according to treatment phase and was higher during induction and ARA-C–containing consolidation courses. The most common event was febrile neutropenia (42% induction; 60% consolidation). Clinically documented infections occurred in 30% of patients during induction and in 11% during the ARA-C–containing consolidation blocks; microbiologically documented infections were reported in 5% of children (11 bacteremia; 2 Aspergillus pneumonia) during induction and in 6% during consolidation (11 bacteremia; 1 Escherichia coli cellulitis; 1 Aspergillus pneumonia). With the exception of 1 patient who presented with cerebral thrombosis and died during subsequent treatment and 1 t-AML–related death, no other consolidation and maintenance–related deaths were observed. Symptoms and signs of DS were observed in 11% of patients during induction, with no difference between SR and HR patients. These patients required dexamethasone and temporary ATRA withholding; all restarted ATRA at a reduced dose and then proceed to full dosing. ATRA-associated PC occurred in 6% of patients during induction; in one child, severe cranial hypertension precluded further ATRA use during consolidation and maintenance. Acute cardiac and liver toxicities were analyzed. There were no cardiac deaths; 1 patient developed cardiotoxicity during induction, contraindicating further anthracycline treatment, and is alive 4 years from diagnosis. Liver toxicity was minimal during induction and consolidation; 1% of patients had grade 3 increased aspartate/alanine aminotransferase levels, and 2 patients showed elevated bilirubin levels.
Discussion
The present study describes the largest pediatric APL cohort treated with a risk-adapted protocol. In past years, the combination of ATRA and intensive chemotherapy has clearly demonstrated its effectiveness in pediatric APL. In the present report, comparing 2 consecutive multicenter studies (International ICC-APL-01 and Italian AIDA2000, the former with a shorter follow-up), induction with ATRA and single-agent chemotherapy (idarubicin) confirmed its high efficacy, with hematologic response rates of 97% and 96%, respectively, and with the virtual absence of resistant disease in pediatric patients. The ED rate was similar (3% vs 4%) in the 2 cohorts. The leading cause of ED was ICH (8 and 4 patients, respectively) occurring early after diagnosis; ICH was present before starting treatment in almost all patients. All but 1 patient who died presented with a WBC count >10 × 109/L. In agreement with other reported APL trials where ATRA was combined with ≥1 chemotherapeutic agents during induction, a higher WBC count at diagnosis was an unfavorable risk factor for induction deaths.2,9,13,14 In a recent international retrospective study, including 683 children with newly diagnosed APL (236 were Italian patients), the initial WBC count was significantly associated with ED, and patients with ICH had a significantly higher probability of death.14 ED resulting from hemorrhage remains a major cause of induction failure. Earlier referral to specialized centers, short delay in recognizing APL and, consequently, in starting ATRA, and optimized coagulopathy management, especially in HR patients, should be pursued in order to minimize the ED risk. Whether substitution of standard ATRA-chemotherapy regimens with ATO-based treatments will result in a further reduction in ED requires to be proven through ongoing trials.
Risk-adapted consolidation strategies, with ATRA extended to consolidation and maintenance, resulted in an excellent outcome in SR children in ICC-APL-01 and AIDA2000 (OS, 98.4% and 96.4%; EFS, 89.4% and 88.1%, respectively). In the ICC-APL-01 trial, 94% of 125 SR patients were MRD negative after the second consolidation. Despite the limitation of being obtained in a nonrandomized trial, the outcome of the ICC-APL-01 SR patients who were given a reduced cumulative anthracycline dose (355 mg/m2) was not inferior to that reported in other APL studies employing significantly higher anthracycline doses (AIDA0493: SR OS, 96%; PETHEMA/LPA-2005: SR OS, 88%).1,3,15 Our results confirm observations in a smaller series of 81 pediatric APL patients treated with an anthracycline-ARA-C regimen in combination with extended ATRA.5 SR children treated with the risk-adapted AIDA2000 regimen had a favorable outcome, which is comparable to that of children treated with the more intensive consolidation of the first historical Italian AIDA0493 protocol.1 As reported for adults, intercalating agents can be safely reduced from post-induction treatment of non-HR APL pediatric patients, sparing treatment-related toxicity in this subset of patients.9 No child treated in the AIDA2000 trial had to discontinue consolidation for therapy-related toxicities in contrast to the AIDA0493, where 6 children withdrew from the study.1
The benefit of prolonged ATRA in consolidation was also observed in HR patients. OS and EFS for these patients were 89.4% and 74.2% in ICC-APL-01 and 88.1% and 78.6% in AIDA2000, and values were superior to that achieved in our historical comparator AIDA0493 trial (OS, 81%; EFS, 59%).1 HR group results are comparable with those of adults (<61 years) treated with the AIDA2000 protocol (6-year OS in HR patients, 87.4%).9 Despite the absence of consolidation-toxic deaths in children, the incidence of neutropenic fever episodes (60%) and clinical and microbiologically documented infections (11% and 6%) were still high, with 5 life-threatening episodes in HR patients. In the more recent studies, mostly conducted in adults, the combination of ATO and ATRA during consolidation is associated with significantly less myelosuppression and fewer infections.16-18 The recently published COG-AAML0631 front-line protocol, including 101 children, combined ATO and low-dose anthracycline for consolidation of SR and HR patients.18 This trial clearly demonstrated that ATO consolidation allowed significant reduction in anthracycline doses while maintaining excellent results (3-year OS and EFS, 94% and 91%, respectively) with a low relapse rate for SR and HR patients (3-year relapse incidence, 4%). The use of ATO, which appears to be the most active single agent, needs to be extended to pediatric patients with newly diagnosed disease.
MRD monitoring to detect residual disease after consolidation and at different time points during maintenance and thereafter for timely detecting early relapse, was adopted in our trials. In the ICC-APL-01, 94% of SR children who tested PCR negative after the second consolidation block proceeded to maintenance with results not inferior to those achieved in the same category of patients treated with more intensive consolidation chemotherapy (EFS, 84.3% and 82.6% in ICC-APL-01 and AIDA0493, respectively).1 Furthermore, preemptive ATO salvage therapy was administered to the 16 patients experiencing molecular relapse, leading to a prolonged second mCR in 87% of them.
In summary, our data confirm that limiting the anthracycline cumulative dose does not compromise the outcome of childhood APL, especially in SR. Current efforts to reduce or even avoid front-line chemotherapy in APL children rests on the front-line use of ATO. Recent results suggest that the ATO + ATRA combination may allow highly effective treatment without systemic chemotherapy in SR patients.16-18 The chemo-free treatment of APL has proved, in adults and in limited childhood series,19-21 to be associated with a better outcome and more manageable toxicity. Currently ongoing pediatric trials (North-American COG-AAML1331 and European ICC-APL-02) are testing a chemo-free approach (ATO + ATRA) for SR patients and ATO, ATRA, and low-dose anthracycline for those at HR. Long-term toxicity of ATO-based APL therapy in pediatric patients also needs to be monitored.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ursula Creutzig for discussing the outlines of the trial and for having participated in the design of the protocol.
The work was supported by AIEOP.
Authorship
Contribution: A.M.T., A. Pession, B.G., O.S., F.L., and G.J.L.K. designed the study; A.M.T., A. Pession, B.G., A.C.d.A., L.M., G.L., S.E., H.H., J.v.d.W.t.B., O.S., F.L., and G.J.L.K. contributed materials and clinical data; D.D. and D.G. performed most of the molecular tests; M.D.R. was the data manager; A.M.T., F.L., F.L.C., and G.J.L.K. analyzed and interpreted the data; A. Piciocchi carried out statistical analysis; A.M.T. wrote the paper; A. Pession, B.G., F.L.C., F.L., R.F., and G.J.L.K. reviewed the manuscript and contributed to the final draft; A.M.T., A. Pession, M.D.R., R.F., F.L., and G.J.L.K. supervised the study; and all authors performed critical review of the manuscript and gave their final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
David Grimwade died on 17 October 2016.
Correspondence: Anna Maria Testi, Department of Cellular Biotechnologies and Hematology, Sapienza University of Rome, Via Benevento 6, 00161 Rome, Italy; e-mail: testi@bce.uniroma1.it; and Franco Locatelli, Department of Pediatric Hematology/Oncology, IRCCS Bambino Gesù Children’s’ Hospital, Rome, University of Pavia, Piazza di Sant’Onofrio 4, 00165 Rome, Italy; e-mail: franco.locatelli@opbg.net.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal