Key Points
Formation of more compact plasma fibrin clots associated with impaired susceptibility to lysis predisposes to recurrent DVT.
Determination of plasma clot phenotype before anticoagulation withdrawal might help identify patients at elevated risk of DVT recurrence.
Abstract
It has been demonstrated that fibrin clots generated from plasma samples obtained from patients with prior thromboembolic events are denser and less susceptible to lysis. Such a prothrombotic fibrin clot phenotype has been suggested as a new risk factor for venous thromboembolism, but its prognostic value is unclear. To assess whether abnormal clot properties can predict recurrent deep vein thrombosis (DVT), we studied 320 consecutive patients aged 18 to 70 years following the first-ever DVT. Plasma clot properties were evaluated after 3 months of anticoagulant treatment since the index event. A mean duration of anticoagulation was 10 months (range, 4-20). Recurrent DVT was observed in 77 patients (25%; 6.6%/year) during a median follow-up of 44 months. Recurrences of DVT were associated with faster formation (−9% lag phase) of denser fibrin networks (−12% fibrin clot permeability [Ks]) and 4% higher maximum absorbance of plasma clots that displayed impaired fibrinolytic degradation (+25% prolonged clot lysis time [CLT]) and a 5% slower rate of increase in D-dimer levels during clot degradation (D-Drate; all P < .05). Proximal DVT alone, higher C-reactive protein, D-dimer, peak thrombin, lower Ks, shorter lag phase, decreased D-Drate, and prolonged CLT were independent predictors of recurrences (all P < .05). Individuals characterized by low Ks (≤7.3 × 10−9 cm2) and prolonged CLT (>96 min) were at the highest risk of recurrent DVT (odds ratio, 15.8; 95% confidence interval, 7.5-33.5). Kaplan-Meier curves showed that reduced Ks and prolonged CLT predicted recurrent DVT. We demonstrate that unfavorably altered clot properties may predict recurrent DVT after anticoagulation withdrawal.
Introduction
Venous thromboembolism (VTE) encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE) occurs for the first time among people of European ancestry in 104 to 183 individuals per 100 000 person-years.1 The annualized event rate is 7.9% per patient-year for unprovoked VTE and 1.0% per patient-year for VTE provoked by surgery in the first 12 months after the withdrawal of anticoagulation therapy.2 The cumulative incidence rate of VTE recurrence reaches 10% to 11% at 1 year, 21.5% to 30% at 5 years, and 39.9% after 10 years.3-5 Risk factors of VTE recurrences include unprovoked events, male sex, proximal DVT, concomitant PE, and the presence of a filter in the vena cava.6-9 Moreover, severe thrombophilia, increased thrombin generation, and elevated D-dimer levels are associated with VTE recurrences.6,7,9,10 However, prediction of recurrent VTE following anticoagulation withdrawal remains challenging.
Growing evidence, largely derived from case-control studies, suggests that formation of more compact clots, relatively resistant to lysis, may predispose to arterial thromboembolism and VTE.11,12 The so-called prothrombotic clot phenotype is characterized by a small size of pores in fibrin networks reflected by low fibrin clot permeability coefficient (Ks) and reduced overall plasma clot fibrinolysis, expressed as clot lysis time (CLT).13 Unfavorably altered fibrin clot features have been reported in patients following unprovoked VTE14 and complications of VTE, including chronic thromboembolic pulmonary hypertension,15 residual vein obstruction,16 and postthrombotic syndrome.17 Alterations similar to clot characteristics have been observed in several diseases associated with an increased risk of thromboembolic events, including cancer, diabetes, and antiphospholipid syndrome.13
Recently, Zabczyk et al have shown that lower Ks and prolonged CLT are associated with recurrent PE.7
There is inconsistent evidence that prolonged CLT can predispose to recurrent VTE in some patient groups17-20 but longitudinal studies that comprehensively assess a predictive value of various clot features are lacking. We hypothesized that reduced Ks, combined with prolonged CLT, predicts recurrent DVT. The aim of this cohort study was to investigate whether fibrin clot properties measured 3 months since the DVT event predict recurrences.
Patients and methods
We screened 368 white patients aged 18 to 70 years with a history of the first-ever isolated DVT or combined with PE who were referred to an outpatient clinic between October 2008 and June 2010. The diagnosis of DVT was established by a positive finding in color duplex sonography (visualization of an intraluminal thrombus in calf, popliteal, femoral, or iliac veins). The diagnosis of PE was based on the presence of characteristic symptoms and positive results of high-resolution spiral computed tomography. All patients received initially unfractionated or low-molecular-weight heparin (LMWH) and then vitamin K antagonists (VKAs) were continued for at least 3 months in patients with DVT trigged by transient risk factors and 6 months or longer in patients with unprovoked DVT. We excluded subjects with known malignancy (n = 12), chronic kidney disease stage 4 or 5 (n = 4), severe thrombophilia (including antiphospholipid syndrome, antithrombin deficiency, homozygous factor V (FV) Leiden, or prothrombin 20210A mutations) (n = 24), pregnancy (n = 1), international normalized ratio (INR) >1.2 (n = 2), acute coronary syndrome or ischemic stroke within the previous 3 months (n = 2), and acute infection or exacerbated chronic inflammation (n = 3). Definitions of VTE types, comorbidities and smoking status were shown in supplemental Materials, Appendix A (available on the Blood Web site). The University Bioethical Committee approved the study, and all participants provided informed consent in accordance with the Declaration of Helsinki.
Follow-up
The follow-up started at the time of blood collection for clot analysis and was carried out on the 6-month basis (a visit at the center or a telephone contact). All subjects were asked about current management. The primary end point of the study was symptomatic recurrent DVT. The diagnosis of DVT was established based on positive finding in color duplex sonography. In cases of suspected DVT recurrence in the same leg as the index event, noncompressibility of a previously compressible venous segment or an increase of at least 4 mm in the residual diameters was applied to confirm the diagnosis.
The end of follow-up was defined as the date of a recurrent thromboembolic event or death. The minimal follow-up of 36 months was chosen to observe the sufficient number of DVT recurrences.
Laboratory investigations
Fasting blood samples were drawn from an antecubital vein with minimal stasis at 8 to 10 am, after ∼3 months (12 to 15 weeks) of anticoagulant treatment since DVT diagnosis based on routine practice at our center where, at that time, most patients are referred for further laboratory workup and clinical evaluation (including the decision of extended anticoagulation therapy). Patients treated with VKA were switched to an LMWH for 10 to 14 days, and blood samples were collected 16 to 24 hours after the last injection. In a subset of patients (n = 167), we determined anti-Xa activity at the time of blood draw (Siemens). After that, patients continued anticoagulation with VKA according to local guidelines. Blood cell count, lipid profiles, glucose, creatinine, and INR were assessed by routine laboratory techniques. Blood samples (vol/vol, 9:1 of 3.2% trisodium citrate) were centrifuged at 2000g for 10 minutes within 30 minutes of the draw, and supernatant was aliquoted and stored at −80°C until analysis. Fibrinogen was determined using the Clauss method. High-sensitivity CRP was measured by nephelometry (Siemens). Immunoenzymatic assays were used to assess D-dimer, tPa and plasminogen activator inhibitor-1 antigens (American Diagnostica).
Plasma clot permeability, the kinetics of clot formation, and lysis were determined in duplicate by technicians blinded to the origin of the samples (intraassay and interassay coefficients of variation, 5% to 7%).
Fibrin clot permeability
Fibrin clot permeation in the assay involving addition of tissue factor (TF) was assessed as described previously.21 Briefly, we mixed 60 µL plasma with 60 µL coagulation trigger containing 1 IU/mL human thrombin and 20 mM CaCl2. 100 µL of this mixture was immediately added to a plastic cylinder prepared from a serological pipette (Sarstedt, Nümbrecht, Germany). After incubation, the plasma clot was percolated with Tris buffer. The permeability coefficient was calculated by using the formula Ks (×10−9 cm2) = Q × L × η/t × A × ∆P, where Q (cm3) is the flow rate at time t (s), L (cm) is the length of the fibrin gel, η (dyne × s/cm2) is the viscosity of the liquid, A (cm2) is the cross-sectional area, and ∆p (dyne/cm2) is the differential pressure. The intraassay coefficient of variation was 6.8%.
Turbidity measurements
Plasma citrated samples were mixed 2:1 with a Tris buffer containing 0.6 IU/mL human thrombin (Sigma) and 50 mM CaCl2, and polymerization was initiated. The absorbance was read at 405 nm. A lag phase before start of fibrin polymerization, the slope of the polymerization curve, and maximum absorbance at the plateau were recorded.14,22
Plasma clot lysis assays
To assess the efficiency of clot lysis, 2 methods were used. In the first assay, CLT was measured. Briefly, citrated plasma was mixed with 15 mmol/L CaCl2, 10 000-diluted human TF (Innovin, Siemens) with a final concentration of 0.6 pM, 12 µmol/L phospholipid vesicles, and 60 ng mL−1 recombinant tissue plasminogen activator (Boehringer Ingelheim, Ingelheim, Germany). The turbidity was measured at 405 nm at 37°C. CLT was defined as the time from the midpoint of the clear-to-maximum-turbid transition, which represents clot formation, to the midpoint of the maximum-turbid-to-clear transition (representing the lysis of the clot). In the second assay, fibrin clots, formed as for the permeability evaluation, were perfused with a Tris buffer containing 0.2 µmol/L recombinant tissue plasminogen activator.23 D-dimer levels were measured every 20 minutes in the effluent using an enzyme-linked immunosorbent assay (American Diagnostica). The experiment was stopped, usually after 80 to 120 minutes, when the fibrin gel collapsed under the pressure. The maximum rate of increase in D-dimer levels (D–Drate) and maximum D-dimer concentrations (D–Dmax) were analyzed.
Calibrated automated thrombogram
The calibrated automated thrombogram assay was performed using commercial reagents (Thrombinoscope, BV, Maastricht, Netherlands).24 Briefly, 80 µL plasma was mixed with 20 µL of a reagent containing recombinant relipidated TF and phospholipids at final concentrations of 5 pM and 4 mM, respectively. A starting reagent contains 100 mM CaCl2 and 2.5 mM fluorogenic substrate. Fluorescence intensity was recorded by the Fluoroskan Ascent microplate fluorometer (Thermo Fisher Scientific, Vantaa, Finland). We assessed the maximum concentration of thrombin formed (peak thrombin), the area under the curve (AUC) representing the endogenous thrombin potential (ETP), and the time from start of thrombin generation to the maximum thrombin value (the time to thrombin peak).
Genotyping
Thrombophilia screening was performed in all study participants. FV Leiden and prothrombin 20210A polymorphisms were determined by the polymerase chain reaction followed by restriction-fragment length polymorphism analysis as described previously.14
Statistical analysis
The study was powered to have a 90% chance of detecting a 10% difference in CLT using an α value of 0.05 based on the CLT values presented previously (mean ± standard deviation [SD], 84.4 ± 10.5 minutes).17 In order to demonstrate such a difference or greater, 34 patients were required in each group. In turn, to demonstrate such a difference or greater in Ks using an α value of 0.05, based on the previous values (mean ± SD, 7.4 ± 1.3 × 10−9 cm2),25 at least 74 patients were required in each group.
Continuous variables were expressed as mean ± SD or median and interquartile range (IQR). The categorical and qualitative variables are expressed as number (percentages). The normality of variable distribution was assessed by the Shapiro-Wilk test. The differences between the groups were compared using the Student or Welch t test depending on the equality of variances for normally distributed variables. The Mann-Whitney U test was used for nonnormally distributed continuous variables. Categorical variables were compared by the Fisher exact test. The association between 2 continuous variables was assessed by the Spearman’s rank correlation test or the Pearson correlation test. Receiver operating characteristic (ROC) curves and AUC were used to analyze the discriminatory power of variables in respect to the risk of recurrent DVT. To evaluate which fibrin clot feature is the best predictor of recurrent DVT, pairwise comparisons of AUC in ROC curves with the Bonferroni correction were used. The Kaplan-Meier method was applied to estimate the event free rates for patients with recurrent DVT in reference to the risk factors of recurrent DVT. The optimal cutoffs points for coagulation, fibrinolysis, and fibrin clot parameters were determined in the ROC analysis. The Kaplan-Meier survival curves were compared by the log-rank test. The univariate and multivariate Cox proportional hazards regression models, based on backward stepwise regression with a potential confounders locked in the models a priori were used to establish the risk factors for recurrent DVT. The results were presented as hazard ratios (HR) with 95% confidence intervals (CI). The logistic regression was applied to assess odds ratio (OR) with 95% CI for recurrent DVT with regard to the fibrin clot phenotype. The level of significance for the 2-sided tests was set below 0.05. Statistical analyses were performed with package R,26 G*Power 3.1,27 and Statistica 12.5 software (StatSoft, Tulsa, OK).
Results
At baseline
A total of 320 DVT patients (155 men and 165 women) at a median age of 46 were included in the final analysis (Table 1). There were 249 (77.8%) patients with isolated DVT, including 172 (53.8%) with proximal DVT, and 71 (22.2%) subjects with PE combined with DVT. Patients with a provoked episode did not differ from those with unprovoked DVT in terms of baseline characteristics (data not shown).
Baseline characteristics
| . | DVT patients (n = 320) . | Recurrent DVT (n = 77) . | Nonrecurrent DVT (n = 231) . | P* . |
|---|---|---|---|---|
| Variable | ||||
| Age (y) | 46 (36-54) | 48 (39-58) | 45 (36-53) | .046 |
| Male sex, n (%) | 155 (48.44) | 30 (39.0) | 119 (51.5) | .07 |
| BMI, kg/m2 | 26.0 (23.6-29.1) | 26.3 (24.2-28.6) | 25.9 (23.4-29.4) | .45 |
| Unprovoked VTE, n (%) | 159 (49.69) | 45 (58.4) | 109 (47.18) | .11 |
| Risk factors, n (%) | ||||
| Trauma/surgery | 91 (28.4) | 15 (19.5) | 74 (32.0) | .04 |
| Hospitalization | 23 (7.2) | 6 (7.8) | 15 (6.5) | .79 |
| Pregnancy/postpartum† | 16 (9.70) | 5 (10.6) | 10 (8.9) | .77 |
| Contraceptives† | 42 (25.45) | 9 (19.1) | 32 (28.6) | .24 |
| Current smokers | 112 (35.0) | 15 (19.5) | 92 (39.8) | .001 |
| Smokers (≥10 cigarettes daily) | 18 (5.6) | 13 (16.9) | 5 (2.2) | <.0001 |
| Family history of VTE | 51 (15.94) | 12 (15.6) | 38 (16.5) | .99 |
| Comorbidities, n (%) | ||||
| Hypertension | 96 (30.0) | 21 (27.3) | 73 (31.6) | .57 |
| Diabetes | 13 (4.1) | 4 (5.2) | 9 (3.9) | .74 |
| COPD | 16 (5.0) | 4 (5.2) | 12 (5.2) | .99 |
| Heart failure | 10 (3.1) | 2 (2.6) | 8 (3.5) | .99 |
| Medications, n (%) | ||||
| Aspirin | 46 (14.4) | 4 (5.2) | 40 (17.3) | .008 |
| Sulodexide alone | 37 (11.6) | 11 (14.3) | 22 (10.8) | .42 |
| ACEI | 53 (16.6) | 8 (10.4) | 43 (18.6) | .11 |
| β-blockers | 12 (3.7) | 3 (3.9) | 8 (3.5) | .99 |
| Statins | 144 (45.0) | 28 (36.4) | 109 (47.2) | .11 |
| Laboratory parameters | ||||
| Creatinine, µmol/L | 70.00 (61.88-79.56) | 70.72 (62.00-80.00) | 70.00 (61.88-79.56) | .38 |
| Glucose, mmol/L | 5.06 (4.70-5.50) | 5.07 (4.80-5.50) | 5.1 (4.70-5.50) | .69 |
| TG, mmol/L | 1.20 (0.80-1.70) | 1.26 (0.79-1.79) | 1.20 (0.79-1.67) | .49 |
| TC, mmol/L | 5.2 (4.4-5.8) | 5.04 (4.43-5.78) | 5.20 (4.33-5.84) | .54 |
| LDL-C, mmol/L | 3.19 (0.89) | 3.09 (0.84) | 3.23 (0.91) | .22 |
| HDL-C, mmol/L | 1.46 (1.19-1.71) | 1.43 (1.17-1.68) | 1.45 (1.19-1.74) | .35 |
| CRP, mg/L | 1.49 (0.92-2.25) | 1.71 (1.17-2.26) | 1.46 (0.89-2.25) | .04 |
| INR | 0.98 (0.90-1.04) | 0.98 (0.91-1.04) | 0.97 (0.89-1.03) | .35 |
| Fibrinogen, g/L | 3.03 (2.53-3.89) | 2.90 (2.50-3.59) | 3.09 (2.55-3.94) | .16 |
| D-dimer, mg/dL | 279 (226-337) | 336 (254-432) | 259 (218-311) | <.0001 |
| tPA, ng/Ml | 9.6 (7.2-11.5) | 8.9 (6.8-11.7) | 9.7 (7.7-11.4) | .35 |
| PAI-1, ng/mL | 11.20 (8.5-14.5) | 10.87 (8.4-16.3) | 11.40 (8.7-13.9) | .91 |
| Peak thrombin, nM | 245.4 (210.0-291.8) | 286.43 (241.0-352.0) | 239.0 (202.0-279.5) | <.0001 |
| ETP, nM×min | 1433 (1346-1671) | 1622 (1465-1791) | 1510 (1304-1638) | <.0001 |
| Time to thrombin peak, min | 4.68 (4.23-5.33) | 4.67 (4.22-5.33) | 4.67 (4.21-5.52) | .26 |
| Genotyping, n (%) | ||||
| FV Leiden | 42 (13.12) | 8 (10.39) | 31 (13.42) | .56 |
| Prothrombin 20210A | 15 (4.69) | 4 (5.19) | 11 (4.53) | .99 |
| . | DVT patients (n = 320) . | Recurrent DVT (n = 77) . | Nonrecurrent DVT (n = 231) . | P* . |
|---|---|---|---|---|
| Variable | ||||
| Age (y) | 46 (36-54) | 48 (39-58) | 45 (36-53) | .046 |
| Male sex, n (%) | 155 (48.44) | 30 (39.0) | 119 (51.5) | .07 |
| BMI, kg/m2 | 26.0 (23.6-29.1) | 26.3 (24.2-28.6) | 25.9 (23.4-29.4) | .45 |
| Unprovoked VTE, n (%) | 159 (49.69) | 45 (58.4) | 109 (47.18) | .11 |
| Risk factors, n (%) | ||||
| Trauma/surgery | 91 (28.4) | 15 (19.5) | 74 (32.0) | .04 |
| Hospitalization | 23 (7.2) | 6 (7.8) | 15 (6.5) | .79 |
| Pregnancy/postpartum† | 16 (9.70) | 5 (10.6) | 10 (8.9) | .77 |
| Contraceptives† | 42 (25.45) | 9 (19.1) | 32 (28.6) | .24 |
| Current smokers | 112 (35.0) | 15 (19.5) | 92 (39.8) | .001 |
| Smokers (≥10 cigarettes daily) | 18 (5.6) | 13 (16.9) | 5 (2.2) | <.0001 |
| Family history of VTE | 51 (15.94) | 12 (15.6) | 38 (16.5) | .99 |
| Comorbidities, n (%) | ||||
| Hypertension | 96 (30.0) | 21 (27.3) | 73 (31.6) | .57 |
| Diabetes | 13 (4.1) | 4 (5.2) | 9 (3.9) | .74 |
| COPD | 16 (5.0) | 4 (5.2) | 12 (5.2) | .99 |
| Heart failure | 10 (3.1) | 2 (2.6) | 8 (3.5) | .99 |
| Medications, n (%) | ||||
| Aspirin | 46 (14.4) | 4 (5.2) | 40 (17.3) | .008 |
| Sulodexide alone | 37 (11.6) | 11 (14.3) | 22 (10.8) | .42 |
| ACEI | 53 (16.6) | 8 (10.4) | 43 (18.6) | .11 |
| β-blockers | 12 (3.7) | 3 (3.9) | 8 (3.5) | .99 |
| Statins | 144 (45.0) | 28 (36.4) | 109 (47.2) | .11 |
| Laboratory parameters | ||||
| Creatinine, µmol/L | 70.00 (61.88-79.56) | 70.72 (62.00-80.00) | 70.00 (61.88-79.56) | .38 |
| Glucose, mmol/L | 5.06 (4.70-5.50) | 5.07 (4.80-5.50) | 5.1 (4.70-5.50) | .69 |
| TG, mmol/L | 1.20 (0.80-1.70) | 1.26 (0.79-1.79) | 1.20 (0.79-1.67) | .49 |
| TC, mmol/L | 5.2 (4.4-5.8) | 5.04 (4.43-5.78) | 5.20 (4.33-5.84) | .54 |
| LDL-C, mmol/L | 3.19 (0.89) | 3.09 (0.84) | 3.23 (0.91) | .22 |
| HDL-C, mmol/L | 1.46 (1.19-1.71) | 1.43 (1.17-1.68) | 1.45 (1.19-1.74) | .35 |
| CRP, mg/L | 1.49 (0.92-2.25) | 1.71 (1.17-2.26) | 1.46 (0.89-2.25) | .04 |
| INR | 0.98 (0.90-1.04) | 0.98 (0.91-1.04) | 0.97 (0.89-1.03) | .35 |
| Fibrinogen, g/L | 3.03 (2.53-3.89) | 2.90 (2.50-3.59) | 3.09 (2.55-3.94) | .16 |
| D-dimer, mg/dL | 279 (226-337) | 336 (254-432) | 259 (218-311) | <.0001 |
| tPA, ng/Ml | 9.6 (7.2-11.5) | 8.9 (6.8-11.7) | 9.7 (7.7-11.4) | .35 |
| PAI-1, ng/mL | 11.20 (8.5-14.5) | 10.87 (8.4-16.3) | 11.40 (8.7-13.9) | .91 |
| Peak thrombin, nM | 245.4 (210.0-291.8) | 286.43 (241.0-352.0) | 239.0 (202.0-279.5) | <.0001 |
| ETP, nM×min | 1433 (1346-1671) | 1622 (1465-1791) | 1510 (1304-1638) | <.0001 |
| Time to thrombin peak, min | 4.68 (4.23-5.33) | 4.67 (4.22-5.33) | 4.67 (4.21-5.52) | .26 |
| Genotyping, n (%) | ||||
| FV Leiden | 42 (13.12) | 8 (10.39) | 31 (13.42) | .56 |
| Prothrombin 20210A | 15 (4.69) | 4 (5.19) | 11 (4.53) | .99 |
Values are given as mean ± SD (for LDL) or median (IQR, for other variables), unless indicated otherwise.
ACEI, angiotensin-converting enzyme inhibitor; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PAI-1, plasminogen activator inhibitor-1; TC, total cholesterol; TG, triglyceride; tPA, tissue plasminogen activator.
Recurrent versus nonrecurrent groups.
Females only.
Laboratory variables measured in blood samples taken 3 months after DVT are shown in Tables 1 and 2. No differences in fibrin clot properties were found between men and women (data not shown) or between patients with provoked and unprovoked DVT (supplemental Table 1). As expected, fibrinogen was inversely correlated with Ks (r = −0.36), whereas it was positively correlated with maximum absorbance of plasma clots (ΔAbs) (r = 0.61) and CLT (r = 0.25) (all P < .05). There were more potent correlations of ETP with fibrin clot parameters compared with those for peak thrombin generated (supplemental Table 2). Of note, CRP and D-dimer were weakly associated with both Ks and CLT (supplemental Table 2).
Comparison of fibrin clot features
| Variable . | All patients (n = 308) . | Recurrent DVT (n = 77) . | Nonrecurrent DVT (n = 231) . | P* . |
|---|---|---|---|---|
| Ks, 10−9 cm2 | 7.40 (6.55-7.90) | 6.60 (6.20-7.20) | 7.50 (6.90-8.10) | <.0001 |
| Lag phase, s | 43 (39-46) | 40 (35-44) | 44 (40-47) | <.0001 |
| ΔAbs | 0.81 (0.77-0.85) | 0.83 (0.79-0.88) | 0.80 (0.77-0.85) | .01 |
| D-Dmax, mg/L | 4.07 (3.69-4.39) | 3.98 (3.69-4.41) | 4.07 (3.66-4.33) | .45 |
| D-Drate, mg/L/min | 0.073 (0.068-0.079) | 0.069 (0.066-0.072) | 0.073 (0.069-0.080) | <.0001 |
| CLT, min | 86 (74-99) | 101 (92-110) | 81 (70-94) | <.0001 |
| Variable . | All patients (n = 308) . | Recurrent DVT (n = 77) . | Nonrecurrent DVT (n = 231) . | P* . |
|---|---|---|---|---|
| Ks, 10−9 cm2 | 7.40 (6.55-7.90) | 6.60 (6.20-7.20) | 7.50 (6.90-8.10) | <.0001 |
| Lag phase, s | 43 (39-46) | 40 (35-44) | 44 (40-47) | <.0001 |
| ΔAbs | 0.81 (0.77-0.85) | 0.83 (0.79-0.88) | 0.80 (0.77-0.85) | .01 |
| D-Dmax, mg/L | 4.07 (3.69-4.39) | 3.98 (3.69-4.41) | 4.07 (3.66-4.33) | .45 |
| D-Drate, mg/L/min | 0.073 (0.068-0.079) | 0.069 (0.066-0.072) | 0.073 (0.069-0.080) | <.0001 |
| CLT, min | 86 (74-99) | 101 (92-110) | 81 (70-94) | <.0001 |
Values are given as median (IQR). All variables were adjusted for fibrinogen.
Recurrent vs nonrecurrent DVT.
Anti-Xa activity, available in 167 (52.2%) patients, was analyzed to confirm washout of LMWH; in 27 (16.2%) subjects, detectable anti-Xa activity was found (maximum, 0.15 IU/mL; median [IQR], 0.1 [0.07-0.11] IU/mL). No differences in fibrin clot properties and thrombin generation parameters were found between patients with detectable and nondetectable anti-Xa (data not shown).
Follow-up
Twelve patients (3.8%) were lost to follow-up. Four patients (1.3%) died during follow-up; the known causes included gastrointestinal bleeding, a traffic accident, lung cancer, and sudden death. Mean duration of anticoagulation was 10 months (range, 4-20) and did not differ with regard to the DVT recurrence (data not shown). After anticoagulation withdrawal, 36 patients (11.7%) were treated with sulodexide 250 to 500 mg twice a day and 44 subjects (14.3%) with low-dose aspirin (3 patients used both medications).
During a median follow-up of 44 months (range, 2-72 months), encompassing 1159 patient-years, 77 subjects had recurrent DVT (25%; 6.6% per year; Table 1). Proximal DVT was recognized more often than distal DVT in patients who experienced recurrent DVT (81.8% vs 18.2%) and in those free of this event (73.63% vs 26.4%). Nonsmokers were overrepresented among patients without recurrent DVT (Table 1). However, in the group with recurrent DVT, patients who smoked ≥10 cigarettes daily were more likely to experience recurrent DVT than those who smoked <10 cigarettes daily (13 [16.9%] vs 2 [2.6%], P < .01).
Patients with recurrent DVT had 17.1% higher CRP levels, 29.7% higher D-dimer levels, 19.8% higher peak thrombin, and 7.4% longer ETP compared with the remainder (Table 1). Other routine laboratory test results, including fibrinogen, were similar. The distribution of the FV Leiden and prothrombin 20210A polymorphisms studied was similar in both groups (Table 1).
Fibrin clots and DVT recurrence
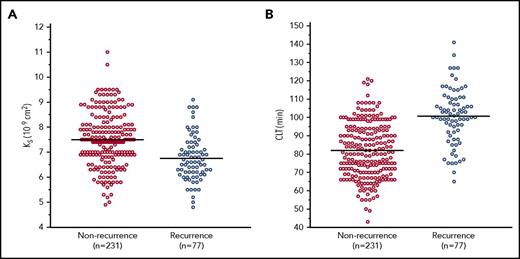
Patients with DVT recurrences compared with the remainder had lower Ks (−12%), indicating formation of more compact clots, shorter lag phase (−9%), higher ΔAbs (+4%), and impaired fibrinolysis, as evidenced by reduced maximum rate of increase in D-dimer levels in the lysis assay (D-Drate) (−5%) and prolonged CLT (+25%) (Table 2; Figure 1). Fibrin clot variables did not differ in patients treated with sulodexide or low-dose aspirin vs the remainder (supplemental Table 3).
Ksand CLT for patients with recurrent and nonrecurrent DVT. Horizontal lines represent the means of each group.
Ksand CLT for patients with recurrent and nonrecurrent DVT. Horizontal lines represent the means of each group.
Predictors of recurrent DVT
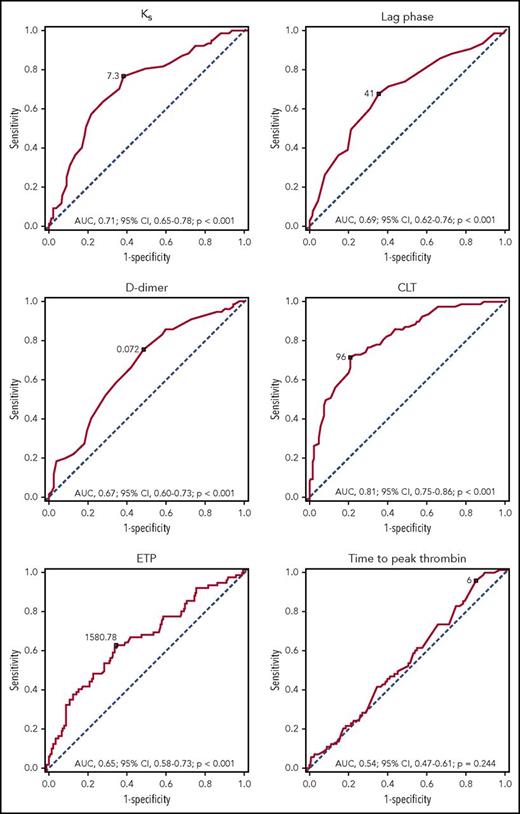
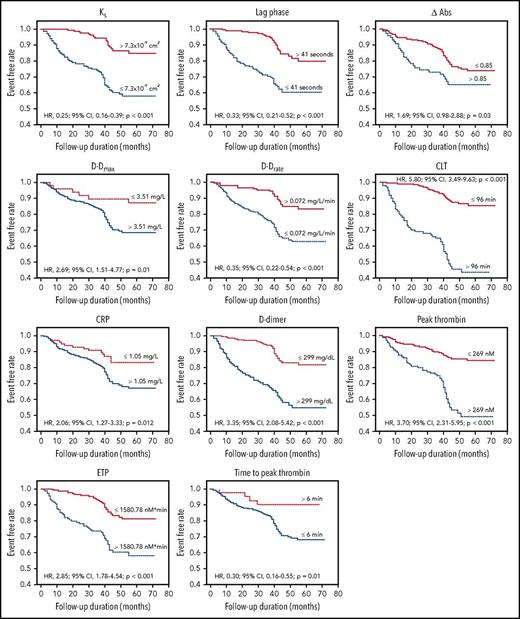
We observed a moderate accuracy for Ks, lag phase, and D-Drate, together with high accuracy for CLT, in the prediction of recurrent DVT (Table 3; Figure 2). The Kaplan-Meier event-free survival curves showed that reduced Ks, shorter lag phase, higher ΔAbs, and reduced D-Drate, together with elevated maximum D-dimer levels in the lysis assay (D-Dmax), prolonged CLT, increased CRP and D-dimer levels, elevated peak thrombin, higher ETP, and shorter time to thrombin peak were predictors of recurrent DVT (Figure 3).
Measures of performance to predict the DVT recurrence during follow-up based on optimal cutoffs investigated in the ROC analysis
| Variable . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | PPV, % (95% CI) . | NPV, % (95% CI) . | Positive LR (95% CI) . | Negative LR (95% CI) . |
|---|---|---|---|---|---|---|
| Ks, 7.3 × 10−9 cm2 | 76.62 (65.6-85.5) | 61.47 (54.9- 67.8) | 39. 86 (31.9- 48.2) | 88.75 (82.8- 93.2) | 1.99 (1.62- 2.44) | 0.38 (0.25-0.58) |
| Lag phase, 41 s | 67.53 (55.9- 77.8) | 64.50 (57.9- 70.7) | 38.8 (30.5-47.6) | 85.63 (79.5- 90.5) | 1.90 (1.51- 2.40) | 0.50 (0.36-0.70) |
| ΔAbs, 0.85 | 45.45 (34.1- 57.2) | 70.12 (63.8-76.0) | 33.65 (24.7- 43.6) | 79.41 (73.2- 84.7) | 1.52 (1.11- 2.08) | 0.78 (0.62-0.97) |
| D-Dmax, 3.51 mg/L | 93.51 (85.5- 97.9) | 18.18 (13.4- 23.8) | 27.58 (22.3- 33.4) | 89.36 (76.9- 96.5) | 1.14 (1.05- 1.24) | 0.36 (0.15-0.87) |
| D-Drate, 0.072 mg/L/min | 75.32 (64.2- 84.4) | 51.51 (44.9- 58.1) | 34.11 (27.0- 41.8) | 86.23 (79.3- 91.5) | 1.55 (1.29- 1.87) | 0.48 (0.32-0.72) |
| CLT, 96 min | 71.43 (60.0-81.2) | 79.22 (73.4- 84.3) | 53.39 (43.3- 63.3) | 89.26 (84.2-93.2) | 3.44 (2.58- 4.59) | 0.36 (0.25-0.52) |
| Peak thrombin, 269 nM | 68.83 (57.26-78.91) | 71.00 (64.68-76.76) | 44.17 (35.11-53.52) | 87.23 (81.60-91.65) | 2.37 (1.85-3.05) | 0.44 (0.31-0.62) |
| ETP, 1580.78 nM × min | 62.34 (50.56-73.13) | 65.37 (58.85-71.49) | 37.5 (29.10-46.49) | 83.89 (77.69-88.94) | 1.8 (1.4-2.31) | 0.58 (0.43-0.78) |
| Time to thrombin peak, 6 min | 94.81 (87.23-98.57) | 16.02 (11.54-21.40) | 27.34 (22.09-33.11) | 90.24 (76.87-97.28) | 1.13 (1.05-1.22) | 0.32 (0.12-0.88) |
| Variable . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | PPV, % (95% CI) . | NPV, % (95% CI) . | Positive LR (95% CI) . | Negative LR (95% CI) . |
|---|---|---|---|---|---|---|
| Ks, 7.3 × 10−9 cm2 | 76.62 (65.6-85.5) | 61.47 (54.9- 67.8) | 39. 86 (31.9- 48.2) | 88.75 (82.8- 93.2) | 1.99 (1.62- 2.44) | 0.38 (0.25-0.58) |
| Lag phase, 41 s | 67.53 (55.9- 77.8) | 64.50 (57.9- 70.7) | 38.8 (30.5-47.6) | 85.63 (79.5- 90.5) | 1.90 (1.51- 2.40) | 0.50 (0.36-0.70) |
| ΔAbs, 0.85 | 45.45 (34.1- 57.2) | 70.12 (63.8-76.0) | 33.65 (24.7- 43.6) | 79.41 (73.2- 84.7) | 1.52 (1.11- 2.08) | 0.78 (0.62-0.97) |
| D-Dmax, 3.51 mg/L | 93.51 (85.5- 97.9) | 18.18 (13.4- 23.8) | 27.58 (22.3- 33.4) | 89.36 (76.9- 96.5) | 1.14 (1.05- 1.24) | 0.36 (0.15-0.87) |
| D-Drate, 0.072 mg/L/min | 75.32 (64.2- 84.4) | 51.51 (44.9- 58.1) | 34.11 (27.0- 41.8) | 86.23 (79.3- 91.5) | 1.55 (1.29- 1.87) | 0.48 (0.32-0.72) |
| CLT, 96 min | 71.43 (60.0-81.2) | 79.22 (73.4- 84.3) | 53.39 (43.3- 63.3) | 89.26 (84.2-93.2) | 3.44 (2.58- 4.59) | 0.36 (0.25-0.52) |
| Peak thrombin, 269 nM | 68.83 (57.26-78.91) | 71.00 (64.68-76.76) | 44.17 (35.11-53.52) | 87.23 (81.60-91.65) | 2.37 (1.85-3.05) | 0.44 (0.31-0.62) |
| ETP, 1580.78 nM × min | 62.34 (50.56-73.13) | 65.37 (58.85-71.49) | 37.5 (29.10-46.49) | 83.89 (77.69-88.94) | 1.8 (1.4-2.31) | 0.58 (0.43-0.78) |
| Time to thrombin peak, 6 min | 94.81 (87.23-98.57) | 16.02 (11.54-21.40) | 27.34 (22.09-33.11) | 90.24 (76.87-97.28) | 1.13 (1.05-1.22) | 0.32 (0.12-0.88) |
LR, likelihood ratio; NPV negative predictive value; PPV, positive predictive value.
ROC curves for Ks, lag phase,D-dimer level, CLT, ETP, and time to peak thrombin. The outcome investigated in the ROC analysis was DVT recurrence. Optimal cutoffs and AUC (with 95% CIs) are presented.
ROC curves for Ks, lag phase,D-dimer level, CLT, ETP, and time to peak thrombin. The outcome investigated in the ROC analysis was DVT recurrence. Optimal cutoffs and AUC (with 95% CIs) are presented.
Kaplan-Meier curves of recurrent DVT during follow-up. Event-free survival in patients with recurrent DVT following discontinuation of anticoagulation with regard to permeability coefficient, Ks (log-rank P < .001), lag phase (log-rank P < .001), difference between maximum and minimum absorbance in the turbidimetric clotting assay, ΔAbs (log-rank P = .03), maximum D-dimer levels in the lysis assay, D-Dmax (log-rank P = .01), maximum rate of D-dimer release from fibrin clots, D-Drate (log-rank P < .001), CLT (log-rank P < .001), CRP (log-rank P = .01), D-dimer (log-rank P < .0001), peak thrombin (log-rank P < .0001), ETP (log-rank P < .001), and time to thrombin peak (log-rank P = .01). HRs (with 95% CIs) and P values are presented.
Kaplan-Meier curves of recurrent DVT during follow-up. Event-free survival in patients with recurrent DVT following discontinuation of anticoagulation with regard to permeability coefficient, Ks (log-rank P < .001), lag phase (log-rank P < .001), difference between maximum and minimum absorbance in the turbidimetric clotting assay, ΔAbs (log-rank P = .03), maximum D-dimer levels in the lysis assay, D-Dmax (log-rank P = .01), maximum rate of D-dimer release from fibrin clots, D-Drate (log-rank P < .001), CLT (log-rank P < .001), CRP (log-rank P = .01), D-dimer (log-rank P < .0001), peak thrombin (log-rank P < .0001), ETP (log-rank P < .001), and time to thrombin peak (log-rank P = .01). HRs (with 95% CIs) and P values are presented.
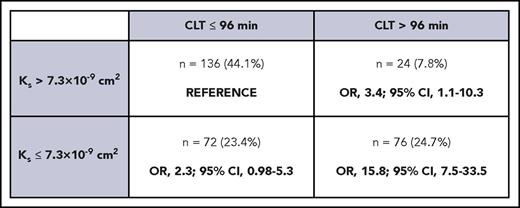
Patients were divided into 4 groups according to Ks and CLT cutoffs were estimated using ROC analysis, as shown in Figure 4, to establish the least favorable plasma clot phenotype. Individuals with low Ks (≤7.3 × 10−9 cm2) combined with prolonged CLT (>96 min) had the highest risk of recurrent DVT (OR, 15.8; 95% CI, 7.5-33.5).
Phenotypes of plasma fibrin clots in DVT patients based on Ksand CLT measurements, with calculation of OR of developing recurrent thrombosis during follow-up. The so-called prothrombotic phenotype was associated with a 15.8-fold higher risk of DVT recurrence.
Phenotypes of plasma fibrin clots in DVT patients based on Ksand CLT measurements, with calculation of OR of developing recurrent thrombosis during follow-up. The so-called prothrombotic phenotype was associated with a 15.8-fold higher risk of DVT recurrence.
The univariate Cox proportional hazards analysis showed that older age, female sex, higher CRP levels, elevated D-dimer levels, increased peak thrombin generated, Ks ≤7.3 × 10−9 cm2, lag phase ≤41 seconds, ΔAbs >0.85, D-Dmax >3.51 mg/L, D-Drate ≤0.072 mg/L per minute, and CLT >96 minutes were associated with recurrent DVT (Table 4). The model incorporating all fibrin clot properties showed that proximal DVT alone, higher CRP levels, increased D-dimer, elevated peak thrombin, Ks ≤7.3 × 10−9 cm2, lag phase ≤41 seconds, D-Drate ≤0.072 mg/L per minute, and CLT >96 minutes were independent predictors for DVT recurrences (Table 4, P = .0004 compared with the traditional model in supplemental Table 4). The Cox analysis with categorical fibrin clot properties after adjustment for the use of aspirin and sulodexide showed similar results (data not shown).
The Cox proportional hazards model for risk factors of DVT recurrence: model with categorical fibrin clot properties
| Variable . | HR per . | Univariate . | Multivariate* . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Age | 1 y | 1.02 (1-1.04) | .017 | 1.02 (0.99-1.04) | .11 |
| Male sex | No/yes | 0.63 (0.40- 0.99) | .045 | 0.72 (0.43-1.20) | .21 |
| BMI | 1 kg/m2 | 1.02 (0.97-1.08) | .449 | — | — |
| Time of anticoagulation | 1 mo | 1.05 (0.99-1.11) | .111 | — | — |
| Unprovoked VTE | No/yes | 1.46 (0.93-2.29) | .100 | — | — |
| Proximal DVT alone | No/yes | 1.19 (0.76-1.87) | .45 | 1.86 (1.13-3.06) | .01 |
| Smoking | No/yes | 0.41 (0.23-0.72) | .001 | 0.54 (0.29-1.02) | .06 |
| Family history of VTE | No/yes | 1.0035 (0.54-1.86) | .99 | 0.58 (0.26-1.24) | .16 |
| Creatinine† | 1 µmol/L | 1.01 (0.99-1.03) | .22 | 0.99 (0.97-1.01) | .24 |
| Glucose† | 1 mmol/L | 1.14 (0.82-1.58) | .45 | 1.04 (0.7-1.52) | .87 |
| CRP | 1 mg/L | 1.16 (1.03-1.30) | .027 | 1.20 (1.04-1.40) | .02 |
| D-dimer | 100 mg/dL | 1.94 (1.6-2.35) | <.001 | 2.05 (1.59-2.66) | <.001 |
| tPA | 1 ng/mL | 0.97 (0.90-1.05) | .42 | — | — |
| PAI-1 | 1 ng/mL | 1.005 (0.97-1.05) | .81 | 0.95 (0.9-1.01) | .09 |
| Peak thrombin | 100 nM | 2.67 (2.03-3.53) | <.0001 | 1.46 (1.09-1.96) | .01 |
| Ks | >7.3 vs ≤7.3 × 10−9 cm2 | 4.06 (2.40-6.89) | <.001 | 2.18 (1.17-4.06) | .01 |
| Lag phase | >41 vs ≤41 s | 3.07 (1.9-4.95) | <.001 | 2.87 (1.66-5.02) | <.001 |
| ΔAbs | ≤0.85 vs >0.85 | 1.69 (1.05-2.72) | .038 | — | — |
| D-Dmax | ≤3.51 vs >3.51 mg/L | 2.71 (1.18-6.26) | .007 | — | — |
| D-Drate | >0.072 vs ≤0.072 mg/L/min | 2.91 (1.73-4.88) | <.0001 | 3.71 (2.03-6.77) | <.001 |
| CLT | ≤96 vs >96 min | 5.91 (3.66-9.54) | <.001 | 3.52 (2.01-6.16) | <.001 |
| Variable . | HR per . | Univariate . | Multivariate* . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Age | 1 y | 1.02 (1-1.04) | .017 | 1.02 (0.99-1.04) | .11 |
| Male sex | No/yes | 0.63 (0.40- 0.99) | .045 | 0.72 (0.43-1.20) | .21 |
| BMI | 1 kg/m2 | 1.02 (0.97-1.08) | .449 | — | — |
| Time of anticoagulation | 1 mo | 1.05 (0.99-1.11) | .111 | — | — |
| Unprovoked VTE | No/yes | 1.46 (0.93-2.29) | .100 | — | — |
| Proximal DVT alone | No/yes | 1.19 (0.76-1.87) | .45 | 1.86 (1.13-3.06) | .01 |
| Smoking | No/yes | 0.41 (0.23-0.72) | .001 | 0.54 (0.29-1.02) | .06 |
| Family history of VTE | No/yes | 1.0035 (0.54-1.86) | .99 | 0.58 (0.26-1.24) | .16 |
| Creatinine† | 1 µmol/L | 1.01 (0.99-1.03) | .22 | 0.99 (0.97-1.01) | .24 |
| Glucose† | 1 mmol/L | 1.14 (0.82-1.58) | .45 | 1.04 (0.7-1.52) | .87 |
| CRP | 1 mg/L | 1.16 (1.03-1.30) | .027 | 1.20 (1.04-1.40) | .02 |
| D-dimer | 100 mg/dL | 1.94 (1.6-2.35) | <.001 | 2.05 (1.59-2.66) | <.001 |
| tPA | 1 ng/mL | 0.97 (0.90-1.05) | .42 | — | — |
| PAI-1 | 1 ng/mL | 1.005 (0.97-1.05) | .81 | 0.95 (0.9-1.01) | .09 |
| Peak thrombin | 100 nM | 2.67 (2.03-3.53) | <.0001 | 1.46 (1.09-1.96) | .01 |
| Ks | >7.3 vs ≤7.3 × 10−9 cm2 | 4.06 (2.40-6.89) | <.001 | 2.18 (1.17-4.06) | .01 |
| Lag phase | >41 vs ≤41 s | 3.07 (1.9-4.95) | <.001 | 2.87 (1.66-5.02) | <.001 |
| ΔAbs | ≤0.85 vs >0.85 | 1.69 (1.05-2.72) | .038 | — | — |
| D-Dmax | ≤3.51 vs >3.51 mg/L | 2.71 (1.18-6.26) | .007 | — | — |
| D-Drate | >0.072 vs ≤0.072 mg/L/min | 2.91 (1.73-4.88) | <.0001 | 3.71 (2.03-6.77) | <.001 |
| CLT | ≤96 vs >96 min | 5.91 (3.66-9.54) | <.001 | 3.52 (2.01-6.16) | <.001 |
BMI, body mass index; tPA, tissue plasminogen activator.
Multivariate model was fitted using backward stepwise regression. Adjusted for fibrinogen. c-statistic = 0.87 (P = .0004 compared with the traditional model in supplemental Table 4).
Variable locked in the model.
Because smoking reported by patients was found to decrease the risk of recurrent DVT (Table 4), a model with the categorical variable of cigarettes smoked daily was built. The univariate and the multivariate models showed that smoking ≥10 cigarettes per day increased the risk of DVT recurrences (HR, 4.59; 95% CI, 2.53-8.35 and HR, 3.87; 95% CI, 1.91-7.83, respectively; supplemental Table 5).
The Cox proportional hazards model with Ks as the only fibrin clot feature included in the model showed that reduced Ks predicted recurrent DVT (supplemental Table 6). The Cox proportional hazards model with the most prothrombotic phenotype presented in Figure 4 showed that higher CRP levels, increased D-dimer, elevated peak thrombin, and the protrombotic phenotype (defined as Ks ≤7.3 × 10−9 cm2 and CLT >96 minutes) predicted recurrent DVT (supplemental Table 7).
Discussion
To our knowledge, this is the first study to show that the prothrombotic fibrin clot phenotype, including poorly permeable fibrin fiber networks resistant to lysis, could be a novel risk factor for DVT recurrence in patients after anticoagulation withdrawal in a follow-up of more than 3 years. Our study was the first to comprehensively assess plasma clot structure and function using 6 established parameters 3 months since the DVT event. Denser, faster-formed clots that were lysed at a slower rate were observed ex vivo in blood obtained from patients who experienced DVT recurrences. Importantly, the prothrombotic phenotype defined as reduced Ks and prolonged CLT, but not a single clot feature, predicted recurrent DVT, which is a novel observation.
Our findings provided evidence that in DVT, like in PE,7 unfavorable clot characteristics can predict recurrent DVT regardless of several known risk factors.9 In our study, CLT was an independent predictor of DVT recurrence. Of note, patients with reduced Ks and prolonged CLT had an ∼16 times higher risk of DVT recurrence. Our study extends observations regarding a value of CLT in the prediction of VTE recurrences.17-20 Traby et al have showed that women with CLT values in the top vs the first quartile had a 3.28 higher risk of recurrent DVT.18 Recently, Siudut et al observed that subjects with recurrent VTE during a 1-year follow-up were characterized by 8.7% reduced Ks, 4.2% reduced D-Drate, and 15.3% prolonged CLT measured at 3 months since the index event, which is in line with our findings.17 Karasu et al reported a weak, insignificant association between prolonged CLT (above the 90th percentile) and recurrent VTE (HR, 1.5; 95% CI, 0.9-2.6).19 However, Meltzer et al found no association between CLT and risk of recurrent DVT (HR, 1.0; 95% CI, 0.5-1.9) when the 90th percentile was used as a cutoff point.20 Inconsistent findings could result from differences in patient characteristics and study design. Moreover, Meltzer et al analyzed blood samples collected 3 months after anticoagulation withdrawal,20 whereas Traby et al assessed samples 3 weeks after withdrawal of therapy, which lasted for 3 to 18 months since the DVT index.18
Of note, in the present study, there were no differences in fibrin clot properties ex vivo between patients after provoked or unprovoked DVT episodes. It might suggest that unfavorably altered fibrin clot features are universal predictors of recurrent DVT, regardless of whether transient risk factors are present. Because a substantial proportion of the study's patients with recurrent VTE either were observed following surgery or trauma or were hospitalized subjects who received pharmacological thromboprophylaxis, it is plausible that the prothrombotic phenotype contributes to DVT as a failure of such prophylaxis. Our group has recently shown such links (A.U., unpublished data).
A potential impact of the antithrombotic medications used during follow-up on DVT recurrence deserves a comment. Efficacy of aspirin28 for preventing VTE recurrences has been demonstrated, although the data are still controversial.29 Aspirin has been shown to improve fibrin clot properties.30 However, we failed to show any favorable effect of aspirin in preventing DVT recurrence. Another option after anticoagulation cessation is sulodexide, which when used orally at 500 lipasemic units twice daily for 2 years decreased VTE recurrence in a randomized, double-blind trial.31 Sulodexide did not reduce the risk of DVT recurrence in our cohort. Of note, most participants of this study were taking sulodexide 250 mg twice a day, whereas the assessed doses of this medication in the trial in 2015 were twofold higher.31 It is likely that the small subgroups taking aspirin or sulodexide are not representative of all individuals with a history of DVT who preferred cessation of anticoagulation.
Several known and potential risk factors for VTE recurrences were investigated in this study. We observed that higher D-dimer levels and higher peak thrombin predicted DVT recurrence, which is in line with previous reports.9,32-34 We also demonstrated that a reduced D-Drate predicted DVT recurrence. A reduced D-Drate indicates impaired transport of tissue plasminogen activator in fibrin clots and slower degradation, which is typically observed in dense clots with low Ks, and this might predispose to residual vein obstruction35 and increased risk of DVT recurrence. Higher CRP levels also predicted recurrent DVT, but the findings on CRP as a risk factor of DVT recurrence are inconsistent.36,37 It cannot be excluded that inflammation plays a more significant role in recurrent DVT in a middle-aged population.
It has been reported that fibrin clots formed from plasma of smokers are more compact and resistant to lysis compared with those from plasma of never-smokers.38 Moreover, following acute exposure to cigarette smoke, fibrin clots are denser and composed of thinner fibers.39,40 Paradoxically, we observed a larger proportion of smokers in nonrecurrent patients, but most of them smoked <10 cigarettes daily. Interestingly, our data suggest that higher tobacco exposure (defined as smoking ≥10 cigarettes daily) leads to DVT recurrence, indicating the links between smoking and DVT are complex and warrant further investigation.
It remains to be established that the plasma clot properties may predict abnormal fibrin clot structure within intravascular thrombi. Among patients in the acute phase of ST-elevation myocardial infarction who underwent thrombectomy, plasma obtained from subjects with high fibrin content in intracoronary thrombi generated in vitro more compact and resistant to lysis clots.41 It might be speculated that the prothrombotic plasma fibrin clot properties predispose to increased amount of fibrin within venous thrombi. Venous thrombectomy could provide the material to test this hypothesis.
Our study had several limitations. Although the number of study participants was limited, the study was adequately powered to detect differences in recurrent DVT events related to clot properties. All variables were assessed at a single time point; therefore, we cannot exclude their changes over time, especially after a few years of follow-up. We did not analyze the impact of blood cells on fibrin clot properties. Moreover, asymptomatic VTE events could have be omitted during follow-up, because patients without any signs or symptoms of PE or DVT were not assessed by imaging in this study. Of note, despite our guidelines, all DVT patients following the first event discontinued VKA during follow-up, and the results of our study cannot be extrapolated to patients who continue anticoagulation with VKA or are switched to newer anticoagulants. It remains to be established whether clot phenotype retains its predictive value in patients on long-term anticoagulation.
The strengths of our study included a well-characterized patient population, measurements done in the same laboratory using validated assays, and a relatively long follow-up, with a low number of subjects lost to follow-up.
In conclusion, we have shown that a prothrombotic fibrin clot phenotype including lower Ks and prolonged CLT characterizes patients with recurrent DVT. Our study provides new insights into DVT recurrence that might have practical implications. Longitudinal studies are needed to confirm our findings and assess the clinical value of Ks and CLT in the prediction of recurrent DVT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Jagiellonian University School of Medicine (K/ZDS/005802) (A.U.) and the Polish National Science Centre (UMO-2013/09/B/NZ5/00254) (A.U.).
Authorship
Contribution: J.C. and S.M. interpreted data and wrote the article; E.B. performed statistical analysis; and A.U. designed the study, recruited patients, collected data, and approved the article for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anetta Undas, Institute of Cardiology, Jagiellonian University Medical College, 80 Pradnicka St, 31-202 Krakow, Poland; e-mail: mmundas@cyf-kr.edu.pl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal