Abstract
RUNX1 is a member of the core-binding factor family of transcription factors and is indispensable for the establishment of definitive hematopoiesis in vertebrates. RUNX1 is one of the most frequently mutated genes in a variety of hematological malignancies. Germ line mutations in RUNX1 cause familial platelet disorder with associated myeloid malignancies. Somatic mutations and chromosomal rearrangements involving RUNX1 are frequently observed in myelodysplastic syndrome and leukemias of myeloid and lymphoid lineages, that is, acute myeloid leukemia, acute lymphoblastic leukemia, and chronic myelomonocytic leukemia. More recent studies suggest that the wild-type RUNX1 is required for growth and survival of certain types of leukemia cells. The purpose of this review is to discuss the current status of our understanding about the role of RUNX1 in hematological malignancies.
Introduction
It is timely that Blood publishes a review article on RUNX1 since 2016 marks the 25th anniversary of the identification of RUNX1’s involvement in the t(8;21) translocation in acute myeloid leukemia (AML). In 1991, Miyoshi et al localized the breakpoints on chromosome 21 to a gene called AML1, which was an unknown gene at the time.1 In the following two years, groups led by Nancy Speck and Yoshiaki Ito independently identified and characterized AML1 in the context of Moloney murine leukemia virus and polyomavirus, respectively.2-4 It was shown that AML1, also known as CBFa2 and PEBP2aB, is a mammalian homolog of Drosophila runt, which was cloned and characterized by Peter Gergen in the 1980s.5,6 Therefore, a unifying name “Runt-related transcription factor” or RUNX was proposed and accepted in the field.7 There are three related RUNX genes in the mammalian genomes, RUNX1, RUNX2, and RUNX3. All RUNX proteins contain the runt-homology domain (RHD), which is responsible for DNA-binding and interaction with a common heterodimeric partner, CBFβ. The identification of CBFB as the target of chromosome 16 inversion in human AML by our group in 19938 further indicated this group of genes as key players in leukemia. It has been demonstrated that the RUNX1-RUNX1T1 (aka ETO and MTG8) fusion generated by t(8;21) and the CBFB-MYH11 fusion generated by inv(16) are leukemia initiating or driver mutations.9,10
In addition to t(8;21), more than 50 chromosome translocations affect RUNX1.11 The more common, recurrent translocations include t(12;21) in pediatric acute lymphoblastic leukemia (ALL) and t(3;21) in therapy-related AML and myelodysplastic syndrome (MDS), which generate ETV6-RUNX1 (also known as TEL-AML1) and RUNX1-MECOM (including MDS1 and EVI1) fusions, respectively.12,13
RUNX1 point mutations in leukemia were first identified in 1999.14 Many subsequent studies documented frequent somatic mutations in RUNX1 in MDS, AML, ALL, and chronic myelomonocytic leukemia (CMML).15 Germ line mutations of RUNX1 are associated with familial platelet disorder with associated myeloid malignancy (FPDMM).16
Finally, recent studies suggest that normal RUNX1 plays an important role during leukemogenesis. It has been demonstrated that normal RUNX1 is required for leukemia development by both RUNX1-RUNX1T1 and CBFB-MYH11. Similarly, several reports suggest that RUNX1 is important for mixed-lineage leukemia (MLL). The proposed hypothesis is that wild-type RUNX1 is required for survival of these leukemia cells.17
Here, we provide a comprehensive review of the recent literature describing the ways by which RUNX1 is altered that lead to hematologic diseases, including chromosome translocations, somatic and germ line point mutations, and the potential role of normal RUNX1 in leukemogenesis.
Genomic organization and functional domains of RUNX1
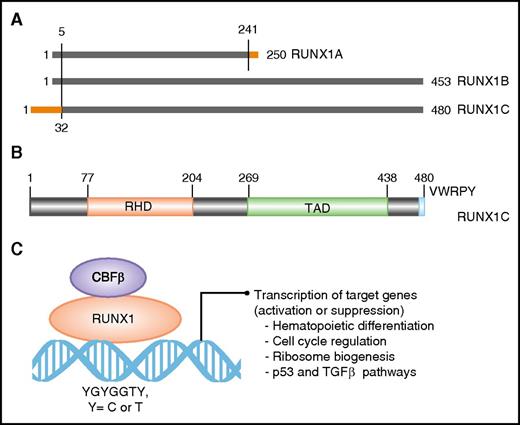
The RUNX1 gene spans ∼261 kb on the long arm of chromosome 21. Three major isoforms are transcribed by use of two promoters and alternative splicing (Figure 1A). Isoforms 1A (250 amino acids) and 1B (453 amino acids) are transcribed from the proximal promoter P2 and differ at their carboxyl termini (Figure 1A). Isoform 1C (483 amino acids) is transcribed from the distal promoter P1 and is identical to isoform 1B except for 32 amino acids encoded by alternative exons at its amino terminus (Figure 1A). The three isoforms are expressed in a temporal and tissue-specific manner. Isoform 1C is expressed at the time of emergence of definitive hematopoietic stem cells, whereas isoforms 1A and 1B are expressed throughout hematopoietic differentiation.18 All 3 isoforms share the conserved 128 amino acid RHD (Figure 1B-C). Isoforms 1B and 1C share another large domain, termed TAD, which consists of activating and inhibitory domains that bind to a number of activating and repressor proteins (Figure 1B). The last five amino acids of isoforms 1B and 1C make the VWRPY motif that interacts with mammalian homolog of Groucho, or TLE1.19 Through interaction with multiple proteins through its domains, RUNX1 controls the expression of its target genes involved in hematopoietic differentiation, ribosome biogenesis, cell cycle regulation, and p53 and transforming growth factor β signaling pathways.20,21
Domain architect of RUNX1 protein and its role as a transcription factor. (A) A schematic depicting the 3 major isoforms of RUNX1 (1A, 1B, and 1C). Isoforms 1A and 1B are transcribed from P2, and isoform 1C is transcribed from P1, thus differing by 32 amino acids at its 5′ end (marked in orange). Isoform 1A contains only the RHD and differs by 9 amino acids at its 3′ end (marked in orange). (B) Schematic of the protein encoded by the largest isoform, 1C, with major functional domains marked: RHD and transactivation domain (TAD). The numbers above the lines represent the amino acid residues. (C) A schematic of RUNX1 heterodimerization with its binding partner, CBFβ, and interaction with DNA at promoters of target genes that carry the specific binding site: YGYGGTY, where Y is C or T.
Domain architect of RUNX1 protein and its role as a transcription factor. (A) A schematic depicting the 3 major isoforms of RUNX1 (1A, 1B, and 1C). Isoforms 1A and 1B are transcribed from P2, and isoform 1C is transcribed from P1, thus differing by 32 amino acids at its 5′ end (marked in orange). Isoform 1A contains only the RHD and differs by 9 amino acids at its 3′ end (marked in orange). (B) Schematic of the protein encoded by the largest isoform, 1C, with major functional domains marked: RHD and transactivation domain (TAD). The numbers above the lines represent the amino acid residues. (C) A schematic of RUNX1 heterodimerization with its binding partner, CBFβ, and interaction with DNA at promoters of target genes that carry the specific binding site: YGYGGTY, where Y is C or T.
RUNX1-RUNX1T1, the fusion gene product of t(8;21)
As mentioned above, RUNX1 and CBFB are frequent targets of chromosome abnormalities in human AML and ALL. In particular, de novo AMLs with translocations affecting either RUNX1 or CBFB are known as core-binding factor (CBF) leukemia. The CBF leukemia chromosomal rearrangements include t(8;21)(q22;q22) in AML subtype M2 and inv(16)(p13.1;q22)/t(16;16)(p13.1;q22) in AML subtype M4Eo.22 Both CBF leukemia subtypes are associated with younger age, with incidence ranging from 20% in pediatric AML23 to less than 5% in older AML.24 Moreover, CBF leukemias are generally associated with relatively good prognosis.
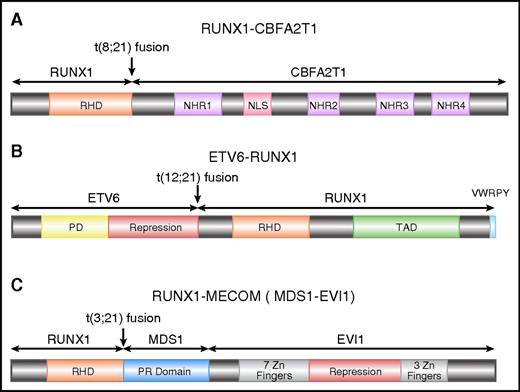
RUNX1-CBFA2T1 (AML1-eight-twenty one oncoprotein [ETO]), the protein product of RUNX1-RUNX1T1, contains the N-terminal 177 amino acids of RUNX1, including the entire RHD, fused with almost the entire CBFA2T1 protein (also known as ETO and myeloid translocation gene on 8q22 [MTG8]) (Figure 2A). CBFA2T1 contains four conserved domains termed nervy homology regions (NHR) 1 to 4.25 NHR2 mediates oligomerization of RUNX1–CBFA2T1, a process that is critical for leukemogenesis.26-28 CBFA2T1 also contains a nuclear localization signal between NHR1 and NHR2.29,30
Schematics of RUNX1 fusion proteins. (A) Schematic diagram of full-length RUNX1-CBFA2T1, illustrating the site of fusion between the 2 proteins. RUNX1-CBFA2T1 comprises the RHD from RUNX1 and 4 Nervy homology regions (NHR1–4) from CBFA2T1. The location of the nuclear localization signal (NLS) is also indicated. (B) Schematic diagram of ETV6-RUNX1, illustrating the site of fusion between the 2 proteins. The ETV6-RUNX1 fusion protein contains the N-terminal non-DNA binding moiety of ETV6 fused to almost the entire RUNX1 protein, including its RHD and TAD domains, and the VWRPY motif. (C) Schematic diagram of RUNX1-MECOM, illustrating the site of fusion between the 2 proteins. In the RUNX1-MECOM fusion, RUNX1 fuses with one or both of the “MDS1 and EVI1 complex locus” genes present at chromosome 3q26.
Schematics of RUNX1 fusion proteins. (A) Schematic diagram of full-length RUNX1-CBFA2T1, illustrating the site of fusion between the 2 proteins. RUNX1-CBFA2T1 comprises the RHD from RUNX1 and 4 Nervy homology regions (NHR1–4) from CBFA2T1. The location of the nuclear localization signal (NLS) is also indicated. (B) Schematic diagram of ETV6-RUNX1, illustrating the site of fusion between the 2 proteins. The ETV6-RUNX1 fusion protein contains the N-terminal non-DNA binding moiety of ETV6 fused to almost the entire RUNX1 protein, including its RHD and TAD domains, and the VWRPY motif. (C) Schematic diagram of RUNX1-MECOM, illustrating the site of fusion between the 2 proteins. In the RUNX1-MECOM fusion, RUNX1 fuses with one or both of the “MDS1 and EVI1 complex locus” genes present at chromosome 3q26.
Isoforms of RUNX1-RUNX1T1 have been identified because of alternative splicing of RUNX1T1 exons. RUNX1T1 contains 14 exons, including 2 alternative exons, 9a and 11a.31 The RUNX1-CBFA2T19a protein lacks NHR3 and NHR4 domains and therefore has reduced capacity to inhibit RUNX1-mediated transcriptional activation. Surprisingly, RUNX1-CBFA2T19a was a more potent inducer of leukemia than was the full-length RUNX1-CBFA2T1 in mouse models.10 It has been hypothesized that the deleted region inhibits leukemogenic potential of RUNX1-CBFA2T1.10 However, the functional significance of RUNX1-CBFA2T19a in human leukemia remains unclear.
RUNX1-CBFA2T1 interacting proteins
Many RUNX1-CBFA2T1 interacting proteins have been identified. As was predicted, RUNX1-CBFA2T1 interacts with the heterodimeric partner of RUNX1, CBFβ. However, the importance of the CBFβ interaction for leukemogenesis by RUNX1-CBFA2T1 is still not completely resolved.26,32 RUNX1-CBFA2T1 forms a corepressor complex with the nuclear receptor corepressor (NCOR1), histone deacetylase (HDAC1), and SIN3A/HDAC,33-37 which inhibits RUNX1 target genes.38 RUNX1-CBFA2T1 also interacts with transcription coactivators, such as histone acetyltransferases p300 and protein arginine methyltransferase 1 (PRMT1) ,39,40 which may enhance its transcription activation activities. A recent study in the RUNX1-CBFA2T1 leukemic cell line Kasumi-1 showed that RUNX1-CBFA2T1 interacted with coactivators p300 and PRMT1 in physiological conditions, whereas it interacted with corepressors only weakly.41 In addition, RUNX1-CBFA2T1 interacts with E proteins and c-Jun.39,42 RUNX1-CBFA2T1 also binds to GATA1 to dysregulate transcriptional activity of GATA1 by preventing its acetylation.43 Furthermore, RUNX1-CBFA2T1 binds to and inhibits myeloid transcription factors CCAAT/enhancer binding protein alpha (CEBPA) and PU.1, which leads to global suppression of myeloid gene expression.44-47
Secondary mutations in RUNX1-RUNX1T1 leukemia
Secondary chromosome abnormalities in RUNX1-RUNX1T1 leukemia have been described.48 RUNX1-RUNX1T1 leukemia cells frequently (47%) lose the sex chromosomes (either X or Y). In addition, chromosome 9q deletion and trisomy 8 are relatively common findings in RUNX1-RUNX1T1 leukemia. In fact, del(9q) might be a poor prognostic factor in t(8;21) AML.49-51
KIT, RAS, and fms-like tyrosine kinase 3 (FLT3) are frequently mutated in CBF leukemia.51-53 In a recent report, 135 adult patients with RUNX1-RUNX1T1 AML were analyzed for activating mutations involving signal transduction pathways (FLT3-ITD, FLT3-D835, KIT-D816, NRAS codons 12/13/61) and MLL partial tandem duplication (PTD). Strikingly, almost one third of all RUNX1-RUNX1T1 AML patients had mutations affecting RAS pathway, whereas none showed MLL-PTD.48 KIT-D816 mutations had adverse prognostic impact on RUNX1-RUNX1T1 leukemia, whereas the impact of other KIT mutations was less significant.48,52,54 Importantly, most reports suggest an association between these class I mutations55 and worse outcomes in CBF-AML patients.54 In addition, frequent mutations of epigenetic genes, such as ASXL1 and ASXL2, have been found in RUNX1-RUNX1T1 leukemia.48,56 Other genes with frequent mutations include IDH1 and IDH2, identified in approximately 5% of RUNX1-RUNX1T1 leukemia.48
We recently performed whole exome sequencing and single nucleotide polymorphism array analyses of relapsed CBF leukemia to identify mutations that are responsible for relapse.57 In addition to mutations in previously known AML driver genes such as FLT3, KIT, NRAS, and DNMT3A, we found recurrent mutations in DHX15, which encodes an RNA helicase implicated in pre-messenger RNA splicing. Moreover, we found a relapse-specific deletion on chromosome 3, with a minimal overlap region containing 3 genes including GATA2, which is a master regulator of hematopoiesis and also has roles in leukemia.57,58 Our data suggest two potential mechanisms for leukemia relapse, one with a therapy-resistant leukemia clone and the other with a therapy-resistant preleukemia clone, which survived treatments, gained other mutations, and gave rise to relapse.57
Signaling pathways in RUNX1-RUNX1T1 leukemia
Involvement of signaling pathways such as thrombopoietin/myeloproliferative leukemia (TPO/MPL), JAK/STAT, Wnt, and PI3K/AKT in CBF leukemia has been reported. TPO/MPL signaling was important for survival and leukemogenesis by RUNX1-CBFA2T1, likely through upregulating Bcl-xL and activating PI3K/AKT and JAK/STAT pathways.59,60 Loss of CBL function was shown to enhance TPO-mediated proliferation of RUNX1-CBFA2T1 cells.61 RUNX1-CBFA2T1 may upregulate the Wnt signaling pathway,62 through upregulation of γ-catenin.63 In addition, RUNX1-CBFA2T1 upregulates COX-2, which in turn activates Wnt/β-catenin signaling.64 Acetylated RUNX1-CBFA2T1 phosphorylates AKT1 through upregulation of ID1, which interacts with AKT1.65 On the other hand, the PI3K/AKT signaling pathway may be attenuated in RUNX1-RUNX1T1 leukemia.59 It was also reported that NF-κB signaling was inhibited by wild-type RUNX1 through interaction with IκB kinase complex, and RUNX1-CBFA2T1 lost this ability. Consequently, NF-κB signaling was activated in RUNX1-CBFA2T1 cells.66
Dysregulation of tumor suppressors has been observed in RUNX1-RUNX1T1 leukemia. p14ARF (CDKN2A) and NF1 expression were transcriptionally repressed through dominant inhibition of RUNX1 function.67,68 Antiapoptosis genes BCL2 and Bcl-xL are also directly or indirectly upregulated by RUNX1-CBFA2T1.60,69
Specific microRNA signatures in RUNX1-RUNX1T1 leukemia
Recent studies revealed the importance of microRNAs (miRNAs) in the pathogenesis of CBF leukemia. In 2008, 2 groups independently performed the first genome-wide miRNA analyses in CBF leukemia.70,71 In both reports the authors identified signature miRNAs expressed in t(8;21) and inv(16) leukemia, ranging from 2 to 10 in each, that can be used to distinguish them from other types of AML. It was found that miR-126/126* expression was specifically elevated in CBF leukemia, which inhibited apoptosis and increased leukemia cell viability.70
Importantly, miRNAs have been shown to have functional relevance in leukemogenesis, with some miRNAs acting as oncogenes and others as tumor suppressors. Downregulated miRNAs in RUNX1-RUNX1T1 AML, which were shown to act as tumor suppressors, include miR-9,72 miR-223,73 and miR-193a.74 Epigenetic suppression of miR-193a by RUNX1-CBFA2T1 enhances the oncogenic activity of RUNX1-CBFA2T1 by directly enhancing expression of DNA methyltransferase 3A (DNMT3A), HDAC3, KIT, CCND1, and MMDM2 and indirectly decreasing phosphatase and tensin homolog.74 On the other hand, miR-24 is downregulated in RUNX1-CBFA2T1 cells.75 Moreover, miR-126 plays a pivotal role in the regulation of leukemic stem cells and therapeutic resistance in RUNX1-RUNX1T1 leukemia.76,77
ETV6-RUNX1 (TEL-AML1) in childhood ALL
The most common chromosome abnormality in childhood ALL is t(12;21), occurring in 17% of the patients78 ; t(12;21) occurs during B-cell differentiation prior to the onset of immunoglobulin gene rearrangement, giving rise to leukemic blasts that appear to be blocked at the pre-B cell stage79-82 . An inframe fusion is generated by t(12;21) between ETV6 (also known as TEL) on chromosome 12 and RUNX1 on chromosome 21 (Figure 2B). ETV6-RUNX1 encodes the N-terminal non-DNA–binding moiety of ETV6 and almost the entire RUNX1 protein.12,79,83
ETV6-RUNX1 is able to dimerize with wild-type ETV684 through interactions between the pointed domains (PD) of both proteins and disrupt ETV6 activity.85 However, the significance of this interaction is not clear, because the wild-type ETV6 allele is lost in many t(12;21) ALL cases.86 Furthermore, ETV6-RUNX1 is able to cooperate with other transcription factors (such as ETS-1, PU.1, and C/EBPα) to drive target gene expression.87,88 The transcriptional activities of ETV6-RUNX1 involve recruitment of NCOR/HDAC complexes to the ETV6 moiety of the fusion protein.89 The PD of ETV6 may be required for repression, which can interact with SIN3A.90 A central repression domain, located between PD and ETS DNA-binding domain, interacts with NCOR1 and HDACs.91-93 It is likely that oligomerization of ETV6 allows for stable formation of repressor–corepressor complexes.94
Studies of identical twins with concordant ALL provide unequivocal evidence that ETV6-RUNX1 arises prenatally.95,96 Monozygotic twins share their blood supplies, so a preleukemia clone arising from one twin will likely be shared with the other twin in utero. Consequently, it has been observed that the leukemia concordance rate for identical twins is much higher than for other sibling combinations, reaching 100% for infant leukemia.96 Molecularly, it has been confirmed that the breakpoints of t(12;21) in the twins are identical to each other,95 consistent with their derivation from a common leukemia clone. Interestingly, twins typically have different sets of secondary mutations in their leukemia cells.97 These findings have led to the hypothesis that ETV6-RUNX1 leads to the generation and expansion of a covert preleukemic clone that can persist for many years before acquiring secondary mutations and producing overt leukemia.96,98
There might not be an exclusive second genetic “hit,” but at diagnosis most cases of ETV6-RUNX1 ALL have deletions of the normal ETV6 allele.99 Deletions are subclonal to ETV6-RUNX1100 and are distinct in their genomic boundaries between twins101 or even between relapse and diagnostic samples from the same individuals.102 Indeed, in 143 ETV6-RUNX1 patient samples analyzed for additional genetic lesions, over half showed complete deletion of the normal ETV6 allele.103 In addition to ETV6, PAX5, which is essential for B-cell commitment, is a frequently mutated gene in ETV6-RUNX1 leukemia.104,105 Deletions have also been found in other genes encoding for transcription factors important for B-cell differentiation and maturation, such as EBF1, IKZF1, E2A, LEF1, and IKZF3.104
Other chromosomal translocations affecting RUNX1
RUNX1 is involved in t(3;21)(q26;q22), which is found in both therapy-related MDS and chronic myeloid leukemia in blast crisis.13 Near the breakpoints on 3q26, there are two genes called EVI1 and MDS1, both of which have been demonstrated to form fusions with RUNX1106,107 (Figure 2C). EVI1 and MDS1 are located close to each other, and some transcripts contain exons from both genes, so they have been recently designated as MECOM, for “MDS1 and EVI1 complex locus.” EVI1 has been recognized as an important player in leukemogenesis. It is involved in other chromosome abnormalities affecting 3q26, and its expression is elevated in many leukemia cases, even those without 3q26 abnormalities, which predict adverse outcome in AML.108
RUNX1 somatic mutations in MDS
It took 8 years since the first report of RUNX1 involvement in leukemias by chromosomal rearrangements to identify somatic and germ line RUNX1 mutations in MDS, AML without translocations, and FPDMM.14,16 Song and colleagues identified a frameshift mutation in RUNX1 in 1 of 14 patients with sporadic MDS.16 They were unable to categorize the mutation as somatic or inherited because of the lack of corresponding germ line DNA. Subsequently, Imai et al109 demonstrated the somatic nature of RUNX1 mutations in 2 out of 37 MDS patients. Harada et al110 reported an increased incidence of RUNX1 mutations in radiation-associated MDS by screening atomic bomb survivors from Hiroshima who developed MDS. These earlier studies did not screen the entire coding region of RUNX1; therefore, the actual incidence of RUNX1 mutations might be higher than reported in these MDS patients. Subsequently, several studies reported somatic mutations in RUNX1 in patients with primary MDS, therapy-related MDS, and AML from progression of MDS.111-118 RUNX1 mutations also occur in ∼20% of Fanconi anemia and 64% of congenital neutropenia (CN) patients who develop MDS.119,120 RUNX1 mutations in MDS are distributed throughout the gene, affecting both major functional domains. RUNX1 is one of the most frequently mutated genes in MDS, accounting for roughly 10% of the cases.115,118,121
Analysis of 132 primary MDS cases115 revealed a positive correlation between RUNX1 mutations and shorter survival (median survival of 11 months vs 28 months). Tsai et al observed that MDS patients with RUNX1 mutations had a higher risk and shorter latency for progression to AML in comparison with MDS patients without RUNX1 mutations.118 The exact role of RUNX1 in progression of MDS to AML is not known. For MDS cases with mutated RUNX1, it is proposed that secondary cytogenetic changes, particularly deletion of the entire chromosome 7 or its long arm (-7/7q-), and activating mutations in the RTK-RAS pathway lead to leukemic transformation.114-116 Several reports suggest that cooperating mutations in FLT3, MLL, and JAK2 cause leukemic transformation of MDS by providing a growth advantage to RUNX1-mutated hematopoietic progenitors.117,122 Using single-cell genomic analysis, Skokowa and colleagues demonstrated that RUNX1 and CSF3R cooperate during leukemogenesis in CN patients.120 Interestingly, they also identified a germ line RUNX1 mutation in familial CN/AML.
RUNX1 somatic mutations in AML
Osato and colleagues were the first to demonstrate occurrence of somatic point mutations in RUNX1 in patients with cytogenetically normal AML (CN-AML).14 They identified missense and nonsense mutations in RUNX1 in 5 of 109 CN-AML patients. Subsequent screening of additional AML patients revealed especially high frequency of RUNX1 mutations in AML-M0, with 27% of AML-M0 patients showing inactivating RHD mutations.123-127 Interestingly, biallelic RUNX1 mutations were observed in many AML-M0 patients, indicating a complete lack of RUNX1 function in their leukemic cells.123,124 Greif and colleagues identified RUNX1 mutations in ∼16% of patients with CN-AML.128 The mutations represent a broad spectrum of missense, nonsense, and framehshift changes distributed throughout the protein, with a higher frequency in the RHD.128
Overall, somatic mutations in RUNX1 are detected in approximately 3% of pediatric and 15% of adult de novo AML patients.120,125,128-130 They are associated with older age, male gender, and poor prognosis when compared with the RUNX1 wild-type AML patients.120,125,126,128,129 Expression profiling has revealed a distinct gene expression profile in RUNX1-mutated versus RUNX1 wild-type AML samples.128,129,131 Furthermore, RUNX1 mutations are frequently observed together with FLT3-ITD, FLT3-TKD, and MLL-PTD.126,128,131,132 In addition, mutations in other AML driver genes (ASXL1, CEBPA, DNMT3A, NRAS, KIT, IDH1, IDH2, WT1) are also observed in RUNX1-mutated AML samples.128,129,131,132 Interestingly, RUNX1 and NPM1 mutations seem to be mutually exclusive.128,129,131 These studies indicate that leukemogenesis is driven by mutations that provide a growth advantage to the hematopoietic progenitor cells with differentiation defects due to mutated RUNX1. Recent studies have proposed molecular subclassification of AML based on mutation spectrum that would help in choosing the proper treatment options.133,134
RUNX1 somatic mutations in ALL
Recently, several independent studies taking different approaches have identified recurrent somatic mutations in RUNX1 in ALL. First, Grossman and colleagues performed a systematic screening of RUNX1 in 128 ALL patients.135 They identified 17 different RUNX1 mutations distributed throughout the RHD and TAD in 15 patients, 13 with T-ALL (18%) and 2 with B-ALL (7%). Interestingly, 2 of the 13 T-ALL patients had biallelic RUNX1 mutations. They further demonstrated association of RUNX1 mutations with higher age and poor prognosis. Next, Della Gatta et al performed global transcriptional network analysis of T-ALL induced by TLX1 and TLX3 and identified RUNX1 as a key mediator of T-ALL.136 Prompted by their data, they screened T-ALL samples for RUNX1 mutations and identified mutations in 4 out of 12 T-ALL cell lines and 5 out of 114 of T-ALL primary samples. A third study137 performed whole-genome sequencing and identified RUNX1 as one of the recurrently mutated genes (3 out of 12 patients) in patients with ETP-ALL (early T-cell precursor ALL). Screening of additional patients revealed RUNX1 mutations or deletions in 12 cases (10 ETP-ALL and 2 non- ETP-ALL). In these studies, the somatic nature of RUNX1 mutations was demonstrated in patients in whom appropriate germ line samples were available. Furthermore, biallelic mutations were observed in some patients, indicating the role of RUNX1 as a tumor suppressor in ALL.
Familial platelet disorder with predisposition to acute myeloid leukemia (FPDMM)
FPDMM or FPD/AML is a rare autosomal dominant disorder with clinical symptoms of mild-to-moderate thrombocytopenia, platelet dysfunction, bleeding propensity, and a significant risk of hematological malignancies, especially MDS and AML. In 1969, Weiss and colleagues138 described the first multigenerational pedigree with symptoms of FPD/AML. Subsequent studies identified additional families with similar clinical symptoms and performed linkage analysis and mutation screening to identify germ line mutations in RUNX1 as the cause of FPD/AML.16,139-142 To date, more than 70 FPD/AML families with inherited RUNX1 mutations have been identified (Table 1). At least one family is reported with symptoms of FPD/AML without a RUNX1 mutation.143 Stockley and colleagues described RUNX1 mutations in 3 families with a phenotype of reduced platelet dense-granule secretion.144 It is not clear whether these families represent a spectrum of FPD/AML phenotype or have a different inherited platelet disorder due to RUNX1 mutations. In addition to the inherited mutations, de novo RUNX1 mutations were reported recently in patients with thrombocytopenia without a family history of FPD/AML.145,146 A de novo translocation, t(16;21)(p13;q22), with a breakpoint in intron 1 of RUNX1, was reported in a patient with storage pool disease, thrombocytopenia, and AML.147
Inherited RUNX1 mutations in FPD/AML families
| Reference . | No. of families . | Family ID* . | RUNX1 mutation (on isoform C) . | Hematological malignancy . |
|---|---|---|---|---|
| 16 | 6 | 1 | Large deletion | AML |
| 2 | V118GfsX11 | AML | ||
| 3 | R204X | AML | ||
| 4 | R201X | AML | ||
| 5 | R201Q | AML | ||
| 6 | R166Q | AML | ||
| 193 | 1 | D198Y | MDS, AML | |
| 148 | 3 | 1 | K110E | Myeloblastic leukemia |
| 2 | R162fsX | AML | ||
| 3 | Y287X | AML | ||
| 194 | 1 | A134P | AML | |
| 143 | 1 | None† | MDS, AML | |
| 195 | 1 | V118GfsX11 | AML | |
| 196, 197, 164 | 1 | T246RfsX8 | CMML, AML | |
| 198 | 1 | T148HfsX9 | AML | |
| 199 | 1 | F330SfsX | MDS | |
| 151 | 5 | A | G363fsX227 | MDS, AML, ALL |
| B | A55fsX81 | AML | ||
| C | D123H | AML | ||
| D | K117fsX101 | MDS, AML, CMML | ||
| E | R320X | MDS, AML | ||
| 200 | 1 | P263LfsX48 | AML | |
| 162 | 1 | R201X | MDS, AML | |
| 163 | 4 | 1 | A156E | AML |
| 2 | R204Q | AML, T-ALL | ||
| 3 | Q335RfsX259 | AML, T-ALL | ||
| 4 | Large deletion | MDS, AML | ||
| 149 | 1 | S388X | MDS, AML | |
| 170 | 2 | 1 | Duplication of exons 2-6 | AML |
| 2 | Deletion of 35 amino acids | MDS, AML | ||
| 153 | 1 | R201X | AML, T-ALL | |
| 150, 165, 164 | 2 | A | R201Q | MDS, AML, T-ALL |
| B | R166X | AML | ||
| 147 | 3 | 1 | G170R | MDS, AML |
| 2 | Q262X | MDS, AML | ||
| 3 | t(16;21)(p13;q22) (de novo) | MDS, AML | ||
| 154 | 1 | G289fsX21 | MDS, CMML | |
| 201 | 1 | L472fsX123 | MDS, AML | |
| 159 | 3 | 1 | G199E | None |
| 2 | G170W | MDS, AML | ||
| 3 | N260fsX50 | MDS, AML | ||
| 155 | 1 | L472P | AML, HCL | |
| 166 | 7 | 16 | N260fs | None |
| 18 | F330fs | MDS, AML | ||
| 19 | R201X | AML | ||
| 32 | L472P | HCL | ||
| 53 | G289fs | MDS | ||
| 54 | S167N | MDS, AML | ||
| 57 | G199E | None | ||
| 168 | 1 | R166X | AML | |
| 145 | 4 | 4 | V118GfsX6 | None |
| 5 | R166Q (de novo) | None | ||
| 6 | D198N (de novo) | None | ||
| 7 | R201PfsX12 | None | ||
| 171 | 9 | A | R107H | AML |
| B | A156E | AML | ||
| C | R201Q | AML, T-ALL | ||
| D | R204Q | AML, T-ALL | ||
| E | T196R | MDS, AML | ||
| F | Q335RfsX261 | AML, T-ALL | ||
| G | I364MfsX230 | None | ||
| H | T148HfsX9 | AML | ||
| I | R166X | AML | ||
| 202 | 1 | W279X | None | |
| 164 | 8 | 5 | P245S | None |
| 6 | G135V | T-ALL | ||
| 7 | D332TfsX | AML | ||
| 8 | H404PfsX | AML | ||
| 9 | G135V | None | ||
| 10 | G170RfsX | AML | ||
| 11 | T196R | None | ||
| 14 | T148HfsX | AML | ||
| 172 | 2 | 32 | L472P | HCL |
| 58 | N465K | MDS |
| Reference . | No. of families . | Family ID* . | RUNX1 mutation (on isoform C) . | Hematological malignancy . |
|---|---|---|---|---|
| 16 | 6 | 1 | Large deletion | AML |
| 2 | V118GfsX11 | AML | ||
| 3 | R204X | AML | ||
| 4 | R201X | AML | ||
| 5 | R201Q | AML | ||
| 6 | R166Q | AML | ||
| 193 | 1 | D198Y | MDS, AML | |
| 148 | 3 | 1 | K110E | Myeloblastic leukemia |
| 2 | R162fsX | AML | ||
| 3 | Y287X | AML | ||
| 194 | 1 | A134P | AML | |
| 143 | 1 | None† | MDS, AML | |
| 195 | 1 | V118GfsX11 | AML | |
| 196, 197, 164 | 1 | T246RfsX8 | CMML, AML | |
| 198 | 1 | T148HfsX9 | AML | |
| 199 | 1 | F330SfsX | MDS | |
| 151 | 5 | A | G363fsX227 | MDS, AML, ALL |
| B | A55fsX81 | AML | ||
| C | D123H | AML | ||
| D | K117fsX101 | MDS, AML, CMML | ||
| E | R320X | MDS, AML | ||
| 200 | 1 | P263LfsX48 | AML | |
| 162 | 1 | R201X | MDS, AML | |
| 163 | 4 | 1 | A156E | AML |
| 2 | R204Q | AML, T-ALL | ||
| 3 | Q335RfsX259 | AML, T-ALL | ||
| 4 | Large deletion | MDS, AML | ||
| 149 | 1 | S388X | MDS, AML | |
| 170 | 2 | 1 | Duplication of exons 2-6 | AML |
| 2 | Deletion of 35 amino acids | MDS, AML | ||
| 153 | 1 | R201X | AML, T-ALL | |
| 150, 165, 164 | 2 | A | R201Q | MDS, AML, T-ALL |
| B | R166X | AML | ||
| 147 | 3 | 1 | G170R | MDS, AML |
| 2 | Q262X | MDS, AML | ||
| 3 | t(16;21)(p13;q22) (de novo) | MDS, AML | ||
| 154 | 1 | G289fsX21 | MDS, CMML | |
| 201 | 1 | L472fsX123 | MDS, AML | |
| 159 | 3 | 1 | G199E | None |
| 2 | G170W | MDS, AML | ||
| 3 | N260fsX50 | MDS, AML | ||
| 155 | 1 | L472P | AML, HCL | |
| 166 | 7 | 16 | N260fs | None |
| 18 | F330fs | MDS, AML | ||
| 19 | R201X | AML | ||
| 32 | L472P | HCL | ||
| 53 | G289fs | MDS | ||
| 54 | S167N | MDS, AML | ||
| 57 | G199E | None | ||
| 168 | 1 | R166X | AML | |
| 145 | 4 | 4 | V118GfsX6 | None |
| 5 | R166Q (de novo) | None | ||
| 6 | D198N (de novo) | None | ||
| 7 | R201PfsX12 | None | ||
| 171 | 9 | A | R107H | AML |
| B | A156E | AML | ||
| C | R201Q | AML, T-ALL | ||
| D | R204Q | AML, T-ALL | ||
| E | T196R | MDS, AML | ||
| F | Q335RfsX261 | AML, T-ALL | ||
| G | I364MfsX230 | None | ||
| H | T148HfsX9 | AML | ||
| I | R166X | AML | ||
| 202 | 1 | W279X | None | |
| 164 | 8 | 5 | P245S | None |
| 6 | G135V | T-ALL | ||
| 7 | D332TfsX | AML | ||
| 8 | H404PfsX | AML | ||
| 9 | G135V | None | ||
| 10 | G170RfsX | AML | ||
| 11 | T196R | None | ||
| 14 | T148HfsX | AML | ||
| 172 | 2 | 32 | L472P | HCL |
| 58 | N465K | MDS |
Identification of each pedigree is based on the publication describing it.
Family without RUNX1 mutations.
Most FPD/AML patients carry point mutations or small indels in RUNX1 that cause missense, nonsense, or frameshift changes in the protein (Table 1). A few cases with large intragenic deletions and duplications have also been reported (Table 1). Interestingly, mutations are clustered in the RHD and TAD domains with a few exceptions (Table 1, Figure 1). Each FPD/AML pedigree carries a unique mutation, and recurrent mutations are observed in only a few amino acid residues (Table 1). Functional studies showed that most mutations are dominant-negative, loss of function, or hypermorphic.148-150 In general, missense and nonsense mutations are dominant-negative, whereas frameshift mutations and large deletions are loss of function, leading to haploinsufficiency. The broad spectrum of mutations and their effects on RUNX1 function indicate that a proper RUNX1 dosage is critical for thrombocyte differentiation.
Overall lifetime risk of MDS and AML in FPD patients is 35% to 40%, and average age of onset is 33 years (range, 6–77 years).151,152 In addition, FPD/AML patients may develop other types of leukemias, such as T-ALL, Hairy cell leukemia (HCL), and CMML.151,153-155 Interestingly, higher incidence of MDS and AML have been observed in families with certain types of mutations.148,151 The exact mechanism of thrombocytopenia and leukemia in FPD/AML patients is not clear. Animal models (mouse and zebrafish) with heterozygous RUNX1 knockout alleles do not display FPD/AML phenotypes.156,157 Therefore, we and others have generated induced pluripotent stem cells (iPSCs) from FPD/AML patients to study disease mechanism.158-161 Together, these studies demonstrated that megakaryopoiesis defects in FPD/AML patients are caused by RUNX1 mutations.
RUNX1 mutations in FPD/AML patients are not sufficient for leukemogenesis. Progression to malignant disease likely occurs by acquisition of additional somatic mutations followed by clonal evolution.162 Several studies have identified loss-of- function somatic mutations in the normal RUNX1 allele as a frequent second hit during leukemogenesis in FPD/AML patients.146,163,164 Antony-Debre and colleagues reported trisomy 21 with duplication of the RUNX1-mutated chromosome or loss of the normal RUNX1 allele in 5 FPD/AML families.164 It is proposed that complete loss of RUNX1 function, either by somatic mutations of the normal copy or by dominant-negative function of the inherited mutations, leads to genome instability.160 In addition, cooperating somatic mutations in more than 20 genes and copy number changes are observed in MDS and leukemic samples from FPD/AML patients.154,155,162,164-169 ASXL1, CBL, CDC25C, FLT3, PHF6, SRSF2, and WT1 were identified as recurrently mutated genes. Additional studies using unbiased genomic approaches are required to identify recurrently mutated genes that cooperate with RUNX1.
Diagnosis of FPD/AML can be missed, because not all patients display clinical symptoms until the malignant disease develops.151,170-172 Furthermore, anticipation leads to occurrence of MDS and AML in younger individuals in subsequent generations. Therefore, a molecular diagnosis by sequencing of all RUNX1 coding exons and copy number analysis for deletions is recommended in families with young patients with MDS and AML.167,170,173 This is of particular concern if bone marrow transplantation from a presumably healthy family member is considered for the treatment of disease. The ability to correct RUNX1 mutation in iPSCs by genome-editing technology may open new avenues for gene therapy in FPD/AML patients in the near future.158,161
Requirement of normal RUNX1 for leukemogenesis
In recent years there is an increasing recognition of the importance of normal RUNX1, as well as its heterodimeric partner, CBFβ, in leukemogenesis.
The story starts with CBF leukemia, those with RUNX1-RUNX1T1 and CBFB-MYH11 fusion genes. The initial hint came from clinical sequencing studies, which showed that RUNX1 mutations do not occur in CBF leukemia patients, even though such mutations are relatively common in other subtypes of AML.125,131,174 Experimentally, RUNX1-RUNX1T1 and CBFB-MYH11 were initially thought to induce leukemia through inhibiting normal RUNX1/CBFB, because Runx1-Runx1t1 and Cbfb-MYH11 knockin mice had embryonic hematopoietic defects similar to those found in Runx1−/− and Cbfb−/− embryos.175,176 Molecular studies seemed to support a RUNX1 dominant suppression mechanism for the fusion genes as well.177-179 However, more recent studies revealed a complicated picture, because both fusion genes were found to have RUNX1-independent functions. For example, Cbfb-MYH11 knockin embryos have defects in primitive hematopoiesis, which is absent in Runx1−/− and Cbfb−/− embryos.175,180 Moreover, it was found that the dysregulated genes by Cbfb-MYH11 were quite different from those dysregulated in Runx1−/− and Cbfb−/− mice.180 More important, Cbfb-MYH11 knockin mice with a mutated Runx1 developed leukemia much more slowly than did those with wild-type Runx1.181 These data suggest that normal Runx1 is required for leukemogenesis by Cbfb-MYH11. Interestingly, RUNX1 knockdown in ME-1, a cell line derived from an inv(16) patient, led to significant apoptosis.17
Similar findings have been reported for the RUNX1 requirement in leukemia cells with RUNX1-RUNX1T1. Ben-Ami and colleagues showed that the t(8;21) leukemia cell line, Kasumi-1, required normal RUNX1 for survival.17 They believed that a delicate balance between the wild-type RUNX1 and RUNX1-RUNX1T1 is important for leukemia cell survival. Similarly, in a model for leukemogenesis using human CD34+ cells transduced with leukemia fusion genes, Goyama and colleagues showed that wild-type RUNX1 is required for RUNX1-RUNX1T1–expressing cells to grow.182 Moreover, Ptasinska and colleagues demonstrated that transcriptional network in t(8;21) leukemia cells is regulated by a dynamic equilibrium between RUNX1-CBFA2T1 and RUNX1 complexes, which compete for binding of identical DNA target sites.46 Li and colleagues showed that RUNX1 was a member of the transcription factor complex containing RUNX1-CBFA2T1, and the relative binding signals of RUNX1 and RUNX1- CBFA2T1 on chromatin determine which genes are repressed or activated by RUNX1- CBFA2T1.39 Recently, Staber and colleagues demonstrated that functional RUNX proteins are required for maintaining an adequate level of PU.1 in hematopoietic cells, which is important for leukemia development by RUNX1-RUNX1T1 in a murine transplantation model.183
Interestingly, Goyama and colleagues found that RUNX1 may also be required by leukemia cells with MLL fusion genes.182 Knocking down RUNX1 in human CD34+ cells transduced with MLL-AF9 inhibited the growth of these cells in culture. Moreover, inhibition of RUNX1, either through short hairpin RNA knockdown or a RUNX1 chemical inhibitor, Ro5-3335,184 suppressed leukemia development by MLL-AF9 in a mouse model. They also found that additional MLL fusion genes require RUNX1 for leukemogenesis.182
Another recent study by Wilkinson et al185 showed that RUNX1 is a direct target of MLL-AF4. RUNX1 is overexpressed in primary ALLs with the MLL-AF4 fusion gene,185,186 and knocking down MLL-AF4 leads to downregulation of RUNX1 expression. Moreover, RUNX1 is required for the growth of MLL-AF4 leukemia cells.185 In addition, it was found that high RUNX1 expression correlates with a poor clinical outcome in ALL patients with MLL fusion genes.185
The impact of normal RUNX1 on other hematologic malignancies is still unclear. There was a recent study suggesting that high RUNX1 expression is associated with poor prognosis in CN-AML.187 The authors analyzed several publicly available datasets and determined that RUNX1 expression is markedly higher in CN-AML than in normal bone marrow. They also compared the molecular characteristics and clinical outcome of 157 CN-AML patients between RUNX1high and RUNX1low groups. They showed that RUNX1high patients were more likely to carry FLT3-ITD but no CEBPA mutations, and the RUNX1high patients had poorer overall survival and event-free survival. The association between RUNX1 expression level and disease outcome was validated in a separate dataset from 162 CN-AML patients. Further studies and experimental tests need to be carried out to validate these findings and understand the underlying mechanisms.
Concluding remarks
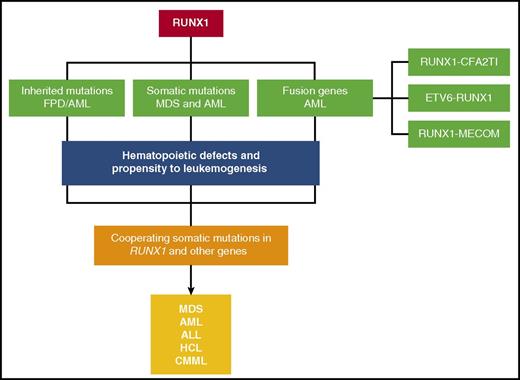
From this review it is clear that RUNX1 is a major player in hematologic malignancies (Figure 3). In general, mutations (both somatic and inherited) are loss of function or dominant-negative. RUNX1 fusion proteins are able to dominantly repress RUNX1 function as well. On the basis of recent molecular studies, it seems that RUNX1 is part of a transcriptional complex that regulates important target genes in hematopoiesis, whereas mutated RUNX1 or RUNX1 fusion proteins disrupt the balance or composition of such a complex. Recent studies suggest that wild-type RUNX1 may also play an active role in leukemia development, at least in the case of CBF leukemia and certain types of MLL leukemia. These findings are consistent with recent observations that other transcription factors such as PU.1, GATA1, and CEBPA also play roles during leukemogenesis.188-190 Overall, these findings all underscore the importance of RUNX1 in maintaining normal hematopoiesis and preventing the development of malignancy.
A schematic depicting the various mechanisms by which RUNX1 gene is altered in hematological malignancies. Inherited mutations cause FPD/AML. Somatic mutations and fusion genes with cooperating mutations in other genes cause hematological malignancies, such as MDS, AML, ALL, HCL, and CMML.
A schematic depicting the various mechanisms by which RUNX1 gene is altered in hematological malignancies. Inherited mutations cause FPD/AML. Somatic mutations and fusion genes with cooperating mutations in other genes cause hematological malignancies, such as MDS, AML, ALL, HCL, and CMML.
RUNX1 sits at the top of the hematopoietic differentiation cascade. The high incidence of RUNX1 somatic mutations in multiple types of hematologic malignancies provides strong support for its essential function in maintaining the proper balance between lineage-specific progenitors during hematopoietic differentiation. The key question that remains to be answered is what mechanisms underlie leukemogenesis and how they can be harnessed to develop potential targeted therapies for these patients. In comparison with the large numbers of publications reporting RUNX1 mutations in various hematological malignancies, there have been only a few papers that study the underlying mechanisms using in vitro or in vivo models.109,148,191,192 Part of the reason for this lack of mechanistic studies of RUNX1 mutations is the lack of suitable animal models. A similar issue exists for the study of FPDMM, because mice and zebrafish heterozygous for RUNX1 knockout mutations do not display the FPDMM phenotype. The application of human-induced pluripotent stem cells and human CD34+ cells as models and the recently developed genome-editing technologies such as CRISPR-Cas9 will, we hope, accelerate the studies of these important mutations, leading to a better understanding of the pathogenesis of leukemia and the next generations of targeted treatments.
Acknowledgments
The authors thank Julia Fekecs for the expert design of the figures.
This work was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Authorship
Contribution: R.S. wrote the sections on RUNX1 mutations in MDS, AML, ALL, and FPDMM; Y.K. wrote the sections on RUNX1-RUNX1T1, ETV6-RUNX1, and other chromosome translocations affecting RUNX1; P.L. wrote the “Introduction,” the section on the requirement of normal RUNX1 in leukemogenesis, and the “Concluding remarks,” and performed the overall editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Liu, 49 Convent Dr, Building 49, Room 3A26, Bethesda, MD 20892; e-mail: pliu@mail.nih.gov.
References
Author notes
R.S. and Y.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal