Subjects:
Free Research Articles
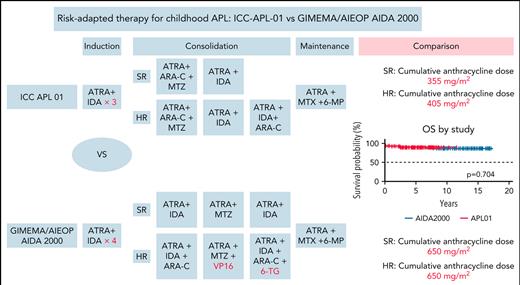
In the visual abstract, for high-risk (HR) patients in the ICC-APL-01 trial, the information for consolidation course 3 should read “ATRA + IDA + ARA-C,” not “ATRA + IDA.” The corrected visual abstract is shown below.
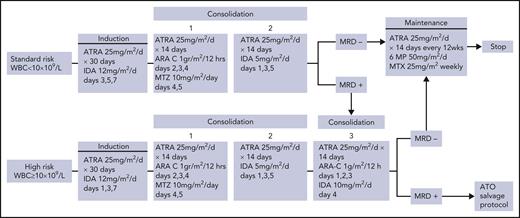
Page 407: In Figure 1, idarubicin (IDA) should not have been listed in consolidation 1 for standard-risk and high-risk patients, cytarabine (ARA-C) should not have been listed in consolidation 2 for standard-risk and high-risk patients, and ARA-C (1 g/m2/12 hours on days 1, 2, and 3) should have been listed in consolidation 3 for high-risk patients. Also, in consolidation 2 for both standard-risk and high-risk patients, “IDA 10mg/m2/d day 4” should read “IDA 5mg/m2/d days 1,3,5.” The corrected Figure 1 is shown below. Close modal
Figure 1.
ICC-APL-01 study protocol design. 6-MP, 6-mercaptopurine; IDA, idarubicin; MTX, methotrexate; MTZ, mitoxantrone.
Figure 1.
ICC-APL-01 study protocol design. 6-MP, 6-mercaptopurine; IDA, idarubicin; MTX, methotrexate; MTZ, mitoxantrone.
© 2023 by The American Society of Hematology
2023

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal