In this issue of Blood, Cieslik et al have added assessments of clot structure as a potentially new and important way to predict recurrent deep vein thromboses (DVT) after withdrawal of anticoagulant therapy.1 Their work represents a departure from the standard approach to understanding blood coagulation, which is typically viewed through the lens of biochemistry as regulated pathways governed by specific clotting factor concentrations and activities. However, as Cieslik et al demonstrate, regulation of enzyme activity through proteolysis represents only 1 aspect of thrombosis and hemostasis. The final product of blood coagulation (ie, the clot) does not exist merely as a result of a ratio between activating and inhibiting enzymes; rather, it is a tangible, complex, and unique biological material.
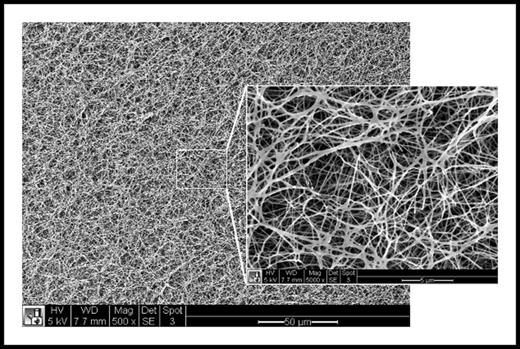
Scanning electron micrograph of the surface of a fibrin clot at ×500 magnification and ×5000 magnification (inset) revealing complex fibrin network architecture at multiple length scales. White scale bar = 50 μm (inset = 5 μm).
Scanning electron micrograph of the surface of a fibrin clot at ×500 magnification and ×5000 magnification (inset) revealing complex fibrin network architecture at multiple length scales. White scale bar = 50 μm (inset = 5 μm).
Recognizing the importance of a clot’s material properties, Cieslik et al have asked the question of whether certain structural characteristics of plasma clots could predict recurrence of DVT. In their long-term prospective study, they found that those subjects having plasma clots with denser fibrin networks, less porosity, and impaired fibrinolytic degradation were at the highest risk of recurrent DVT. These properties used to describe the characteristics of plasma clots may be more familiar to a material scientist than a hematologist. Yet, measurements of lag phase, permeation, maximal absorbance, and lysis time are useful because they reflect both clot structure and, as Cieslik et al have elegantly demonstrated, their potential behavior in vivo.
The unique structure of a thrombus and its relationship to clotting factors becomes apparent when examining its complex microstructure (see figure). The fibrin network structure of a thrombus can be influenced by thrombin and calcium concentration, temperature, and blood flow, each of which can alter fibrin polymerization and crosslinking by factor XIIIa. A dense and crosslinked fibrin network is essential to mechanical stiffness, durability, and retention of important components including red blood cells and proteins such as α-2 antiplasmin.2-5 Platelets also significantly affect clot structure by supporting thrombin generation and by binding and forcefully contracting to compact the fibrin scaffold.6 Under flow, platelet activity also induces hierarchical structuring of the thrombus, producing a core of activated platelets and fibrin having increased local thrombin concentration surrounded by an unstable shell of less activated platelets.7 This unique structure was found to limit entry of plasma molecules by diffusion, a strong regulator of fibrinolysis, and could be regulated by both platelet and thrombin inhibition.7,8 This complex structural organization also imparts global properties of firmness and porosity, which contribute to durability and susceptibility to fibrinolysis. A firm and dense thrombus having reduced permeability may be a favorable trait at sites of wounding where bleeding must be stopped quickly, but is also more likely to propagate and grow over time and thus contribute to thrombotic diseases.
Interestingly, Cieslik et al also found that tobacco exposure through significant cigarette smoking and C-reactive protein were associated with structural changes and recurrence of DVT, suggesting that inflammation may also influence thrombosis by altering clot structure. How might inflammation plausibly alter structure to reduce fibrinolysis and promote recurrence of DVT? Again, clues can be gained by examining the effects of inflammation on fibrin clot structure. Inflammation-induced oxidation can directly affect fibrin polymerization by affecting lateral aggregation of fibrin protofibrils, which can alter the shape of fibrin fibers, reduce clot porosity, and increase resistance to plasma clot lysis.9
So, is it time to begin using a structural approach to quantifying thrombotic risk and guiding antithrombotic therapy? Perhaps the most direct example of using clot structure and mechanics to guide therapy comes from hemostatic management of perioperative bleeding. Standard coagulation tests such as prothrombin and partial thromboplastin times that estimate factor activity have been largely replaced with mechanical measurements such as thrombelastography and rotational thromboelastometry that measure clot formation kinetics, elastic modulus, and lysis. This approach has found particular utility in perioperative situations in which rotational thromboelastometry-guided management has led to often dramatic reductions of transfused blood products.10 However, translating the same approach to detection and treatment of thrombotic diseases such as venous thrombosis has remained elusive.11 Perhaps the answer lies with finding the most appropriate structural property that is both reflective of the pathophysiology of thrombosis and is also clinically feasible. Indeed, as Cieslik et al have elegantly demonstrated, it is clear that there is much to learn from widening our view of thrombosis to include structural and mechanical assessments of clot formation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal