Key Points
Anti-GPIbα antibodies exert a pulling force on platelet GPIbα by crosslinking platelets under shear flow.
A mechanical feature of an anti-GPIbα antibody, rather than affinity or epitope, determines ability to induce Fc-independent clearance.
Abstract
Immune thrombocytopenia (ITP) is a prevalent autoimmune disease characterized by autoantibody-induced platelet clearance. Some ITP patients are refractory to standard immunosuppressive treatments such as intravenous immunoglobulin (IVIg). These patients often have autoantibodies that target the ligand-binding domain (LBD) of glycoprotein Ibα (GPIbα), a major subunit of the platelet mechanoreceptor complex GPIb-IX. However, the molecular mechanism of this Fc-independent platelet clearance is not clear. Here, we report that many anti-LBD monoclonal antibodies such as 6B4, but not AK2, activated GPIb-IX in a shear-dependent manner and induced IVIg-resistant platelet clearance in mice. Single-molecule optical tweezer measurements of antibodies pulling on full-length GPIb-IX demonstrated that the unbinding force needed to dissociate 6B4 from the LBD far exceeds the force required to unfold the juxtamembrane mechanosensory domain (MSD) in GPIbα, unlike the AK2-LBD unbinding force. Binding of 6B4, not AK2, induced shear-dependent unfolding of the MSD on the platelet, as evidenced by increased exposure of a linear sequence therein. Imaging flow cytometry and aggregometry measurements of platelets and LBD-coated platelet-mimetic beads revealed that 6B4 can sustain crosslinking of platelets under shear, whereas 6B4 Fab and AK2 cannot. These results suggest a novel mechanism by which anti-LBD antibodies can exert a pulling force on GPIb-IX via platelet crosslinking, activating GPIb-IX by unfolding its MSD and inducing Fc-independent platelet clearance.
Introduction
Immune thrombocytopenia (ITP) is a common bleeding disorder characterized by increased platelet clearance, primarily via anti-platelet autoantibodies.1-3 Thrombocytopenia leads to an increased risk of bleeding and potentially fatal hemorrhage.4 Common first-line treatments for ITP include intravenous immunoglobulin (IVIg) and corticosteroids.5,6 However, it is estimated that 20% of patients are refractory to these treatments.7 The underlying mechanism of refractoriness is not entirely clear and, to date, there are no clinical tests that can make an accurate prognosis for a given ITP treatment.5,8
Autoantibodies that target platelet surface proteins are the primary factors leading to excessive platelet clearance in ITP.9,10 Common targets of these antibodies are integrin αIIbβ3 and glycoprotein Ib-IX (GPIb-IX),11,12 the 2 most highly expressed receptor complexes on the platelet surface. GPIb-IX is a highly integrated complex consisting of GPIbα, GPIbβ, and GPIX subunits.13 GPIbα contains, starting from its N terminus, a ligand-binding domain (LBD) that binds von Willebrand factor (VWF) and other ligands, a heavily glycosylated macroglycopeptide region, a mechanosensory domain (MSD), a single-span transmembrane domain, and a cytoplasmic domain (Figure 1A). Notably, there is correlation between refractoriness to IVIg or steroids and the presence of anti-GPIb-IX antibodies in patient sera.11,12,14 In addition, infusion of monoclonal antibodies (mAbs) targeting the N-terminal LBD of GPIbα causes fast depletion of nearly all platelets from animals.15-18 Clearance by these anti-LBD mAbs is Fc independent and largely unaffected by IVIg treatment,19,20 consistent with findings that these mAbs can directly activate GPIb-IX, leading to intracellular signaling, particularly deglycosylation and subsequent platelet clearance by hepatocytes or macrophages.21-23 Many studies corroborate a remarkable similarity between patient sera antibodies that target GPIb-IX and murine mAbs that target GPIb-IX. Markers of GPIb-IX activation, especially platelet desialylation, have been seen in mAb-induced ITP as well as patient sera, and the extent of desialylation correlates with patient response to first-line treatments.4,6,21 Studies using mAbs19,20 underscore the relationship between ITP mediated by anti-GPIb-IX antibodies and resistance to IVIg or steroids, which has also been documented in patients.11,12,14
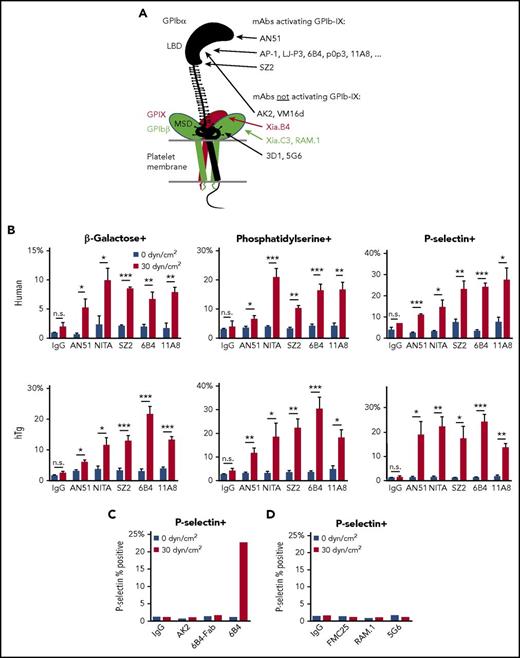
Anti-LBD mAbs, but not others, induce shear-dependent signaling. (A) Illustration of GPIb-IX domains and differential effects of mAbs. Locations of mAb epitopes are noted by arrowheads. (B) Graphs of percentage of positive events for β-galactose, phosphatidylserine, and P-selectin exposure in human (top) and hTg mouse (bottom) PRP treated with control IgG, AN51, NITA, SZ2, 6B4, or 11A8 as indicated under static or sheared conditions. (C) Graph of P-selectin exposure in hTg mouse PRP treated with control IgG, AK2, 6B4-Fab, or 6B4 under static or sheared conditions. (D) Graph of P-selectin exposure in human PRP treated with control IgG, FMC25, RAM.1, or 5G6 under static or sheared conditions. For all graphs, gray bars represent 0 dyn/cm2 and red bars represent 30 dyn/cm2. *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s., not significant.
