To the editor:
Pure erythroid leukemia1 (PEL), or AML-M6b, is a rare form of leukemia characterized by proliferation of >80% undifferentiated or pronormoblastic immature cells committed exclusively to the erythroid lineage.2 Previous reports have described the aggressive nature of erythroid/myeloid leukemia or AML-M6a (acute erythroid leukemia [AEL]) and PEL, as well as their association with high-risk biological features,3-10 including high frequency of TP53 mutations in up to 53% of patients with AEL.11 Despite these advances, the rarity of PEL has so far limited the ability to define its clinical and biological characteristics. Description of the mutational landscape and clinical outcomes of patients with PEL compared with AEL is therefore needed.
We evaluated all patients with newly diagnosed erythroleukemia treated at The University of Texas MD Anderson Cancer Center from 1980 to 2016. Informed consent was obtained according to protocols approved by the institutional review board in accordance with the Declaration of Helsinki. A diagnosis of AEL was considered if >50% of bone marrow nucleated cells were of erythroid lineage and >20% of nonerythroid cells were blasts.2,12,13 Diagnosis of PEL was established if >80% bone marrow nucleated cells consisted of immature erythroid precursors2,13-15 or if bone marrow core biopsy or aspirate clot was composed of sheets of immature undifferentiated blasts positive for glycophorin A.9,10,16-18 In patients evaluated from 2013 to 2016, whole–bone marrow DNA was subject to a 28- or 53-gene targeted polymerase chain reaction–based next-generation sequencing platform19 (available in the data supplement on the Blood Web site).
Twenty-seven (14%) of 189 patients with erythroleukemia met diagnostic criteria for PEL. Median age was 67 years (range, 33-85 years), with male predominance (ratio 2:1). Patients presented with mean hemoglobin of 8.7 g/dL (range, 8.2-9.2 g/dL), platelets of 25 × 109/L (range, 17-32 × 109/L) and leukocyte count of 3 × 109/L (range, 2.2-3.9 × 109/L). Eleven patients (41%) had therapy-related AML, and 11 (41%) had an antecedent hematologic malignancy, including myelodysplastic syndrome in 10 and chronic myeloid leukemia in 1 patient. Eleven patients (41%) received induction chemotherapy, 9 (33%) hypomethylating agents (HMAs), and 1 (4%) a monoclonal antibody. Six patients (22%) did not receive therapy and died before treatment initiation. Detailed descriptions of individual patient characteristics are shown in supplemental Tables 1-3.
Cytogenetic analysis was available in 26 (96%) and 147 patients (91%) with PEL and AEL, respectively, and revealed higher frequency of complex karyotype among patients with PEL (96% vs 61%; P < .001). Among patients with PEL, median number of cytogenetic abnormalities was 20 (range, 9-47), and median number of cytogenetic clones was 2 (range, 1-6). The most frequently involved chromosomes were chr5 (21 [81%] of 26), chr7 (15 [58%] of 26), and chr17 (14 [54%] of 26). Monosomal karyotype was observed in 16 patients (62%).
Targeted sequencing was performed in 12 patients (44%) with PEL. The most frequently mutated gene was TP53, detected in 11 (92%) of 12 patients, followed by ASXL1 (G646fs*), PTPN11 (G503R) and DNMT3A (L798P), each present in 1 patient (8%). Sixteen TP53 mutations were identified among patients with TP53-mutated PEL (Figure 1A). The median variant allele frequency of TP53 mutations detected from whole bone marrow was 0.22 (range, 0.04-0.52). Variant allele frequency estimates were used to evaluate clonal and subclonal relationships within each individual sample,20 with clonal heterogeneity being defined in cases with Pearson goodness-of-fit P values < .05. Among 7 patients evaluable for clonal heterogeneity testing, 3 (43%) were clonally heterogeneous and carried at least 1 subclone (supplemental Figure 1). Allelic frequencies of TP53 mutations suggested that a founder TP53 mutation was always present. Five patients carried 2 different TP53 mutations, with the minor TP53 mutation appearing to be clonally related in 3 and subclonally related in 2 patients (Figure 1B). Among 11 patients (41%) with both sequencing and cytogenetic data, 4 (36%) possessed a co-occurring TP53 mutation and chr17 abnormality (supplemental Figure 2). More than 1 TP53 anomaly, either resulting from mutation or loss of chr17, was detected in 8 (73%) of 11 of these patients. Targeted sequencing was available in 17 patients (10.5%) with AEL. Mutations in TP53 were observed in 7 patients (41%), 3 of whom had 2 different TP53 mutations (supplemental Table 4). Among the patients with AEL for whom sequencing was available, 10 (59%) had complex karyotype, but only 1 had a chr17 abnormality, and this patient did not have any TP53 mutation detected.
Landscape of TP53 mutations and clonal architecture of patients with pure erythroid leukemia. (A) Landscape of TP53 mutations identified in patients with PEL. Mutations highlighted in dark gray in the included table represent transitions, and those highlighted in light gray represent transversions. (B) Clonal architecture of patients with PEL. Y-axis represents variant allele frequency for identified mutations. Bone marrow erythroblasts include normoblasts, pronormoblasts, and undifferentiated leukemic erythroblasts present on bone marrow aspirates and are represented as a frequency of total bone marrow scaled to a range from 0 to 1 from original percentage. Chr17 abnormalities are expressed as a 0:1 ratio of metaphases harboring the alteration from the total analyzed metaphases. Patients 15, 16, 21, 24, and 25 carried 2 different TP53 mutations, and among these, the minor TP53 mutation seemed to be clonally related in 3 patients (15, 24, 25) and subclonally related in 2 patients (16 and 21). In patient 13, the TP53 mutation was observed with a variant allele frequency of 0.92 with no detectable chr17 abnormalities, suggesting loss of heterozygosity with uniparental disomy of chr17 harboring a somatic TP53 mutation.
Landscape of TP53 mutations and clonal architecture of patients with pure erythroid leukemia. (A) Landscape of TP53 mutations identified in patients with PEL. Mutations highlighted in dark gray in the included table represent transitions, and those highlighted in light gray represent transversions. (B) Clonal architecture of patients with PEL. Y-axis represents variant allele frequency for identified mutations. Bone marrow erythroblasts include normoblasts, pronormoblasts, and undifferentiated leukemic erythroblasts present on bone marrow aspirates and are represented as a frequency of total bone marrow scaled to a range from 0 to 1 from original percentage. Chr17 abnormalities are expressed as a 0:1 ratio of metaphases harboring the alteration from the total analyzed metaphases. Patients 15, 16, 21, 24, and 25 carried 2 different TP53 mutations, and among these, the minor TP53 mutation seemed to be clonally related in 3 patients (15, 24, 25) and subclonally related in 2 patients (16 and 21). In patient 13, the TP53 mutation was observed with a variant allele frequency of 0.92 with no detectable chr17 abnormalities, suggesting loss of heterozygosity with uniparental disomy of chr17 harboring a somatic TP53 mutation.
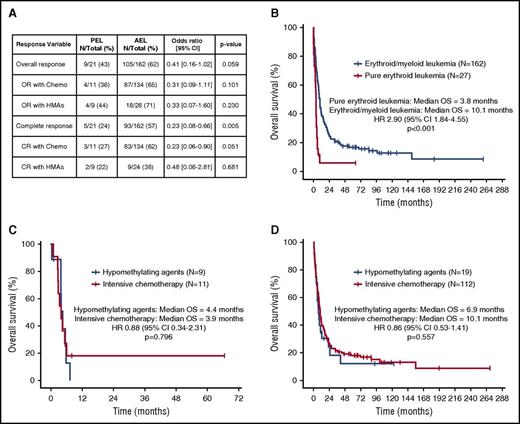
The median follow-up of the cohort was 8 months (range, 0-269 months). Twenty-four (15%) and 134 patients (83%) with AEL received HMAs or induction chemotherapy, respectively. No differences in overall response rate were observed based on therapy (Figure 2A). Patients with PEL treated with chemotherapy had lower CR rates compared with patients with AEL (27% vs 62%; odds ratio, 0.23; 95% confidence interval, 0.58-0.90; P = .051), but not with HMA therapy (22% vs 38%; P = .681). Twenty patients (12%) with AEL and 1 (3.6%) with PEL underwent allogeneic stem-cell transplantation at first CR (P = .323). Median OS of patients with PEL was 3.8 months compared with 10.1 months for those with AEL (P < .001; Figure 2B). No differences in median OS were observed between patients with PEL and those with TP53-mutant AEL (3.8 vs 4.5 months; P = .627). No differences in median OS were observed with chemotherapy compared with HMAs for patients with PEL (4.4 vs 3.9 months; P = .796; Figure 2C) or AEL (10 vs 6.9 months; P = .557; Figure 2D).
Clinical outcomes of patients with erythroleukemia. (A) Response outcomes in patients with AEL based on French-American-British subtype (PEL vs AEL) and therapy. (B) Kaplan-Meier estimate curves for overall survival (OS) of patients with PEL compared with AEL. Kaplan-Meier estimate curves for OS based on type of therapy for patients with PEL (C) and AEL (D). CI, confidence interval; CR, complete response; HR, hazard ratio; OR, overall response.
Clinical outcomes of patients with erythroleukemia. (A) Response outcomes in patients with AEL based on French-American-British subtype (PEL vs AEL) and therapy. (B) Kaplan-Meier estimate curves for overall survival (OS) of patients with PEL compared with AEL. Kaplan-Meier estimate curves for OS based on type of therapy for patients with PEL (C) and AEL (D). CI, confidence interval; CR, complete response; HR, hazard ratio; OR, overall response.
To the best of our knowledge, this study, which includes the largest reported series of patients with PEL, is the first to analyze its mutational landscape; it reveals a higher proportion of TP53 mutations compared with any other subset of leukemia. Importantly, cooccurrence of TP53 mutations and TP53 deletions resulting from chr17 abnormalities was detected in 73% of evaluable patients, suggesting more than a single TP53 abnormality may represent a pathognomonic signal associated with PEL. Presence of preexisting TP53-mutant clones in patients receiving chemotherapy has been associated with development of therapy-related myeloid malignancies,21 which are known to have higher frequency of these mutations.22,23 Given the high frequency of therapy-related and secondary disease among these patients with PEL, it is possible that the high frequency of TP53 abnormalities is in part related to successive clonal selection of preexisting TP53-mutant clones. Importantly, few additional common AML mutations were identified, supporting the notion that multiple TP53 alterations represent a central biological hallmark in PEL development. Recent data suggest hyperactivation of ERK1/2 signaling via NRAS G12D may synergize with homozygous TP53 loss, leading to leukemic transformation of megakaryocyte-erythroid progenitors.24 Although we did not observe NRAS mutations, 1 patient did have a cooccurring PTPN11 mutation, suggesting potential involvement of the RAS/MAPK pathway in PEL leukemogenesis.
Survival of these patients is unfavorable, with low CR rates with induction chemotherapy. Treatment with HMAs did not improve survival outcomes, and unlike recent data25 reporting high response rates to decitabine in patients with TP53-mutant AML, the observed response rates of patients with PEL treated with HMAs were low and similar to those of patients treated with chemotherapy.
Although this cohort is the largest, most comprehensively studied to date, the sample size is small, partly because of the rarity of PEL, and our sequencing technique focused on a limited number of candidate genes. Whole-exome sequencing will better identify cooperating genomic abnormalities in PEL. In conclusion, presence of more than a single TP53 abnormality seems to be a strong influential factor for PEL pathogenesis. Accumulative impaired TP53 function is consistent with severe genomic instability and may significantly contribute to accelerated deterioration of patients with PEL irrespective of currently available therapies. There is a continued need for therapeutic interventions targeting TP53-mutated AML and its quintessential example, PEL.
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 4 December 2016.
The online version of the article contains a data supplement.
Authorship
Acknowledgments: This work was supported by the American Society of Hematology, the American Society of Hematology Research Training Award for Fellows, a grant from the National Institutes of Health, National Cancer Institute (NCI) (P30 CA016672), the Cancer Prevention Research Institute of Texas (RP100202) (G.G.-M.), and generous philanthropic contributions to Anderson’s AML Moon Shot Program (K.T., H.K., G.G.-M., and A.F.), the NCI Leukemia Specialized Programs of Research Excellence Career Development Grant (K.T.), and the Charif Souki Cancer Research Fund.
Contribution: G.M.-B. and C.B.B. designed the study, collected and analyzed data, and wrote the manuscript; S.A.W. performed histopathological analysis and helped analyze data; F.R., T.K., J.C., N.D., K.T., C.D., E.J., G.B., M.K., S.K., and H.K. included patients and wrote the manuscript; S.P. collected and analyzed the data; K.H.Y. and C.B.-R. performed histopathological analysis; K.P. performed all the sequencing studies and participated in writing the manuscript; and G.G.-M. and M.A. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing conflicts of interest.
Correspondence: Michael Andreeff, Anderson Cancer Center, Department of Leukemia, 1515 Holcombe Blvd, Unit 448, Houston, TX 77030; e-mail: mandreef@mdanderson.org.
References
Author notes
G.M.-B. and C.B.B. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal