Abstract
GATA family proteins play essential roles in development of many cell types, including hematopoietic, cardiac, and endodermal lineages. The first three factors, GATAs 1, 2, and 3, are essential for normal hematopoiesis, and their mutations are responsible for a variety of blood disorders. Acquired and inherited GATA1 mutations contribute to Diamond-Blackfan anemia, acute megakaryoblastic leukemia, transient myeloproliferative disorder, and a group of related congenital dyserythropoietic anemias with thrombocytopenia. Conversely, germ line mutations in GATA2 are associated with GATA2 deficiency syndrome, whereas acquired mutations are seen in myelodysplastic syndrome, acute myeloid leukemia, and in blast crisis transformation of chronic myeloid leukemia. The fact that mutations in these genes are commonly seen in blood disorders underscores their critical roles and highlights the need to develop targeted therapies for transcription factors. This review focuses on hematopoietic disorders that are associated with mutations in two prominent GATA family members, GATA1 and GATA2.
The GATA transcription factor family
Mammalian genomes encode six structurally related members of the GATA family, which function as tissue-specific master transcriptional regulators.1,2 Each factor contains two essential Cys4-type zinc fingers. The C-terminal zinc finger imparts the bulk of the DNA binding activity, whereas the N-terminal finger (N-finger) binds some DNA sites and interacts with critical transcriptional cofactors including friend of GATA1 (FOG1). GATA1 and GATA2 each play critical roles in hematopoiesis. Germ line mutations of GATA1, which resides on the X chromosome, cause a variety of sex-linked recessive forms of hereditary thrombocytopenia and dyserythropoietic anemia.3 Furthermore, acquired somatic mutations in GATA1 accompany the development of Down syndrome (DS) transient abnormal myelopoiesis, hereafter referred to as transient myeloproliferative disorder (TMD), and myeloid leukemia associated with DS, hereafter referred to as DS acute megakaryoblastic leukemia (DS-AMKL).4,5 Germ line GATA2 mutations are responsible for GATA2 deficiency syndrome.6,7 Acquired mutations of GATA2 are occasionally encountered in myelodysplastic syndrome (MDS)8 and acute myeloid leukemia (AML),9,10 and a particular gain-of-function mutation (p.L359V) is associated with blast transformation of chronic myeloid leukemia.11 GATA3 is expressed in several tissues, including immune cells, where it controls the maintenance and proliferation of T cells,12 and its germ line mutation is responsible for the syndrome of hypoparathyroidism, deafness, and renal anomalies,13 whereas its somatic mutations are seen in breast cancer.14 Common GATA3 variants are also associated with predisposition to acute lymphoblastic leukemia.15 Finally, GATA4, GATA5, and GATA6 are involved in heart formation, and their germ line mutations and common variants are associated with congenital heart disease.16 Notably, rare variants of GATA5 occur more commonly among a subset of patients with congenital heart disease who have DS.17 The identification of two links between GATA factors and trisomy 21 raises the question of whether there is a link between altered hematopoiesis and cardiac development in DS. In this review, we focus on the hematopoietic factors GATA1 and GATA2 and the contributions of their alterations in benign and malignant blood diseases.
GATA1 in hematopoiesis
GATA1 is an X-linked gene that encodes a DNA binding protein with two zinc fingers and a transactivation domain. The C-terminal zinc finger provides the predominant DNA binding activity to allow GATA1 to associate with its (A/T)GATA(A/G) consensus sequence.18-20 The N-finger is important for increasing the stability of GATA1 bound to DNA and also facilitates GATA1 binding to a subset of binding sites that contain a palindromic motif.21 In addition, the N-finger is critical because it is the domain that interacts with the key cofactor FOG1.22 Finally, the N-terminus of GATA1 contains a classically defined transcriptional activation domain, which can activate reporter genes containing the consensus GATA binding site in fibroblasts.23
Studies performed in multiple mouse models have revealed a requirement for GATA1 in erythroid cells, megakaryocytes, mast cells, eosinophils, and basophils. The first studies with Gata1-null animals and embryonic stem cells demonstrated that the gene is essential for erythropoiesis; in the absence of Gata1, mouse embryos died at E10.5-E11.5 from anemia.24 Without GATA1, erythroid progenitors undergo apoptosis, in part through reduced expression of Bcl-xL.25,26 More recently, an inducible knockout mouse model revealed an additional requirement for Gata1 in stress erythropoiesis, likely through a similar pathway as the constitutive knockout.27
Interestingly, loss of GATA1 has the opposite effect on megakaryocytes; in the absence of GATA1, megakaryocytes fail to undergo terminal differentiation but expand dramatically. This effect was most clearly seen in mice that were engineered to have a deletion in an upstream hypersensitive site, which is now recognized as a megakaryocyte-selective enhancer. These Gata1 ΔneoΔHS (also known as Gata1low) mice also have reduced expression of GATA1 in erythroid cells, but there are sufficient erythroid cells made to allow the animals to survive.28 These mice are thrombocytopenic and harbor a significant increase in the numbers of immature megakaryocytes in the bone marrow and the spleen.29 A similar expansion was observed in the inducible Gata1-knockout mouse.27 Megakaryocytes without GATA1 have multiple abnormalities; they fail to upregulate CD42 or reach the high ploidy states of wild-type cells, have impaired cytoplasmic maturation with a reduction in demarcation membranes, express megakaryocyte-associated genes at low levels, and shed very few platelets.30 Both the FOG-interaction domain and the N-terminus are required for normal maturation and growth control of megakaryocytes.31,32
Other notable features of Gata1low mice are that they have impaired mast cell differentiation33 and uniformly develop bone marrow fibrosis, with an accompanying poikilocytosis and anemia that appear in older animals. The latter phenotype is in some ways akin to primary myelofibrosis and likely results from the accumulation of immature megakaryocytes with impaired differentiation (see “The myeloproliferative neoplasms”). Finally, other studies implicate GATA1 in development of eosinophils,34,35 basophils,36 and even dendritic cells.37
GATA1 in disease
Given that GATA1 is an essential gene, it was initially suspected that mutations would cause embryonic lethality and that mutant clones would be at a selective disadvantage. However, there are several types of GATA1 mutations that occur in humans. First, inherited GATA1 mutations, which lead to a spectrum of congenital red cell and megakaryocyte disorders or Diamond-Blackfan anemia (DBA), have been observed in numerous families. Second, acquired GATA1 mutations are found in nearly all cases of TMD and AMKL in children with Down syndrome (DS-AMKL). Third, downregulation of GATA1 likely contributes to the development of fibrosis in patients with the myeloproliferative neoplasms.
Benign hematologic disorders
Congenital dyserythropoietic anemia
One of the most important cofactors of GATA1 is the zinc finger–containing protein FOG1, which binds the N-finger of GATA1 (Figure 1A) and is essential for normal hematopoiesis. Mice lacking Fog1 die in utero from anemia at a similar stage as Gata1-deficient mice.38 Of note, in contrast to Gata1-deficient animal models, Fog1-null mice completely lack megakaryocytes. This observation revealed that there is a critical GATA1-independent function of GATA1; subsequent studies demonstrated that this function depends on its interaction with GATA2.39
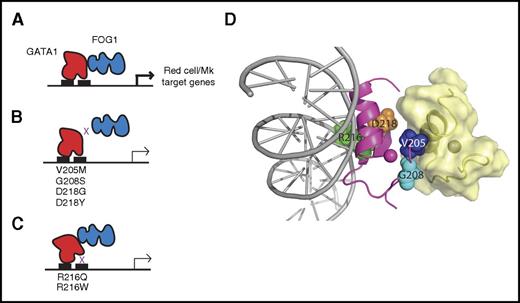
In 2000, Weiss and colleagues40 reported that a V205M mutation in GATA1 is the cause of a rare form of dyserythropoietic anemia and thrombocytopenia in humans. The V205M mutation was identified in two half brothers whose mother developed a mild thrombocytopenia during pregnancy and was heterozygous for the allele. This GATA1 mutation acts by reducing the affinity of GATA1 for FOG1, but does not affect binding to DNA (Figure 1B,D). Additional FOG1 noninteracting mutations in GATA1 have been identified. These include alterations in G20841 and D218,42,43 which, like V205, lie on the surface of GATA1 that faces away from DNA (Figure 1D). Changes in these residues lead to a spectrum of benign hematologic disorders that include aberrant megakaryocyte and/or erythroid cell disorders (Table 1). Furthermore, GATA1 mutations that impair the ability of GATA1 to bind DNA, including R216Q and R216W, were found in individuals with macrothrombocytopenia and β-thalassemia, with additional features of gray platelet syndrome and congenital porphyria, respectively44-47 (Figure 1C-D).
GATA1 mutations in benign hematologic disorders often affect the function of N-terminal zinc finger. (A) The normal function of the N-finger is to bind DNA at complex GATA motifs and to recruit FOG1; together, GATA1 and FOG1 drive expression of numerous red cell and megakaryocyte (Mk) genes. (B) Mutations in the FOG1-binding face of GATA1 disrupt the protein-protein interaction and diminish the expression of target genes. (C) Mutations in the N-finger that reduce the affinity of GATA1 for DNA also suppress expression of GATA1 target genes. (D) Structural representation of mutants in the GATA1 N-finger (magenta). Residues that are mutated in these disorders are indicated as spheres. The positions of FOG1 (yellow, derived from the structure of the GATA1:FOG1 structure111 ) and DNA (gray, inferred from the structure of the GATA1-DNA complex112 ) are shown. The NMR structure of the N-finger of GATA1 confirms the localization of the mutations to the FOG-interacting (left) or DNA binding surface (right).
GATA1 mutations in benign hematologic disorders often affect the function of N-terminal zinc finger. (A) The normal function of the N-finger is to bind DNA at complex GATA motifs and to recruit FOG1; together, GATA1 and FOG1 drive expression of numerous red cell and megakaryocyte (Mk) genes. (B) Mutations in the FOG1-binding face of GATA1 disrupt the protein-protein interaction and diminish the expression of target genes. (C) Mutations in the N-finger that reduce the affinity of GATA1 for DNA also suppress expression of GATA1 target genes. (D) Structural representation of mutants in the GATA1 N-finger (magenta). Residues that are mutated in these disorders are indicated as spheres. The positions of FOG1 (yellow, derived from the structure of the GATA1:FOG1 structure111 ) and DNA (gray, inferred from the structure of the GATA1-DNA complex112 ) are shown. The NMR structure of the N-finger of GATA1 confirms the localization of the mutations to the FOG-interacting (left) or DNA binding surface (right).
GATA1 mutations in hematologic disorders
| Mutation . | Disease . | Notable features . | Reference(s) . |
|---|---|---|---|
| V205M | X-linked dyserythropoietic anemia and thrombocytopenia | Severe fetal anemia and postnatal abnormalities in both erythroid and platelet lineages. Also, hypercellular bone marrow with an abundance of large, multinucleated erythroid precursors and small dysplastic megakaryocytes | 40 |
| G208S | X-linked macrothrombocytopenia | Macrothrombocytopenia with severe bleeding, and mild dyserythropoiesis | 41, 42 |
| D218G | |||
| D218Y | X-linked macrothrombocytopenia with severe anemia | More severe phenotype than the D218G mutation due to a stronger block in the GATA1-FOG1 interaction | 43 |
| R216Q | X-linked thrombocytopenia with β-thalassemia | Individuals with the R216Q mutation share notable features with patients with gray platelet syndrome. Patients with the R216W mutation show features of congenital erythropoietic porphyria | 44,,-47 |
| R216W | |||
| GATA1s without +21 | Inherited macrocytic anemia and neutropenia | Erythroid hypoplasia with mild neutropenia and mild defects in megakaryopoiesis | 48 |
| GATA1s with +21 | TMD | Transient expansion of megakaryoblasts; hepatosplenomegaly, petechiae, and effusions | 5, 58 |
| GATA1s with +21 and a third mutation | AMKL | Fulminant megakaryocytic leukemia | 4, 73 |
| Mutation . | Disease . | Notable features . | Reference(s) . |
|---|---|---|---|
| V205M | X-linked dyserythropoietic anemia and thrombocytopenia | Severe fetal anemia and postnatal abnormalities in both erythroid and platelet lineages. Also, hypercellular bone marrow with an abundance of large, multinucleated erythroid precursors and small dysplastic megakaryocytes | 40 |
| G208S | X-linked macrothrombocytopenia | Macrothrombocytopenia with severe bleeding, and mild dyserythropoiesis | 41, 42 |
| D218G | |||
| D218Y | X-linked macrothrombocytopenia with severe anemia | More severe phenotype than the D218G mutation due to a stronger block in the GATA1-FOG1 interaction | 43 |
| R216Q | X-linked thrombocytopenia with β-thalassemia | Individuals with the R216Q mutation share notable features with patients with gray platelet syndrome. Patients with the R216W mutation show features of congenital erythropoietic porphyria | 44,,-47 |
| R216W | |||
| GATA1s without +21 | Inherited macrocytic anemia and neutropenia | Erythroid hypoplasia with mild neutropenia and mild defects in megakaryopoiesis | 48 |
| GATA1s with +21 | TMD | Transient expansion of megakaryoblasts; hepatosplenomegaly, petechiae, and effusions | 5, 58 |
| GATA1s with +21 and a third mutation | AMKL | Fulminant megakaryocytic leukemia | 4, 73 |
+21, trisomy 21.
Hollanda et al48 identified a second class of GATA1 mutations in a family in Brazil. Affected individuals harbor a mutation in exon 2 of GATA1, which encodes the N-terminal transactivation domain. This mutation results in loss of full-length GATA1, but continued expression of its shorter isoform, named GATA1s, which lacks this activation domain but retains the two zinc fingers and the entire C-terminus.49 Similar mutations had previously been discovered in children with DS who have myeloid leukemia (see “Myeloid malignancies in children with DS”). The children with this mutation displayed erythroid hypoplasia with neutropenia and mild defects in megakaryocytes, indicating that the N-terminus of GATA1 is necessary for its full activity.
DBA
More than half of patients with DBA harbor mutations in a ribosomal gene, with the most common being RPS19.50,51 In an effort to discover the genetic basis of DBA cases that lack a ribosome mutation, Gazda and colleagues52 performed exome sequencing on two siblings and discovered a mutation in GATA1. On the surface, the mutation, a G to C transversion that results in L74V, might appear not to have a pathogenic effect. However, the substitution led to a change in the last nucleotide of exon 2 that causes a splicing defect, the consequence of which is expression of GATA1s in the absence of full-length GATA1. Sequencing of a larger cohort identified two other cases,52,53 whereas other groups also found similar GATA1 mutations in DBA.54 One of these mutations resulted in the loss of the translation initiation codon for the full-length isoform, again implicating loss of GATA1, and/or unique expression of GATA1s as the culprit. In their efforts to determine if there is a link between the GATA1-mutant cases and those with ribosomal gene mutations, Sankaran and colleagues53 made the surprising observation that knocking down different ribosomal subunits led to a selective impaired production of full-length GATA1 and GATA1s. Moreover, they showed that ectopic expression of GATA1 in primary DBA patient samples improved erythroid differentiation, suggesting that its impaired expression is a key factor in the erythroid defects.
Why do some patients with GATA1s develop DBA, whereas others develop a less severe disease? It has been speculated that the difference in the severity of the GATA1s-mutant cases is a result of differences in the level of GATA1s expression.53 Support for this model comes from studies with GATA1s transgenic mice, in which high levels of transgene expression fully rescue a Gata1 deficiency, whereas lower levels of gene expression were associated with impaired hematopoiesis.55 An alternative explanation is semantics; perhaps GATA1-mutant cases should be classified as belonging to a single disease entity with various features.56
Malignant hematologic disorders
Myeloid malignancies in children with DS
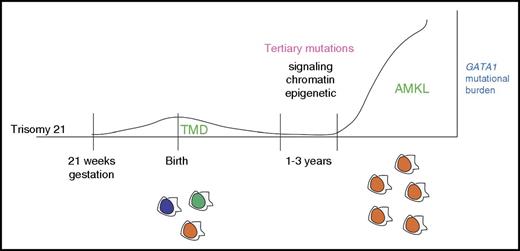
Children with DS are at an increased risk for leukemia, especially AMKL and B-cell acute lymphoblastic leukemia.57 Moreover, they uniquely develop a self-limiting leukemia known as TMD or transient abnormal myelopoiesis.57 TMD is a fascinating disease that presents at birth and resolves in one to two months in the majority of cases.58,59 However, TMD is also a preleukemic condition, which evolves to AMKL in one to three years in as many as 20% of cases (Figure 2). The incidence of TMD is unclear, but it has been estimated to affect as many as 10% of infants with DS.57
Progression of myeloid leukemia in children with DS.GATA1 mutations commonly occur in utero as early as week 21 of gestation in hematopoietic progenitors with trisomy 21. The resulting TMD is initially polyclonal, with multiple distinct GATA1-mutant clones. The GATA1 mutational burden diminishes shortly after birth as the TMD resolves. Over the course of one to three years, a persistent GATA1-mutant clone may acquire a tertiary mutation, which leads to clonal expansion and acute megakaryocytic leukemia.
Progression of myeloid leukemia in children with DS.GATA1 mutations commonly occur in utero as early as week 21 of gestation in hematopoietic progenitors with trisomy 21. The resulting TMD is initially polyclonal, with multiple distinct GATA1-mutant clones. The GATA1 mutational burden diminishes shortly after birth as the TMD resolves. Over the course of one to three years, a persistent GATA1-mutant clone may acquire a tertiary mutation, which leads to clonal expansion and acute megakaryocytic leukemia.
The observation that the murine Gata1 deficiency drives proliferation of megakaryocyte progenitors while simultaneously impairing terminal differentiation provided the basis for analyzing the GATA1 gene and its activity in humans with hematologic malignancies. Sequencing of GATA1 in patients led to the seminal finding that mutations in GATA1 are defining events in megakaryocytic leukemia in children with DS.4 GATA1 mutations have now been found in nearly every case of DS-AMKL, but are rarely seen in AMKL in euploid individuals and have not been reported in any other malignancies.60
Although TMD and DS-AMKL blasts resemble one another morphologically and immunophenotypically and have similar gene expression programs,61 the extent to which the two diseases are related was unclear until the discovery of GATA1 mutations.57 Sequencing revealed that GATA1 mutations are present in nearly all, if not all, patients with TMD,5 and that these mutations resemble the types seen in DS-AMKL. Evidence for a direct evolution of TMD to AMKL was provided by Izraeli and colleagues,62 who found an identical GATA1 mutation in TMD and subsequent AMKL in a single patient. Other interesting aspects of the disease include the finding that GATA1 mutations can be detected as early as week 21 of gestation, that more than five mutant clones can be seen in TMD, and that GATA1 mutations can occur in individuals without DS who acquire trisomy 21 in their hematopoietic cells.57 That GATA1 mutations in TMD also lead to eosinophil hyperproliferation is consistent with the role of GATA1 in this lineage.63 Recently, using a sensitive next-generation sequencing approach, Roberts, Vyas and colleagues64 discovered that nearly 30% of newborns with DS are born with a GATA1 mutation. This is a remarkable mutational frequency in a population, which suggests that GATA1 mutations are strongly selected for in the background of trisomy 21. The absence of GATA1 mutations in newborns without DS further underscores the inextricable link between trisomy 21 and GATA1.
DS-AMKL is distinct from other subtypes of AMKL with respect to genetics and outcome. AMKL in children without DS is characterized by a number of chromosomal abnormalities, including 11q23 rearrangements, t(1;22), and inv(16)(p13.3q24.3), the last 2 of which lead to expression of the RBM15-MKL1 (aka OTT-MAL) and CBFA2T3-GLIS2 chimeric oncogenes, respectively.60,65,66 The outcomes for this class of AMKL vary among the different genetic subtypes, but on average, the 5-year event-free survival and overall survival were reported to be 43% and 49%, respectively.67 In contrast, the genetic basis of adult AMKL is poorly defined and the outcome is dismal, with a median survival of 10.4 months.68 Of note, children with DS-AMKL fare well, with 5-year event-free survival and overall survival approaching 80%.69 The increased sensitivity of DS-AMKL blasts to high-dose cytarabine-based therapy has been attributed to decreased expression of cytidine deaminase by the GATA1s-mutant isoform.70
Data from patients and mouse models indicate that GATA1 mutations are not sufficient for DS-AMKL and that the addition of the mouse equivalent of trisomy 21 also does not promote leukemia.71,72 However, a comprehensive next-generation sequencing study of TMD and DS-AMKL by Ogawa and colleagues73 provides strong evidence that GATA1 mutations and trisomy 21 are sufficient for TMD. This study further identified a number of leukemia-associated mutations uniquely in the DS-AMKL phase, demonstrating that the progression of TMD to AMKL is dependent on the acquisition of tertiary mutations. These mutations fall into at least three broad classes: signaling (eg, MPL, JAK2, and NRAS), chromatin (eg, STAG2, RAD21, and CTCF), and epigenetic (eg, EZH2, DNMT1, and ASXL1) (Figure 2).
Despite much effort, there are several unanswered questions regarding the pathogenesis of DS-AMKL. First, how does the loss of the N-terminus of GATA1 contribute to the malignancy? Second, why are GATA1 mutations so common in individuals with DS? Third, how does trisomy 21 predispose children to leukemia and cooperate with GATA1 mutations to cause TMD and subsequent AMKL? To date, data suggest that both GATA1 mutations and trisomy 21 lead to expansion of the erythromegakaryocyte compartment.71,74-77 How they work in concert to transform hematopoietic cells, however, remains to be determined.
The myeloproliferative neoplasms
Perhaps the most understudied aspect of GATA1 biology is its potential contributions to the human myeloproliferative neoplasm primary myelofibrosis (PMF). Migliaccio, Vannucchi, and colleagues78 have provided several important insights into the contributions of GATA1 dysregulation to this disease. For example, they showed that Gata1low mice develop a myeloproliferative disease characterized by profound bone marrow fibrosis, which, as the mice age, is associated with anemia. They also made the striking observation that a majority of megakaryocytes in the bone marrow of patients with PMF do not stain for GATA1.79 These GATA1-deficient cells show an atypical morphology, including bulbous nuclei and an abnormal nuclear:cytoplasmic ratio. On the basis of the idea that the Gata1-mutant cells may contribute by secretion of abnormally high levels of tumor necrosis factor β (TGF-β), Migliaccio and colleagues80 treated the Gata1low mice with TGF-β inhibitors and observed a rescue in megakaryocyte development and gene expression, as well as reversion of fibrosis. The results from this study provide additional rationale for testing TGF-β inhibitors in patients with PMF. Important areas for future studies include determining the link between myeloproliferative neoplasm driver mutations and GATA1 expression with the role of aberrant megakaryocytes in PMF.
GATA2 in hematopoiesis
GATA2 expression is essential for maintenance of the pool of hematopoietic stem cells (HSCs), whereas GATA1 drives HSCs toward erythroid/megakaryocytic differentiation, leading to a loss of self-renewal capacity. During the differentiation of HSCs, through a phenomenon known as “the GATA switch,” GATA2 activates GATA1, and then GATA1, in turn, represses GATA2 while displacing it from chromatin at sites throughout the genome.81 Importantly, GATA1 and GATA2 each autoactivate their own expression. The HSC compartment is therefore exquisitely sensitive to levels of GATA2. Mice that are homozygously deficient for Gata2 exhibit embryonic lethality as a result of failure of definitive hematopoiesis,82 whereas heterozygously deficient mice demonstrate compromised HSC longevity, leading to reduced numbers of progenitor cells.83 Mice remain, however, imperfect models of human GATA2 deficiency, because Gata2+/− mice retain normal bone marrow cellularity and peripheral blood cell counts without developing MDS or AML or otherwise recapitulating features of the disorder.83
GATA2 in disease
Clinical aspects of GATA2 deficiency syndrome
GATA2 deficiency syndrome is a recently described, hereditary, autosomal dominant bone marrow failure disorder with systemic features caused by heterozygous germ line mutation in one of two copies of the gene encoding the GATA2.6
In 2011, different groups first identified heterozygous germ line mutations in GATA2 as the basis for autosomal dominant forms of AML and MDS84 ; Emberger syndrome, which consists of MDS, lymphedema, and warts from human papillomavirus infection85 ; the MonoMAC syndrome of monocytopenia, with predisposition to the nontuberculous Mycobacterium avium complex of infections7 ; and the syndrome of dendritic cell, monocyte, and B and natural killer (NK) lymphoid deficiency, with vulnerability to viral and other types of infections.86 Shortly thereafter, germ line GATA2 mutations were detected among some patients presenting with severe congenital neutropenia,87 suspected aplastic anemia,88 B-cell immunodeficiency,89 juvenile MDS,89,90 chronic myelomonocytic leukemia,91 severe Epstein-Barr virus infections and Epstein-Barr virus–associated cancers,92 at least one case93 of B-cell acute lymphoblastic leukemia, and finally, other unexplained cases of bone marrow failure.94 The clinical manifestation of the disorder defined by heterozygous inactivating germ line mutations in GATA2 has come to be known as “GATA2 deficiency syndrome” (Table 2). Patients with GATA2 deficiency syndrome sometimes additionally exhibit nonhematological and noninfectious complications, including deafness, lymphedema, pulmonary alveolar proteinosis (PAP), miscarriage, hypothyroidism, thrombosis, occasional intellectual disability, and other findings,95-99 including erythema nodosum95 and other autoimmune disorders.100 Interestingly, although PAP is ordinarily associated with circulating antibodies to granulocyte-macrophage colony-stimulating factor, individuals with GATA2-associated PAP do not develop these autoantibodies.
Clinical features of GATA2 deficiency syndrome
| Clinical feature . | Percentage of patients . |
|---|---|
| Hematologic | |
| Cytopenias | |
| B cell | 86 |
| NK cell | 82 |
| Monocytopenia | 78 |
| CD4 | 51 |
| Neutropenia | 47 |
| Malignant blood disorders | |
| MDS* | 84 |
| AML | 14 |
| CMML | 8 |
| Infectious | |
| Viral | 70 |
| HPV | 63 |
| Herpesvirus | 35 |
| Herpes simplex virus | 16 |
| Varicella Zoster virus | 11 |
| EBV | 11 |
| Cytomegalovirus | 4 |
| Molloscum contagiosum | 3.5 |
| Bacterial | |
| Disseminated nontubercular mycobacteria† | 53 |
| Other severe bacterial | 49 |
| Severe fungal | 16 |
| Aspergillosis | 9 |
| Histoplasmosis | 5 |
| Candidiasis | 5 |
| Solid tumors | |
| HPV-related neoplasia | 35 |
| EBV-related neoplasia‡ | |
| Breast cancer§ | 22 |
| Pulmonary | |
| Pulmonary alveolar proteinosis | 18 |
| Pulmonary function test abnormalities | 79 |
| Lymphedema | 11 |
| Venous thrombosis | 25 |
| Mild to severe sensorineuronal hearing loss|| | 76 |
| Miscarriage¶ | 33 |
| Hypothyroidism | 14 |
| Clinical feature . | Percentage of patients . |
|---|---|
| Hematologic | |
| Cytopenias | |
| B cell | 86 |
| NK cell | 82 |
| Monocytopenia | 78 |
| CD4 | 51 |
| Neutropenia | 47 |
| Malignant blood disorders | |
| MDS* | 84 |
| AML | 14 |
| CMML | 8 |
| Infectious | |
| Viral | 70 |
| HPV | 63 |
| Herpesvirus | 35 |
| Herpes simplex virus | 16 |
| Varicella Zoster virus | 11 |
| EBV | 11 |
| Cytomegalovirus | 4 |
| Molloscum contagiosum | 3.5 |
| Bacterial | |
| Disseminated nontubercular mycobacteria† | 53 |
| Other severe bacterial | 49 |
| Severe fungal | 16 |
| Aspergillosis | 9 |
| Histoplasmosis | 5 |
| Candidiasis | 5 |
| Solid tumors | |
| HPV-related neoplasia | 35 |
| EBV-related neoplasia‡ | |
| Breast cancer§ | 22 |
| Pulmonary | |
| Pulmonary alveolar proteinosis | 18 |
| Pulmonary function test abnormalities | 79 |
| Lymphedema | 11 |
| Venous thrombosis | 25 |
| Mild to severe sensorineuronal hearing loss|| | 76 |
| Miscarriage¶ | 33 |
| Hypothyroidism | 14 |
Percentage of patients manifesting noted clinical features. Data abstracted from Spinner et al95 from 57 patients, 40 of whom were diagnosed on the basis of clinical presentation, with an additional 17 through family screening. Similar data are tabulated by Collin et al.6
CMML, chronic myelomonocytic leukemia; EBV, Epstein-Barr virus; HPV, human papillomavirus.
Of 50 patients who underwent bone marrow biopsy.
Reflects potential ascertainment bias before clinical phenotype expanded.
Per Cohen et al.92
Of 18 women ≥35 years old, including one with a concurrent BRCA2 mutation.
Not controlled for aminoglycoside exposure for treatment of bacterial infections.
Of 43 known pregnancies.
In accord with the disorder’s variable clinical manifestations, patients may exhibit a range of peripheral cytopenias, perhaps the most reliable of which are B-cell and NK-cell lymphopenia and monocytopenia.88 Consistent with the role of GATA2 in maintaining a reservoir of HSCs, sequential bone marrow monitoring by flow cytometry of patients with GATA2 deficiency syndrome reveals progressive depletion of granulocyte-macrophage and multilymphoid progenitor populations, as well as clonal hematopoiesis when evaluated by nonrandom X-chromosome inactivation in female patients.96 A reduction in the pool of HSCs also likely contributes to pathogenesis in GATA2 deficiency syndrome. Cytopenias tend to be progressive, with loss of bone marrow progenitor populations over time and a tendency toward clonal hematopoiesis.6,96 Immunoserology can demonstrate elevated FMS-like tyrosine kinase 3 ligand, which may be an indicator of clinical progression.96,101
Bone marrow histopathological changes can be subtle, not always present before onset of overt bone marrow failure, and characteristically include hypolobated micromegakaryocytes, large osteoclastlike megakaryocytes, dyserythropoiesis (consisting of nuclear budding, binucleation, and megaloblastoid changes), and myeloid dysplasia (as evidenced by maturation asynchrony and hypogranularity).88,102,103 Hypocellularity can also be observed. In accord with peripheral cytopenias, flow cytometric analysis of bone marrow from patients with GATA2 deficiency reveals significant reductions in monocytes, B cells, and NK cells.88 Additional bone marrow flow cytometric abnormalities include an inverted CD4:CD8 ratio, atypical plasma cell subsets, a reduced CD34+ population, and reduced hematogones (CD19+CD10+CD20− precursor B cells).88,101 Clonal cytogenetic abnormalities have been reported in more than half of patients and include trisomy 8, monosomy 7, trisomy 21, and other changes, alone or in combination with each other.95
GATA2 deficiency syndrome has proven to be unexpectedly common. Among children and young adults with MDS, its prevalence is about 7% to 15% overall, and in cases of MDS with monosomy of chromosome 7, it is found in as many as 72% of patients.89,90 Genetic testing in patients with idiopathic bone marrow failure employing a panel of 85 genes associated with different congenital hematological disorders found that heterozygous germ line mutations in GATA2, present in five of 71 patients tested (7%), was the most frequent identifiable hereditary cause of bone marrow failure.96 GATA2 deficiency syndrome often explains sporadic cases of bone marrow failure, even in the absence of a contributory family history, presumably as a result of nonpenetrance and occurrence of de novo germ line GATA2 mutations.104
Hematopoietic stem cell transplantation can be curative, but many patients with GATA2 deficiency syndrome are not transplantation candidates because potential related donors may share a familial GATA2 mutation, rendering them unsuitable, and because outcomes are less favorable following transformation to MDS or leukemia or the onset of chronic infection.105,106 Phenotypic reversion and full immune reconstitution following transplant can take as long as several years.106 Optimal timing in the overall course of disease remains to be determined. There is thus a need for additional therapeutic approaches. Although associated morbidity is significant, many patients nevertheless remain asymptomatic well into their adult years, only later presenting with leukemic or cytopenic complications.6 For many patients with GATA2 deficiency syndrome, there may therefore be a prolonged window of therapeutic opportunity in which to delay or prevent onset of its malignant, infectious, or other complications.
Genetics of GATA2 deficiency syndrome
GATA2 deficiency syndrome is an autosomal dominant disorder caused by heterozygous germ line GATA2 mutations. Although about 100 different mutant alleles are now known, for the most part, GATA2 mutations appear to behave similarly in that they reduce or abrogate transcriptional activity either through deletion, chain termination, point mutations (including amino acid missense substitutions), or promoter/enhancer mutations that lead to decreased expression.6 Mutations that abolish GATA2 activity, such as frameshifts, trend toward an earlier age of onset compared with mutations, such as amino acid missense substitution, that are likely to only partially reduce transcriptional activity,96 consistent with the notion that clinical progression is directly tied to levels of GATA2 activity. An exception may be that second zinc finger mutations are more narrowly associated with the familial MDS/AML phenotype and that a particular MDS/AML-associated GATA2 mutation, T354M, may function as a dominant negative.84 A few cases of GATA2 deficiency syndrome result from large, whole gene deletions, including adjacent contiguous genes on chromosome 3q21.3, and are associated with developmental and neurologic disturbances.97
Among individuals with GATA2 deficiency syndrome that progresses to MDS/AML, acquired secondary mutations in the gene encoding chromatin-binding protein ASXL1 are common.84,91,107 In contradistinction to cancer predisposition syndromes caused by mutations in tumor suppressor genes, however, there is little evidence that, among patients with GATA2 deficiency syndrome, the wild-type copy of GATA2 undergoes an acquired “second hit,” not even with leukemic progression,84,98 as assessed via whole exome107 or whole genome sequencing108 performed at depths of coverage sufficient to reveal hematopoietic subclones.
GATA2 activates its own expression, as strikingly confirmed by the discovery of patients with GATA2 deficiency syndrome who possess a mutation in GATA2 autoregulatory binding sites within its own promoter and enhancer.109,110 Remarkably, patients with these mutations maintain an intact coding sequence on the mutant allele, but they are lacking the regulatory element responsible for maintaining the autoactivation circuit.
Finally, all patients with GATA2 deficiency syndrome appear to retain a functioning, wild-type copy of GATA2, present in all cells throughout their bodies, including malignant blood cells, consistent with the notion that partial loss of GATA2 causes the disorder. This fact suggests that stabilizing or otherwise boosting residual wild-type GATA2 activity may represent a novel therapeutic entry point.
Conclusions
Germ line and somatic mutations in GATA1 and GATA2, two genes that are essential for normal hematopoiesis, are observed in a number of benign and malignant blood disorders that primarily involve erythroid and myeloid lineages, but that sometimes also include lymphoid and nonhematologic systemic features. Although GATA1 mutations in DS-AMKL portend an excellent outcome, mutations in GATA1 and GATA2 are more commonly associated with disorders that are difficult to treat. Although it has been long assumed that targeting transcription factors is not a viable therapeutic option, the advent of gene editing approaches may provide a means to correct GATA mutations, especially in benign hematologic disorders. Given that GATA factor mutations typically consist of monoallelic loss-of-function mutations, stabilization of wild-type protein via pharmacologic inhibition of its rapid turnover might represent another therapeutic avenue ripe for development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joel Mackay (University of Sydney) for providing the structural depiction of the GATA1 N-finger used in Figure 1.
This work was supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK101329) (J.D.C.), National Cancer Institute (R01 CA101774) (J.D.C.), and National Heart, Lung, and Blood Institute (R01 HL130472) (M.S.H.).
Authorship
Contribution: J.D.C. and M.S.H. jointly wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Crispino, Division of Hematology/Oncology, Northwestern University, 303 East Superior St, 5-113, Chicago, IL 60611; e-mail: j-crispino@northwestern.edu.