Anti-LBD mAbs, but not others, induce shear-dependent signaling. (A) Illustration of GPIb-IX domains and differential effects of mAbs. Locations of mAb epitopes are noted by arrowheads. (B) Graphs of percentage of positive events for β-galactose, phosphatidylserine, and P-selectin exposure in human (top) and hTg mouse (bottom) PRP treated with control IgG, AN51, NITA, SZ2, 6B4, or 11A8 as indicated under static or sheared conditions. (C) Graph of P-selectin exposure in hTg mouse PRP treated with control IgG, AK2, 6B4-Fab, or 6B4 under static or sheared conditions. (D) Graph of P-selectin exposure in human PRP treated with control IgG, FMC25, RAM.1, or 5G6 under static or sheared conditions. For all graphs, gray bars represent 0 dyn/cm2 and red bars represent 30 dyn/cm2. *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s., not significant.
Despite these findings, the mechanism by which these antibodies induce GPIb-IX signaling has yet to be elucidated. Regarding the mechanism, 4 key observations have coalesced from the literature. First, the F(ab′)2 but not the Fab fragment of an anti-LBD mAb can induce platelet clearance.17,19 This indicates that the bivalent structure of the antibody is required for activating GPIb-IX. Second, many anti-LBD antibodies clear platelets rapidly, regardless of their epitope in the LBD.15-18,21 This implies that the mechanism of clearance likely does not involve steric occlusion of any binding pockets or regions of interest within the LBD itself. Third, most mAbs targeting the LBD, but not other regions in GPIb-IX, including the juxtamembrane portion of GPIbα, induce Fc-independent clearance (Figure 1A).15,24-26 This indicates that there is something particular about the LBD that allows antibodies targeting this region to activate the receptor and/or clear platelets in an Fc-independent manner. Finally, although almost all anti-LBD antibodies induce platelet clearance, 1 anti-LBD mAb, VM16D, does not (Figure 1A).22 To date, no model has been proposed to fully explain these 4 observations.
It has long been documented that stirring or shear flow is required for triggering ristocetin-induced VWF-mediated platelet aggregation.27 Recently, a juxtamembrane MSD was identified in GPIbα.28 Under physiological shear, binding of soluble VWF induces MSD unfolding on the platelet, which leads to intracellular signaling events, including desialylation, P-selectin expression, and subsequent platelet clearance.29 Moreover, transfected cells and murine platelets that express mutant GPIb-IX complexes in which GPIbα contains an already unfolded MSD exhibit constitutive ligand-free GPIb-IX signaling, and the mutant platelet is cleared much faster than the wild-type platelet.29 In this study, we report evidence that, like VWF, anti-LBD mAbs induce platelet signaling in a shear-dependent manner that entails MSD unfolding, which leads to a new mechanomolecular mechanism to account for all 4 aforementioned observations. Our findings have mechanistic implications for GPIb-IX signaling, particularly in the context of IVIg-resistant ITP, as well as clinical implications in other thrombocytopenic diseases.
Methods
Mice
Transgenic mice expressing only human GPIbα (hTg) have been previously described.30 All experimental procedures were approved by the Institutional Animal Care and Use Committee of Emory University and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In vitro characterization and in vivo clearance measurements were performed as described in the supplemental Methods (available on the Blood Web site).
Human platelets
All procedures using donor-derived human platelets were approved by the Institutional Review Boards at Children’s Healthcare of Atlanta/Emory University and Qilu Hospital. Flow cytometry, aggregometry, and uniform shear treatment were performed as previously described31,32 or as described in the supplemental Methods.
Statistical analysis
Statistical analysis was performed using Graphpad Prism software. Unless otherwise indicated, significance was determined by one-way analysis of variance or Student t test. Flow cytometry data were analyzed by using FlowJo, BD FACSDiva (BD Biosciences), and IDEAS software (Amnis), as indicated. Differences were considered statistically significant when P < .05.
Results
Activation of GPIb-IX by anti-LBD mAbs is epitope independent but shear dependent
A previous report suggested that antibodies targeting an N-terminal portion of the LBD activate GPIb-IX more readily than those targeting other epitopes in the LBD.22 To this end, we analyzed the signaling abilities of several representative anti-LBD mAbs. mAbs AN51 and AK2 bind to the N-terminal portion of the LBD, 6B4 to a site in the middle (residues 230-262), and SZ2 to the sulfated tyrosine region in the C-terminal portion.33,34 Although the epitopes of NIT-A21 and 11A835 are not determined, both can inhibit VWF binding.21 mAb-treated human platelet-rich plasma (PRP) was exposed to static (0 dyn/cm2) and uniform arterial (30 dyn/cm2) shear stress on a cone-plate viscometer. After shear treatment at 22°C for 5 minutes, several markers of GPIb-IX activation (surface exposure of β-galactose, phosphatidylserine, and P-selectin) were detected by flow cytometry via fluorescein isothiocyanate (FITC)–conjugated Erythrina cristagalli lectin, green fluorescent protein–conjugated lactadherin C2 domain (Lact-C2), and allophycocyanin-conjugated anti-P-selectin antibody, respectively. GPIb-IX activation was observed for all anti-LBD mAbs tested (Figure 1B, top). The effect was shear dependent because GPIb-IX signaling was absent in static samples. These experiments were also performed in PRP containing murine hTg platelets, which produced very similar effects (Figure 1B, bottom). In agreement with previous reports that bivalency is required for mAb-induced GPIb-IX activation,17,19 the monovalent 6B4 Fab did not induce signaling (Figure 1C). Given the diversity of epitopes for the mAbs tested, these data indicate that the ability of an anti-LBD mAb to activate GPIb-IX is not determined by its precise epitope in the LBD. Conversely, mAbs FMC25, RAM.1, and 5G6, which target GPIX, GPIbβ, and the MSD of GPIbα, respectively,29,31,36 did not induce P-selectin exposure in human platelets under either static or sheared conditions (Figure 1D), confirming the difference between them and most anti-LBD mAbs in their abilities to activate GPIb-IX.24,31
Anti-LBD mAb AK2 neither activates GPIb-IX nor induces IVIg-resistant clearance of platelets
In our initial screen of anti-LBD mAbs, we found that 1 of them, AK2, behaved differently from the others (Figure 1C). Additional measurements were carried out to characterize and compare the effects of AK2 with those of 6B4, a canonical anti-LBD mAb well-documented to induce platelet clearance.17 Both mAbs bind GPIbα (supplemental Figure 1) and block VWF binding to GPIbα.17,34,37,38 When exposed to shear stresses of 5 or 30 dyn/cm2, human PRP treated with 6B4 includes a subpopulation composed of events of high size (forward scatter [FSC]) and granularity (side scatter [SSC]) (supplemental Figure 2). By comparison, control immunoglobulin G (IgG) did not induce this at any shear level, and the effect of AK2 was notably smaller. In both human and hTg platelets treated with 6B4, expression of all 3 markers of GPIb signaling increased in a shear-dependent manner, an effect not observed in samples treated with IgG or AK2 (Figure 2A).
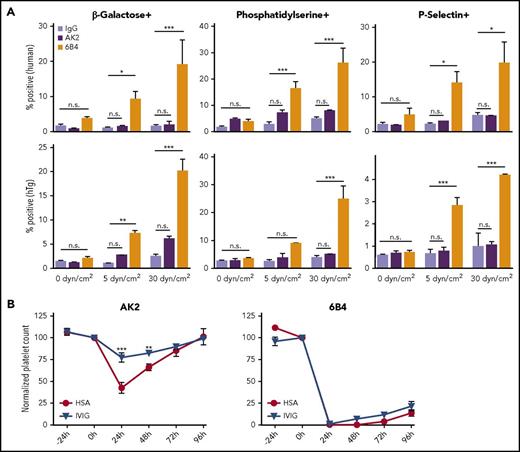
6B4, but not AK2, induces shear-dependent platelet signaling and IVIg-resistant platelet clearance in mice. (A) Graphs of percentage of positive events for Erythrina cristagalli lectin, Lact-C2, and P-selectin exposure in human (top) and hTg (bottom) PRP treated with control IgG, AK2, or 6B4. Samples were exposed to 0, 5, or 30 dyn/cm2 as indicated (n = 5). (B) Platelet survival curves for hTg mice injected retro-orbitally with AK2 or 6B4 24 hours after intraperitoneal IVIg treatment. Blood was drawn from mice at the time of IVIg administration (–24 hours), time of antibody injection (0 hours), and thereafter every 24 hours until 96 hours after antibody injection. Platelet count determined by complete blood count analysis. *P ≤ .05, **P ≤ .01, ***P ≤ .001. HSA, human serum albumin.
6B4, but not AK2, induces shear-dependent platelet signaling and IVIg-resistant platelet clearance in mice. (A) Graphs of percentage of positive events for Erythrina cristagalli lectin, Lact-C2, and P-selectin exposure in human (top) and hTg (bottom) PRP treated with control IgG, AK2, or 6B4. Samples were exposed to 0, 5, or 30 dyn/cm2 as indicated (n = 5). (B) Platelet survival curves for hTg mice injected retro-orbitally with AK2 or 6B4 24 hours after intraperitoneal IVIg treatment. Blood was drawn from mice at the time of IVIg administration (–24 hours), time of antibody injection (0 hours), and thereafter every 24 hours until 96 hours after antibody injection. Platelet count determined by complete blood count analysis. *P ≤ .05, **P ≤ .01, ***P ≤ .001. HSA, human serum albumin.
It has previously been observed that many anti-LBD antibodies can clear platelets in an Fc-independent and IVIg-resistant manner.19,20 To compare the abilities of AK2 and 6B4 to induce platelet clearance, hTg mice were treated with intraperitoneal injection of IVIg or human serum albumin 24 hours before intravenous injection of either AK2 or 6B4. Platelet counts in these mice were measured before mAb injection and over a 4-day period after induction of thrombocytopenia by either mAb. As anticipated,17 6B4 induced robust and long-lasting thrombocytopenia, and its effect was not ameliorated by IVIg pretreatment (Figure 2B). In contrast, AK2 induced clearance to a lesser extent, which was significantly attenuated by pretreatment with IVIg. Overall, these results demonstrate that not all anti-LBD mAbs can effectively activate GPIb-IX and induce IVIg-resistant platelet clearance.
6B4, but not AK2, induces shear-dependent unfolding of the MSD in GPIbα on the platelet
A trigger model of GPIb-IX activation, which describes unfolding of the MSD as the instigating event in VWF-mediated GPIb-IX activation, was recently proposed.29 Given the shear requirements of anti-LBD mAb-induced platelet signaling that was similarly observed for VWF, we next tested whether mAbs induce GPIb-IX activation via unfolding of the MSD.
To detect MSD unfolding on the platelet surface, mAbs 5G6 (which binds a linear epitope in the MSD31,39 ) and WM23 (which binds the macroglycopeptide region of GPIbα) were used. When the MSD is unfolded, 5G6 has greater access to its epitope but WM23 binding remains the same, making the ratio of 5G6 binding to WM23 binding a proxy for the extent of MSD unfolding, as previously established.29 Human PRP was pretreated with fluorescently labeled 5G6 or WM23 in the presence of EDTA, an inhibitor of metalloproteinases that cleave GPIbα. After incubation with 5G6 or WM23, platelets were treated with either 6B4 or AK2 or control IgG under static or shear conditions. Under static conditions, neither 6B4 nor AK2 increased 5G6 binding above the baseline established by control IgG. Under shear conditions (30 dyn/cm2), 6B4 induced a greater than twofold increase in 5G6 binding but AK2 induced little change (Figure 3A-B). WM23 binding did not change between static and sheared samples for all mAbs, indicating that any changes in 5G6 binding were not the result of alterations in overall GPIbα surface expression (Figure 3B). These results suggest that binding of 6B4, but not AK2, induces shear-dependent unfolding of the MSD on the platelet.
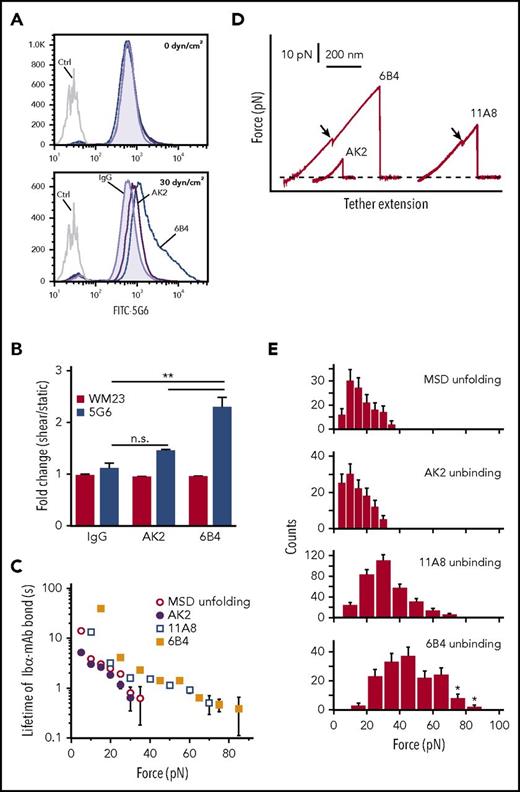
Platelet signaling is triggered by MSD unfolding. (A) Representative histograms illustrating 5G6 binding in PRP treated with control IgG, 6B4, or AK2 under 0 dyn/cm2 (top) and 30 dyn/cm2 (bottom) of uniform shear. EDTA was added to prevent GPIbα shedding via metalloproteinases. (B) Graphs of fold increase (sheared/static) in 5G6 and WM23 binding for PRP treated with IgG, AK2, or 6B4. **P ≤ .01. (C) Plots of lifetimes of noted bonds as a function of force. Lifetimes were obtained by transforming the histograms of unbinding or unfolding forces using the method developed by Dudko et al.53 (D) Overlaid force-distance traces for pulling AK2, 11A8, or 6B4 on GPIb-IX. (E) Force distributions of MSD unfolding or unbinding between LBD mAbs and GPIb-IX. Loading rate was ∼10 pN/s. Error bars are Poisson noise. Asterisks indicate an underestimation of unbinding force resulting from strong bonds that were above the detection limit of our optical tweezers. FITC, fluorescein isothiocyanate.
Platelet signaling is triggered by MSD unfolding. (A) Representative histograms illustrating 5G6 binding in PRP treated with control IgG, 6B4, or AK2 under 0 dyn/cm2 (top) and 30 dyn/cm2 (bottom) of uniform shear. EDTA was added to prevent GPIbα shedding via metalloproteinases. (B) Graphs of fold increase (sheared/static) in 5G6 and WM23 binding for PRP treated with IgG, AK2, or 6B4. **P ≤ .01. (C) Plots of lifetimes of noted bonds as a function of force. Lifetimes were obtained by transforming the histograms of unbinding or unfolding forces using the method developed by Dudko et al.53 (D) Overlaid force-distance traces for pulling AK2, 11A8, or 6B4 on GPIb-IX. (E) Force distributions of MSD unfolding or unbinding between LBD mAbs and GPIb-IX. Loading rate was ∼10 pN/s. Error bars are Poisson noise. Asterisks indicate an underestimation of unbinding force resulting from strong bonds that were above the detection limit of our optical tweezers. FITC, fluorescein isothiocyanate.
Differential unbinding forces of 6B4 and AK2 underlie their disparate abilities to unfold the MSD of GPIb-IX
Faced with the differential abilities of 6B4 and AK2 to unfold the MSD in response to shear, we next measured the unbinding forces between mAbs and the LBD (ie, the force required to pull an mAb apart from the LBD) and their effects on MSD unfolding by single-molecule force spectroscopy. As described earlier,28 recombinant biotinylated GPIb-IX was immobilized on a streptavidin bead held by a fixed micropipette, and the Fab fragment of mAbs (AK2, 11A8, or 6B4) was coupled to a DNA handle–attached bead that was controlled by an optical laser trap. Under typical conditions with contact force of 2 pN, contact time of 0.1 second, and pulling speed of 200 nm/s, adhesion frequencies of 10% to 20% were detected between mAb- and GPIb-IX–coupled beads. In comparison, adhesion frequency was 1% between an uncoupled bead and a GPIb-IX–coupled bead and was undetectable between 2 uncoupled beads or between an mAb-coupled bead and an uncoupled bead (supplemental Figure 3A). Under our experimental conditions, almost 90% of the observed adhesion events should be mediated by a single-molecule bond between the mAb and the LBD in GPIb-IX.40,41 Bond lifetimes and force distributions of the mAb-LBD interactions were measured and compared with those of MSD unfolding obtained from our earlier study28 (Figure 3C-E). Among these, the AK2-LBD interaction had the weakest unbinding force and displayed the shortest lifetime under any given force. The force required for MSD unfolding is slightly stronger than the AK2-LBD bond strength but much weaker than 11A8-LBD or 6B4-LBD bond strength. The 6B4-LBD interaction is the strongest, with 5% of the traces beyond the detection limit of our optical tweezer instrument (about 80-100 pN), because they exhibited DNA overstretching (supplemental Figure 3B). In comparison, pulling the biotin-streptavidin bond on the same instrument produced DNA overstretching in >80% of the traces. At a given force, the 6B4-LBD bond exhibits a five- to tenfold greater lifetime than MSD unfolding (Figure 3C). Thus, the MSD unfolding event was observed in most pulling traces of 11A8 or 6B4 (Figure 3D). In contrast, MSD unfolding was rarely observed when AK2 was used to pull. Together these data indicate that AK2 is much less likely than 6B4 and other anti-LBD mAbs to activate GPIb-IX because of its inability to sustain the interaction necessary to exert enough force to unfold the MSD. This is consistent with our observations that AK2 induced much lower and less frequent platelet signaling or clearance of platelets than 6B4.
Antisera from a patient with chronic ITP produces 6B4-like effects
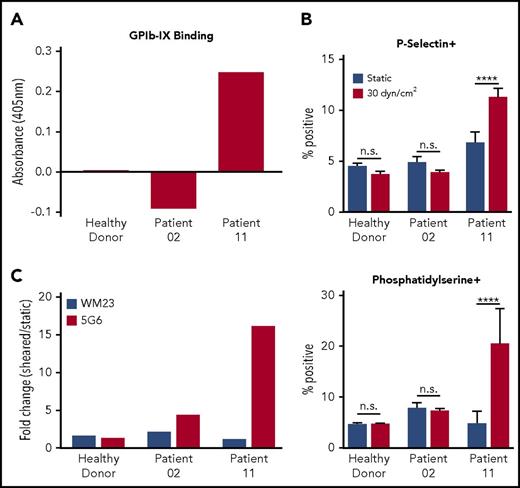
To verify whether human antibody-induced effects are shear dependent in a manner similar to those of murine mAbs such as 6B4, plasma was obtained from 12 patients with chronic ITP, and the presence of anti-GPIb-IX antibodies was assayed via enzyme-linked immunosorbent assay (Figure 4A; supplemental Figure 4). Among these patients, only 1 (patient 11) seemed positive for anti-GPIb-IX antibodies. Washed healthy human platelets were reconstituted in plasma from a healthy donor, an ITP patient lacking anti-GPIb-IX Abs (patient 02), or an ITP patient with anti-GPIb-IX Abs (patient 11) to a normal human platelet count of 150 to 450 × 103/μL and exposed to either static (0 dyn/cm2) or sheared (30 dyn/cm2) conditions. Subsequent testing for platelet signaling showed that healthy donor or patient 02 plasma did not induce shear-dependent expression of P-selectin or phosphatidylserine. In contrast, plasma from patient 11 significantly increased expression of both markers in a shear-dependent manner (Figure 4B; supplemental Figure 5). To assess whether ITP patient antisera could induce MSD unfolding similar to 6B4, the remaining reconstituted PRP samples were pretreated with fluorescently labeled 5G6 or WM23 in the presence of EDTA. In sheared platelets treated with plasma from patient 11, the 5G6 binding increased by ∼16-fold with respect to that under static conditions, which is markedly larger than the ∼1.3-fold change observed for healthy donor plasma (Figure 4C). For comparison, WM23 binding was largely unchanged for both samples. Overall, these results indicate that human antisera from patients with chronic ITP can produce shear-dependent platelet signaling and induce MSD unfolding in a manner similar to that of murine mAbs.
Sera from patients with chronic ITP with anti-GPIb-IX antibodies induce MSD unfolding and shear-dependent platelet signaling. (A) Enzyme-linked immunosorbent assay absorbance values for representative patient samples binding to GPIb-IX. Bars represent the values for plasma binding to purified GPIb-IX plus bovine serum albumin minus the values for bovine serum albumin binding alone. (B) Graphs of percentage of positive events for P-selectin (top) and phosphatidylserine (bottom) exposure in healthy donor platelets reconstituted in plasma from healthy donors, patient 02, or patient 11 under sheared (30 dyn/cm2) or static conditions (n = 4-5). (C) Graphs of fold increase (sheared/static) in 5G6 and WM23 binding to healthy donor platelets reconstituted in plasma from healthy donors, patient 02, or patient 11. Bars represent the mean of duplicate (n = 2) values for each condition. Variance in WM23 fold change was 12% to 15% of total. Variance in 5G6 fold change was 5% to 17% of total. ****P ≤ .0001.
Sera from patients with chronic ITP with anti-GPIb-IX antibodies induce MSD unfolding and shear-dependent platelet signaling. (A) Enzyme-linked immunosorbent assay absorbance values for representative patient samples binding to GPIb-IX. Bars represent the values for plasma binding to purified GPIb-IX plus bovine serum albumin minus the values for bovine serum albumin binding alone. (B) Graphs of percentage of positive events for P-selectin (top) and phosphatidylserine (bottom) exposure in healthy donor platelets reconstituted in plasma from healthy donors, patient 02, or patient 11 under sheared (30 dyn/cm2) or static conditions (n = 4-5). (C) Graphs of fold increase (sheared/static) in 5G6 and WM23 binding to healthy donor platelets reconstituted in plasma from healthy donors, patient 02, or patient 11. Bars represent the mean of duplicate (n = 2) values for each condition. Variance in WM23 fold change was 12% to 15% of total. Variance in 5G6 fold change was 5% to 17% of total. ****P ≤ .0001.
6B4, but not AK2, can crosslink platelets under shear
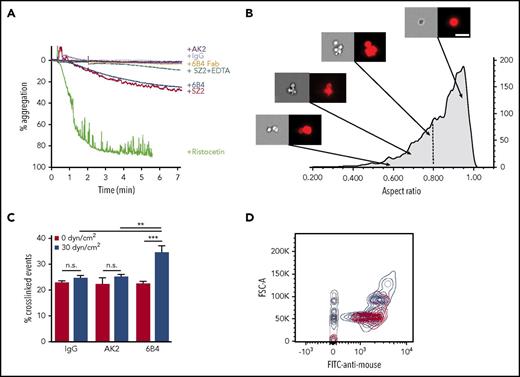
To exert force on platelet GPIb-IX under blood flow, VWF can be anchored to the site of injury, but anti-LBD mAbs clearly produce their shear-dependent activity in solution. This raises the question of how anti-LBD mAbs exert a pulling force on GPIb-IX in platelets. Unlike the platelet, which could exert a dragging force upon attachment to the immobilized VWF under shear flow,42 the size of an anti-LBD mAb is too small to create sufficient drag in shear flow. To test the possibility that anti-LBD mAbs crosslink platelets under shear flow, we analyzed the abilities of AK2, 6B4, 6B4 Fab, SZ2, and control IgG to induce platelet aggregation or agglutination by platelet aggregometry. Compared with the full platelet aggregation induced by ristocetin, 6B4 and SZ2 induced a moderate response, leading to platelet agglutination reflected by ∼20% aggregation. 6B4 Fab did not induce any observable agglutination (Figure 5A), suggesting that the dimeric structure of the antibody is used to crosslink platelets. Anti-LBD mAbs may induce platelet agglutination, in part, by activating integrin αIIbβ3.22 To separate the crosslinking effects of anti-LBD mAbs and integrins, we applied EDTA, a broad inhibitor of integrin binding. The addition of EDTA reduced the extent of anti-LBD mAb-induced agglutination (Figure 5A) but did not completely block it, which confirms that platelet crosslinking is initiated by anti-LBD mAb binding. It should be noted that unlike 6B4 or SZ2, AK2 did not induce any observable agglutination.
mAbs induce platelet signaling via crosslinking platelets. (A) Percentage aggregation of human PRP treated with AK2, IgG, 6B4 Fab, SZ2 + EDTA, 6B4, SZ2, or ristocetin, determined via aggregometry. (B) Representative images of platelets (right: bright field, left: anti-CD41) in PRP treated with 6B4 at low shear. Images obtained via imaging flow cytometry. Arrows indicate the aspect ratio that corresponds to each set of images. Vertical dotted line demarcates the gate used to identify crosslinked events. Scale bar = 10 μm. (C) Frequency of crosslinked events (events with aspect ratio <0.8) in PRP treated with IgG, AK2, or 6B4 at static (0 dyn/cm2) or sheared (30 dyn/cm2) conditions (n = 3). (D) Contour plots of forward scatter (FSC-A) vs anti-mouse antibody fluorescence for platelet-mimetic beads treated with AK2 (red), 6B4 (blue), and IgG (gray) under sheared conditions. Contour lines = 5%. **P < .01, ***P < .001.
mAbs induce platelet signaling via crosslinking platelets. (A) Percentage aggregation of human PRP treated with AK2, IgG, 6B4 Fab, SZ2 + EDTA, 6B4, SZ2, or ristocetin, determined via aggregometry. (B) Representative images of platelets (right: bright field, left: anti-CD41) in PRP treated with 6B4 at low shear. Images obtained via imaging flow cytometry. Arrows indicate the aspect ratio that corresponds to each set of images. Vertical dotted line demarcates the gate used to identify crosslinked events. Scale bar = 10 μm. (C) Frequency of crosslinked events (events with aspect ratio <0.8) in PRP treated with IgG, AK2, or 6B4 at static (0 dyn/cm2) or sheared (30 dyn/cm2) conditions (n = 3). (D) Contour plots of forward scatter (FSC-A) vs anti-mouse antibody fluorescence for platelet-mimetic beads treated with AK2 (red), 6B4 (blue), and IgG (gray) under sheared conditions. Contour lines = 5%. **P < .01, ***P < .001.
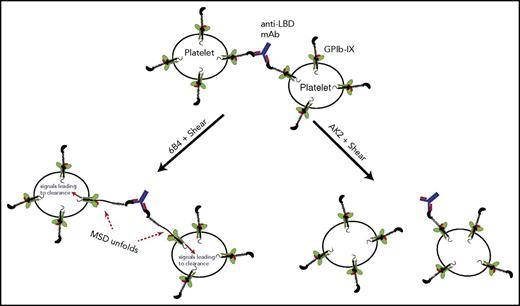
Platelet crosslinking via anti-LBD mAbs was directly visualized and quantitated via imaging flow cytometry. Human platelets treated with 6B4 or AK2 at various shear levels were labeled with fluorescently labeled anti-αIIbβ3 antibody. Each fluorescent particle interrogated by the cytometer was imaged and categorized by the aspect ratio, the ratio of the height and width of the particle. Single platelets have aspect ratios close to 1, whereas clumps of 2 or more platelets have lower aspect ratios, typically below 0.8 (Figure 5B; supplemental Table 1).43,44 Under static conditions, platelets treated with IgG, AK2, or 6B4 contained the same percentage of clumped platelets. When exposed to physiological shear, 6B4 crosslinked a higher percentage of platelets (Figure 5C). This effect is not the result of increased mixing because neither IgG nor AK2 had the effect. In addition, we tested whether anti-LBD mAbs could crosslink platelet-sized beads coated with recombinant LBD (supplemental Figure 6) under shear conditions. Judging by flow cytometry plots, LBD-coated beads incubated with IgG or AK2 produced 2 major populations in which the larger was roughly double the size (FSC) of the smaller (Figure 5D). These 2 populations likely represent single beads and 2 beads stuck together, a phenomenon which occurs at a certain frequency regardless of treatment. In addition to these 2 populations, incubation with 6B4 produced a third major population of beads with even larger size, which represents more highly crosslinked clumps of beads (Figure 5D). Overall, these results suggest that through its binding to the LBD, 6B4 crosslinks significantly more platelets under shear than AK2 does. When crosslinking the platelets, 6B4 must use each of its 2 Fab fragments to engage a copy of GPIb-IX on opposing platelets (Figure 6). In this scenario, a platelet is of sufficient size to generate drag on a linked platelet, thus allowing exertion of tensile force on GPIb-IX and subsequent unfolding of the MSD (Figure 6). It is conceivable that because of the relatively weak AK2-LBD unbinding force, AK2-crosslinked platelets or bead complexes are not stable under shear. Conversely, 6B4 and other anti-LBD antibodies with a strong unbinding force to the LBD can sustain crosslinking of platelets under shear in the bloodstream, allowing tensile force to be exerted on GPIb-IX and leading to GPIb-IX–mediated signaling (Figure 6).
A model of GPIb-IX activation via crosslinking by antibodies against the LBD. In this model, an anti-LBD antibody binds to 2 copies of GPIb-IX on opposing platelets, thereby crosslinking them. Under shear flow, this crosslinking allows the exertion of force on GPIb-IX, subsequent unfolding of the MSD, and signaling into the platelet, which leads to clearance. The ability to crosslink platelets under shear depends on a sufficiently high unbinding force between the antibody and its epitope in the LBD. Antibodies with low unbinding force to the LBD, such as AK2, cannot effectively crosslink platelets and thus cannot exert a shear force to unfold the MSD of GPIb-IX.
A model of GPIb-IX activation via crosslinking by antibodies against the LBD. In this model, an anti-LBD antibody binds to 2 copies of GPIb-IX on opposing platelets, thereby crosslinking them. Under shear flow, this crosslinking allows the exertion of force on GPIb-IX, subsequent unfolding of the MSD, and signaling into the platelet, which leads to clearance. The ability to crosslink platelets under shear depends on a sufficiently high unbinding force between the antibody and its epitope in the LBD. Antibodies with low unbinding force to the LBD, such as AK2, cannot effectively crosslink platelets and thus cannot exert a shear force to unfold the MSD of GPIb-IX.
Discussion
In this study, we provide new evidence that shear is required for anti-LBD antibody-induced GPIb-IX signaling. Following this critical observation, the binding and functional properties of anti-LBD mAbs, particularly those of AK2 and 6B4, were characterized. Although 6B4 and AK2 are similar in their high-affinity binding to the LBD, they differ significantly in their abilities to activate GPIb-IX in the platelet and induce IVIg-resistant clearance of platelets (Figures 1-3). In addition, we demonstrate for the first time that their difference in function is correlated with their difference in the unbinding force for the LBD, and consequently in the ability to sustain platelet crosslinking under shear and induce MSD unfolding (Figures 3 and 5). These results suggest a new mechanomolecular mechanism for Fc-independent platelet clearance induced by anti-LBD mAbs and related IVIg-resistant immune thrombocytopenia. In this mechanism, anti-LBD antibodies crosslink platelets through binding of both Fab domains and generate a pulling force on copies of GPIb-IX on opposing platelets. For most anti-LBD antibodies, their unbinding force for the LBD is sufficiently strong to sustain platelet crosslinking, induce unfolding of the MSD under shear, and subsequently activate GPIb-IX and result in rapid platelet clearance (Figure 6).
Although numerous studies have confirmed GPIb-IX as the endogenous VWF receptor and characterized its structure, the mechanism by which antibodies and ligands activate GPIb-IX is less well defined. An early electron microscopy study indicated that GPIb-IX is uniformly distributed on the surface of the resting platelet, and it undergoes receptor clustering in platelets activated by ristocetin/VWF and thrombin.45 Further work suggested that clustering of GPIb-IX leads to its migration or partition into platelet glycosphingolipid-enriched microdomains and promotes activation of GPIb-IX.46,47 These data led to a clustering model for the mechanism of GPIb-IX activation.48 Regarding antibody-induced GPIb-IX signaling, the clustering model could explain the requirement of bivalency (which is required if an antibody is to laterally dimerize the receptor) and the lack of a specific activating epitope within the LBD. However, it cannot adequately account for the lack of activation by certain anti-LBD (ie, AK2), most anti-GPIbβ, anti-GPIX, and anti-MSD antibodies, which should also be capable of lateral dimerization (Figure 1A).15,24-26 In comparison, the platelet-crosslinking model we propose here is the first model to fully explain the aforementioned 4 observations about anti-GPIb-IX antibodies, in addition to the shear requirement of anti-LBD antibody–induced signaling (Figure 6). First, the dimeric structure of an antibody, but not the Fc region, is used to crosslink platelets, which explains the requirement of bivalency and the feature of Fc independence.19 Second, the location of the binding epitope is not the defining feature of an activating anti-LBD antibody, which explains why these antibodies can have non-overlapping epitopes. Third, the defining feature is instead an unbinding force for the LBD that is sufficiently large to induce MSD unfolding, which explains why certain anti-LBD antibodies such as AK2 are not as effective in activating GPIb-IX as 6B4. Fourth, shear is required to generate a pulling force through an anti-LBD antibody on GPIb-IX and to induce unfolding of the MSD. Finally, compared with anti-LBD antibodies, antibodies that target the other portions of GPIb-IX are not positioned to exert a pulling force on the MSD, which explains why these antibodies generally do not activate GPIb-IX.
VWF engagement with GPIb-IX under shear was reported to induce apoptotic signaling events in human platelets and transfected Chinese hamster ovary cells, in which 14-3-3 protein ζ isoform plays a role.49 Several kinases and other molecules have been identified to mediate GPIb-IX–induced activation of integrin,50-52 but it is not clear whether they also mediate the presentation of clear-me signals on the platelet surface, such as the exposure of β-galactose and other clearance-related cellular changes. Future studies will be needed to clarify the signaling events connecting GPIb-IX activation to platelet clearance.
Overall, this study provides evidence that the bond strength or the force resistance of an antibody to the LBD of GPIb-IX, rather than the location of its binding epitope in the LBD, is the determinant of whether or not the antibody induces GPIb-IX signaling and thrombocytopenia. To the best of our knowledge, this is the first time a mechanical feature of an antibody is the defining pathological feature of a disease. Our results provide insight into the mechanism of IVIg resistance in ITP and inform potential new diagnostic and therapeutic approaches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michael C. Berndt and Francois Lanza for sharing WM23 and RAM.1 antibodies, and the Emory Children’s Pediatric Research Center Flow Cytometry Core for technical support.
This work was supported in part by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grants HL082808, HL123984 (R.L.), and F31HL134241; NIH National Institute of General Medical Sciences grant T32GM008367 (M.E.Q.); and pilot grant funds from Children’s Healthcare of Atlanta and Emory University Pediatric Flow Cytometry Core.
Authorship
Contribution: M.E.Q. performed research, analyzed results, prepared figures, and wrote the paper; M.A.D., W. Chen, A.K.S., W. Cao, and X.L. performed research; W.D., S.F.D.M., G.Z., H.N., J.W., and H.D. provided key reagents; J.P., C.M.B., and M.H. provided patient samples; and X.F.Z. and R.L. designed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renhao Li, Department of Pediatrics, Emory University School of Medicine, 2015 Uppergate Dr NE, Room 440, Atlanta, GA 30322; e-mail: renhao.li@emory.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal