Abstract
The discovery of the GATA binding protein (GATA factor) transcription factor family revolutionized hematology. Studies of GATA proteins have yielded vital contributions to our understanding of how hematopoietic stem and progenitor cells develop from precursors, how progenitors generate red blood cells, how hemoglobin synthesis is regulated, and the molecular underpinnings of nonmalignant and malignant hematologic disorders. This thrilling journey began with mechanistic studies on a β-globin enhancer- and promoter-binding factor, GATA-1, the founding member of the GATA family. This work ushered in the cloning of related proteins, GATA-2-6, with distinct and/or overlapping expression patterns. Herein, we discuss how the hematopoietic GATA factors (GATA-1-3) function via a battery of mechanistic permutations, which can be GATA factor subtype, cell type, and locus specific. Understanding this intriguing protein family requires consideration of how the mechanistic permutations are amalgamated into circuits to orchestrate processes of interest to the hematologist and more broadly.
Introduction
Analyzing gene regulation in the current era almost invariably involves genome-wide studies. However, much of what we know about hematopoietic transcriptional mechanisms emerged from highly focused mechanistic studies at a single locus, β-globin. This work led to the cloning of a β-globin enhancer- and promoter-binding factor, GATA binding protein 1 (GATA-1),1,2 the founding member of the GATA transcription factor family and ushered in the cloning of the related proteins GATA-2-6.3-12 GATA-1, 2, and 3 are expressed in specific hematopoietic cell types, as well as in select nonhematopoietic cells, and regulate the development and function of diverse blood cell lineages.13,14 GATA-4, 5, and, 6 control the development and function of select nonhematopoietic cell types and organogenesis, including cardiovascular development.15,16
The discovery of GATA transcription factors occurred at a time when the first mammalian sequence-specific DNA binding proteins were being purified and cloned.17 Whereas intensive efforts using a wide swath of molecular biological approaches inferred important functions for these factors, it was difficult to ascertain whether they had essential functions. Furthermore, even though millions of copies of the GATA factor-recognized DNA motif (WGATAR) reside in a genome,14 it was unclear whether GATA proteins control a restricted gene cohort or large genetic networks. Rigorous vetting of these foundational questions has yielded lucid answers. Whereas individual GATA factors are often essential for critical biological processes, redundancies can exist. GATA factors establish and maintain complex cell type–specific genetic networks involving dozens to hundreds of activated or repressed target genes,18-20 but these genes represent a very small fraction of those containing GATA motifs.14 The regulation of at least certain genes within the networks requires a specific GATA factor, rather than simultaneous actions of multiple GATA factors.
GATA-1 is expressed in erythroid, megakaryocytic, mast, eosinophil, basophil, and dendritic cells.13,21-23 GATA-1 and GATA-2 expression partially overlap (eg, in yolk sac–derived primitive erythroblasts, megakaryocytes, and eosinophils).24-26 In other contexts, their expression is mutually exclusive or anticorrelative. GATA-2 is uniquely expressed in hematopoietic stem and progenitor cells (HSPCs) and erythroid precursors prior to GATA-1 expression.21,27,28 The distinct patterns infer that these GATA factors control different biological processes. Extremely instructive gene targeting studies strongly supported this concept.
Targeted deletion of Gata1 in mice yields defective erythroid cell development and embryonic day 10.5 (E10.5) to E11.5 lethality.25,29,30 Subsequent studies established the importance of GATA-1 for megakaryocyte, mast cell, eosinophil, and basophil differentiation.31-33 Targeted deletion of Gata2 is lethal at ∼E10 because of a broad collapse of hematopoiesis,24,34 reflecting a GATA-2 requirement for HSPC genesis and function.35-40 Unlike GATA-1 and GATA-2, GATA-3 is expressed in T lymphocytes and controls T-cell lymphopoiesis.41-44 Nonhematopoietic functions include regulating prostate (GATA-2)45,46 and breast (GATA-3)47,48 development and tumorigenesis.
Herein, we discuss how the hematopoietic GATA factors function via a battery of mechanistic permutations to yield complex circuits, with some understood with molecular sophistication and others with only a cursory sketch of the mechanistic fabric. As GATA factor mechanisms can be GATA factor subtype, cell type, and/or locus specific, it is challenging to distill this diversity into a single mechanism that fits into a stringent mold. Understanding this intriguing protein family requires consideration of how the mechanistic permutations are amalgamated into circuits that drive processes of interest to the hematologist and more broadly.
GATA factor mechanisms
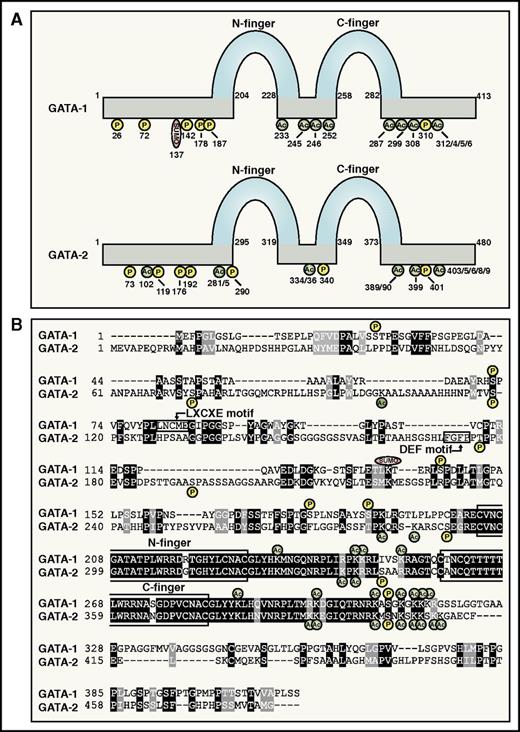
GATA factors bind GATA sequence motifs via a highly conserved dual zinc finger domain (Figure 1A-B).14 This engenders GATA-1 and GATA-2 with similar, if not identical, sequence preferences. The genomic abundance of GATA motifs makes target gene predictions impossible based on sequence alone. Chromatin immunoprecipitation coupled with massively parallel sequencing revealed GATA-1 and GATA-2 occupancy genome-wide, redefined the designation of WGATAR as the GATA motif, and revealed additional motifs enriched at GATA-occupied sites.18-20,49-53 These studies indicated GATA-1 and GATA-2 occupy <1% of GATA motifs, illustrating an exquisite specificity of GATA motif selection.14,54,55
GATA-1 and GATA-2 protein attributes. (A) GATA-1 and GATA-2 protein attributes. N- and C-zinc fingers and posttranslational modification sites are indicated.84,87,88,90,117,122,124,125,170-172 (B) Amino acid sequence alignment of human GATA-1 and GATA-2. Protein domains and posttranslational modification sites are highlighted.
GATA-1 and GATA-2 protein attributes. (A) GATA-1 and GATA-2 protein attributes. N- and C-zinc fingers and posttranslational modification sites are indicated.84,87,88,90,117,122,124,125,170-172 (B) Amino acid sequence alignment of human GATA-1 and GATA-2. Protein domains and posttranslational modification sites are highlighted.
Combining chromatin occupancy with transcriptomic data can enable identification of direct GATA-1 and/or GATA-2 targets. Gene coregulation by different GATA factors can occur through binding identical or distinct GATA motifs within a locus. Multiple GATA factors in the same cell can compete for binding site occupancy, and replacement of one GATA factor by another is termed a GATA switch. GATA switches were initially described for GATA-1 and GATA-2 in erythroid differentiation models28,56-59 (Figure 2A). A genome-wide analysis of enhancer usage during HSPC to erythroid differentiation revealed switches at ∼30% of GATA-bound enhancers,60 and ∼30% of GATA-2-occupied sites are GATA-1-occupied upon megakaryopoiesis.51,61 GATA switching is facilitated by the shorter half-life of GATA-2 vs GATA-1.62,63 As GATA-1 and GATA-2 can exert opposing activities through the same chromatin site, GATA switches can change gene expression and may drive erythroid maturation.14,64 In certain contexts, GATA-2 might prime a chromatin site in preparation for subsequent GATA-1 function.14,64
GATA factor mechanistic principles. (A) GATA switch model. GATA switches involve replacement of one GATA factor by another at a chromatin target site. GATA switches can be associated with an altered transcriptional output. The GATA switch is illustrated at the Gata2 locus. In erythroblasts, friend of GATA-1 (FOG-1) promotes GATA-1-mediated replacement of chromatin-bound GATA-2, instigating repression.64,74 (B) Coregulator dependency matrix model. “Sensitive” and “insensitive” denote whether reductions in the endogenous coregulators impact expression of the GATA-1 target genes. Distinct coregulator ensembles mediate GATA-1-dependent transcription in a locus-specific and context-dependent manner.91,98,99,173
GATA factor mechanistic principles. (A) GATA switch model. GATA switches involve replacement of one GATA factor by another at a chromatin target site. GATA switches can be associated with an altered transcriptional output. The GATA switch is illustrated at the Gata2 locus. In erythroblasts, friend of GATA-1 (FOG-1) promotes GATA-1-mediated replacement of chromatin-bound GATA-2, instigating repression.64,74 (B) Coregulator dependency matrix model. “Sensitive” and “insensitive” denote whether reductions in the endogenous coregulators impact expression of the GATA-1 target genes. Distinct coregulator ensembles mediate GATA-1-dependent transcription in a locus-specific and context-dependent manner.91,98,99,173
GATA-1 and GATA-2 genomic occupancy sites and target genes can be cell type specific and/or overlapping.18,49,51 The milieu of GATA factor–associated coregulators almost certainly contributes to the context dependence65 (Figure 2B). The coregulator ensemble mediating GATA factor activity serves diverse functions, including catalysis of protein modifications (eg, acetylation, sumoylation, and phosphorylation) that directly or indirectly (via chromatin alterations) modulate GATA factor activity (Figure 1A-B). Perhaps the most important GATA-1 coregulator, FOG-1, mediates activation and repression of most GATA-1 target genes.66-68 Resembling Gata1− mice, targeted disruption of Zfpm1, encoding FOG-1, yields failure of primitive and definitive erythropoiesis and embryonic lethality.66,67 A small cohort of GATA-1 targets are insensitive to reduced FOG-1 levels and to GATA-1 mutations that inhibit FOG-1 binding.68-71 Despite its 9 zinc fingers, FOG-1 has not been demonstrated to bind DNA. Four FOG-1 zinc fingers can mediate binding to the GATA-1 N-terminal zinc finger (N-finger).72,73 The GATA-1/FOG-1 interaction promotes GATA-1 occupancy at numerous genomic sites,74,75 and N-finger mutations that inhibit FOG-1 binding can redistribute GATA-1 to ectopic sites.76 FOG-1 recruits the nucleosome remodeling and deacetylase complex77,78 and the repressor C-terminal binding protein (CtBP)73 to GATA-1-bound sites and promotes chromatin looping.79 Although FOG-1 sumoylation enhances binding to CtBP,80 this interaction may be dispensable for erythropoiesis based on studies with mice expressing a FOG-1 mutant lacking a CtBP binding sequence.81 Mice with knock-in mutations in both GATA-1 and GATA-2 that inhibit FOG-1 binding have severely defective megakaryopoiesis,82 resembling FOG-1−/− mice.67 These results led to the proposal that megakarypoiesis requires GATA-1 or GATA-2 to engage FOG-1. We are unaware of evidence indicating that FOG-1 mediates other GATA-2 activities (eg, in HSPCs or GATA-2-expressing erythroid precursors).
As regulation of GATA factor activity is multilayered, a major challenge is to identify and organize these layers into models that can be definitively tested. An intriguing piece of this puzzle involves the selective influence of GATA-1 posttranslational modifications on specific GATA-1 activities, rather than globally at all target loci. This is exemplified by sumoylation of GATA-1 K137 by the PIASy E3 ligase,83 which preferentially impacts FOG-1-sensitive vs FOG-1-insensitive targets.84 Tethering small ubiquitin-like modifier (SUMO) to GATA-1 bypasses the FOG-1 requirement to activate FOG-1-sensitive genes, although SUMO is not required for FOG-1 binding to GATA-1.84 Analysis of subnuclear locus positioning by 3-dimensional immunofluorescence in situ hybridization revealed differential behavior of FOG-1-sensitive and FOG-1-insensitive loci. Transcriptional activation resulted in expulsion of FOG-1/SUMO-sensitive genes from the nuclear periphery, whereas FOG-1-insensitive genes persisted at the periphery.84,85 As locus-specific mechanisms are not commonly elucidated in transcriptional analyses, these studies are likely to yield general principles.
Histone acetyltransferases acetylate histones and nonhistone proteins. The first coregulator interaction described for GATA-1 was with the broadly expressed histone acetyltransferase CREB binding protein and its paralog p300.86 CREB binding protein interacts with numerous transcription factors, binds GATA-1 zinc fingers, and acetylates lysines near the fingers.87,88 This was reported to enhance GATA-1 DNA binding89 ; however, another analysis revealed no impact on DNA binding, but increased chromatin occupancy.87 GATA-1 acetylation promotes Brd3 bromodomain protein binding recruitment to chromatin.90
Another example of context-dependent regulation that impacts a subset of the GATA-1 target gene ensemble involves GATA-1 interactions with histone methyltransferases.65 SetD8, the only known histone H4 K20 monomethyltransferase (H4K20me1), mediates GATA-1-dependent repression of select target genes.91 SetD8 confers erythroblast survival,92 which may or may not reflect its GATA-1 corepressor function. The histone methyltransferases EZH1 and EZH2 catalyze histone H3 K27 trimethylation as part of the polycomb repressive complex (PRC2).93 During erythroid maturation, a switch from EZH2 to EZH1 in PRC2 redistributes the complex from active genes to bivalent or repressed genes.94 GATA-1 interacts with EZH2 and another component of PRC2, Suz12.19,94
Chromatin remodeling impacts local and broad chromatin transitions that control gene activity. GATA-1 recruits BRG1, the catalytic subunit of the SWItch Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex, to chromatin.71,95,96 BRG1 can mediate GATA-1-dependent chromatin looping and transcriptional activation of α- and β-globin loci.71,97 GATA-1 also recruits the multisubunit Mediator complex,98-100 which interacts with numerous transcription factors and basal transcriptional machinery and promotes RNA polymerase II transcription. Mediator-cohesin interactions promote chromatin looping.101 Targeted deletion of Med1 deregulates GATA-1 target genes and impairs erythroid maturation.102 Given the ubiquitous Mediator functions, it is likely integrated into diverse GATA-1 regulatory layers.
Distinct DNA binding protein combinations occupying chromatin can recruit unique coregulator assemblages. Pairing a GATA motif with an E-box (CANNTG) yields a composite element bound by GATA-1 or GATA-2 and the basic helix-loop-helix transcription factor Scl/TAL1.103 GATA-1–Scl/TAL1 recruits non-DNA binding proteins including LMO2 and LDB1, and additional factors are implicated.104-106 A detailed analysis of composite element mechanisms was described recently.107 Certain GATA factor target loci, Gata1, Gata2, Klf1, and Epb4.2, contain functional composite elements, and BRG1 can be recruited to composite elements.107 Although composite elements display a fourfold higher frequency of GATA occupancy vs classical GATA motifs (0.61% vs 0.14%),18,108 many questions remain regarding mechanistic similarities and differences. GATA-1–Scl/TAL1 complex occupancy does not require Scl/TAL1 DNA binding; GATA-1 can recruit Scl/TAL1 to sites lacking an E-box.109 In mouse HPC-7 and human CD34+ cells, GATA-2, Scl/TAL1, Lyl1, LMO2, Runx1, ERG, and Fli-1 co-occupy chromatin, including sites at genes important for HSPCs.53,110
Multiple mechanisms governing GATA factor function are exemplified by Gata2 transcriptional regulation. The Gata2 locus represents the first, and most extensively studied, example of GATA switching. GATA-1-mediated replacement of GATA-2 represses Gata2 transcription during erythroid maturation via FOG-1- and SetD8-dependent mechanisms.65 GATA switching occurs at 5 highly conserved Gata2 sites, −77, −3.9, −2.8, −1.8, and +9.5 kb relative to the 1S promoter.28,56,57,64 As GATA-2 and GATA-1 occupy these sites at the active and repressed locus, respectively, predictions could not be made regarding whether sites contribute to Gata2 activation or repression or if they are dispensable. Knockout mouse strains lacking the individual sites revealed variable and unpredictable consequences vis-à-vis Gata2 expression and hematopoiesis. The −3.9, −2.8, and −1.8 homozygous mutant strains are viable, although Gata2 expression is modestly reduced in −2.8 mutant HSPCs.111-113 The −1.8 site maintains GATA-2 repression late in erythropoiesis.111 Deletion of the −3.9 site yields no alterations in Gata2 expression or hematopoiesis.113 The +9.5 and −77 sites are indispensable regulators of Gata2 transcription with distinct activities.35,40 Comparable results were obtained by targeted deletion of the +9.5 site in mice35 vs deleting Gata2 using Cre recombinase driven by a chromosomal segment containing the +9.5 site.114 The +9.5 site homozygous deletion inactivates the hematopoietic stem cell (HSC) generator in the aorta-gonad-mesonephros (AGM) region during embryogenesis.36 Of the Gata2 GATA switch sites, only the +9.5 site possesses an E-box/GATA composite element,115,116 and interrogation of the genome for “+9.5-like” composite elements revealed additional GATA-2-regulated enhancers active in HSPCs.107,108 The −77 homozygous deletion in mice decreases Gata2 expression in myeloid progenitors, strongly reducing granulocyte macrophage progenitor (GMP) and megakaryocyte erythrocyte progenitor (MEP) populations.40 The distinct +9.5 and −77 site activities provide an opportunity to compare how GATA factors engage regulatory factors/signals through these sites to confer unique transcriptional responses, which will yield principles governing GATA factor mechanisms.
Emerging mechanistic circuits
Signal-dependent mechanisms
Although GATA factors are subjected to multiple posttranslational modifications, in most cases, it is unclear whether signaling mechanisms transduce vital regulation through these modifications. The entry point to this problem was underwhelming, as simultaneous mutation of multiple GATA-1 phosphorylation sites yielded little to no functional consequences.117,118 Akt stimulates GATA-1 serine 310 phosphorylation,119 and this is implicated in activation of a single GATA-1 target gene, Timp1.120 However, without IGF-1 signaling, Gata1S310A knock-in mice exhibit hemolytic anemia.121 Erythropoietin receptor (EpoR)-mediated serine 310 phosphorylation enhances GATA-1 binding to FOG-1 and disrupts a GATA-1/E2F-2/Rb complex that requires a GATA-1 LxCxE motif.121
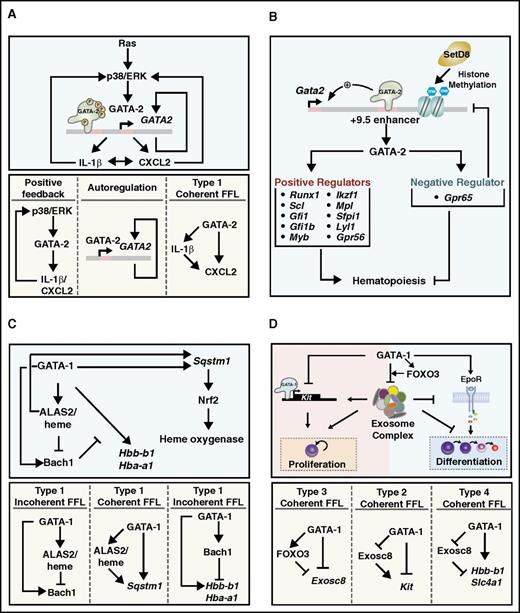
Multisite GATA-2 phosphorylation was discovered from analysis of the activity of a defective GATA-2 mutant (T354M) implicated in the primary immunodeficiency monocytopenia and mycobacterial infection syndrome (MonoMAC), which progresses to myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).122 The T354M mutant migrates with a slower mobility than GATA-2 on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and mass spectrometry revealed the slow mobility reflects hyperphosphorylation that requires S192 phosphorylation. Oncogenic Ras and p38 and/or extracellular signal-regulated kinase signaling stimulates GATA-2 hyperphosphorylation, thereby enhancing GATA-2 chromatin occupancy and activity at a target gene cohort. Unlike wild-type GATA-2, hyperphosphorylation cannot stimulate T354M activity, as this DNA binding domain mutation impairs chromatin occupancy. Thus, T354M hyperphosphorylation appears to be inconsequential and need not be reversed. A GATA-2 MAPK docking module (DEF motif) is essential for oncogenic Ras-induced GATA-2 hyperphosphorylation in AML cells.123 This mechanism increases interleukin-1β and CXCL2 expression, which can activate p38/extracellular signal-regulated kinase and further enhance GATA-2 activity (Figure 3A). As CXCL2 can stimulate AML cell proliferation, the MAPK-GATA-2-interleukin-1β/CXCL2 axis constitutes a positive-feedback circuit implicated in leukemic cell proliferation. GATA-2 is also regulated via insulin-dependent Akt activation, which phosphorylates GATA-2 S401 and suppresses GATA-2 function.124 CDK1 phosphorylates GATA-2 T176 and promotes Fbw7-mediated GATA-2 degradation.125
Emerging GATA factor-dependent mechanistic circuits. (A) Ras-MAPK signaling controls GATA-2 activity.123 (B) GATA-2-GPR65 circuit negatively regulates hematopoiesis.127 (C) GATA-1-heme circuit regulates erythroid differentiation.134 (D) GATA-1-FoxO3-exosome circuit controls erythroid maturation.146,148 FFL, feed forward loop.
Emerging GATA factor-dependent mechanistic circuits. (A) Ras-MAPK signaling controls GATA-2 activity.123 (B) GATA-2-GPR65 circuit negatively regulates hematopoiesis.127 (C) GATA-1-heme circuit regulates erythroid differentiation.134 (D) GATA-1-FoxO3-exosome circuit controls erythroid maturation.146,148 FFL, feed forward loop.
Can the signal-dependent GATA-2 mechanism be extrapolated to other GATA factors? P38 phosphorylates and regulates GATA-3, and this has been suggested to control nuclear translocation and Th2 cytokine gene expression.126 Essentially nothing is known about GATA factor subcellular transitions. Although the phosphorylated GATA-3 amino acid residues were not described, GATA-3 contains a threonine analogous to GATA-2 S192, and we predict GATA-3 also contains a DEF motif.
GATA-2-G-protein-coupled receptor circuit
Ensuring GATA-2 expression within a physiological window.
Gata2 +9.5−/− mouse embryos harbor very few HSPCs,35 as the HSC generator in their AGM is defective.36 This system permitted genomic analyses to establish how the +9.5 enhancer triggers the HSC generator. Although it was hypothesized that the enhancer ensures GATA-2 expression and establishes a genetic network consisting of positive mediators of GATA-2 function, the analysis yielded the discovery of a GATA-2-activated gene, Gpr65, that opposes GATA-2 function.127
GPR65 downregulation in the AGM increases Gata2 expression and AGM-derived CD31+c-Kit+Sca1+ cells, which include HSCs. Mechanistically, GPR65 represses Gata2 transcription and involves SetD8-dependent establishment of repressive chromatin at the +9.5 enhancer. Whereas SetD8 can function as a GATA-1 corepressor, how H4K20me1 alters chromatin function is not understood. Furthermore, how GPR65 interfaces with SetD8 and Gata2 is unknown. Nevertheless, this GATA-2-GPR65 mechanism constitutes a negative-feedback circuit that contributes to maintenance of Gata2 expression within a physiological window (Figure 3B). Either too little or too much GATA-2 is linked to myeloid leukemogenesis,14 and therefore it is exceptionally important to ensure fidelity of the multilayered mechanism dictating normal GATA-2 levels.
GATA-1-heme circuit
Linking heme and globin chain synthesis to control hemoglobin production and erythrocyte development.
Heme biosynthesis in mitochondria128-130 requires GATA-1 target genes encoding heme biosynthesis enzymes. The first and rate-limiting step of heme biosynthesis is catalyzed by 5-aminolevulinic acid synthase (ALAS), which utilizes glycine and succinyl-CoA to form ALA.131 Mammals have 2 ALAS isoforms, ALAS-1 and ALAS-2. Whereas ALAS-1 mediates housekeeping functions in diverse cell types, ALAS-2 is expressed specifically in erythroid cells to generate heme for the developing erythroblast.132,133 GATA-1 strongly activates ALAS2 transcription.18,134 Posttranscriptionally, when iron is low, iron-responsive proteins (IRPs) bind the iron-responsive element (IRE) in the ALAS2 messenger RNA 5′ untranslated region to prevent its translation.135 Excessive heme also induces ALAS-2 protein ubiquitination and degradation,136,137 which establishes a negative feedback circuit to avoid heme toxicity. Furthermore, heme deficiency activates heme-regulated eIF2α kinase, which phosphorylates the eIF2α translational factor to decrease globin translation.138
Considering that GATA-1 activates β- and α-globin gene transcription, as well as ALAS2 and other heme biosynthetic genes,18,139 and colocalizes with a key repressor of fetal hemoglobin expression BCL11A,140 GATA-1 is perhaps the most important factor mediating hemoglobin biosynthesis. Heme binds the transcriptional repressor Bach1, promoting its degradation via the ubiquitin-proteasome system.141 Bach1 only accumulates in a low-heme environment when GATA-1 activates Bach1 transcription.134 GATA-1-mediated Bach1 transcriptional activation is insufficient to elevate Bach1, as the posttranslational destruction system dominates when heme levels are normal. Bach1 represses β-globin transcription,134,142 which helps restore balance between globin chains and heme and prevents excessive globin chains from eliciting toxicity.
Dissecting mechanisms underlying GATA-1-mediated Alas2 transcription revealed an intriguing interconnectivity between heme and GATA-1 that constitutes a new GATA factor mechanism: heme amplifies GATA-1 activity at a cohort of target genes.134 Using CRISPR/Cas9 to delete 2 intronic GATA-1 binding regions at Alas2, GATA-1-null G1E proerythroblasts were engineered to express >10-fold lower heme. Although these mutant cells are viable and proliferate normally, a conditionally active GATA-1 allele (ER-GATA-1) is less effective in activating select GATA-1 target genes. The cell permeable metabolite 5-ALA bypasses this ALAS-2 requirement, rescuing heme levels and increasing GATA-1 target gene expression. This rescue involves Bach1-sensitive and Bach1-insensitive modes, as Bach1 downregulation increases expression of certain, but not all, GATA-1 target genes in the low-heme environment. Thus, GATA-1-mediated induction of Alas2 and Bach1 transcription, and heme downregulation of Bach1, conforms to a Type I incoherent feedforward loop143 (Figure 3C). This network motif is embedded in a complex circuit involving other constituents of the target gene ensemble. As heme functions extend beyond hemoglobin synthesis, this circuit may impact diverse physiological and pathological states. These studies illustrate how changes in a single cofactor (heme) can drastically impact GATA factor function.
GATA-1-exosome complex circuit
Balancing erythroblast proliferation and differentiation through amalgamated feed-forward loops.
Although many studies have described mechanisms promoting erythroid cell development, mechanisms counteracting prodifferentiation factors/signals are less well understood. The studies described previously and others144,145 demonstrate that low heme is not conducive to erythroid maturation. Are there physiological mechanisms that establish maturation roadblocks that are deconstructed by GATA-1 actions? An answer emerged from analyses of how the transcription factor FoxO3 amplifies GATA-1 activity to activate genes encoding autophagy components.50
FoxO3 controls a subset of GATA-1-regulated genes, providing another example of context-dependent GATA-1 factor mechanisms.146 These GATA-1/FoxO3-coregulated genes include those encoding subunits of the exosome complex, an 11 subunit complex mediating the selective degradation of noncoding and coding RNAs.147 The GATA-1/FoxO3-mediated reduction of exosome complex components suggested the complex might create an impediment to erythroid maturation. Beyond controlling RNA degradation and processing, the complex is implicated in transcriptional regulation.147
Loss-of-function studies provided evidence for an exosome complex-mediated erythroid maturation barricade.146 Downregulating exosome complex subunits disrupted the complex, which stimulated primary erythroid precursors to differentiate into more mature erythroblasts and even enucleated erythrocytes. Mechanistic studies revealed a circuit in which the exosome complex confers the barricade via at least 2 mechanisms.148 First, it sustains expression of the receptor tyrosine kinase c-Kit. Stem cell factor–mediated c-Kit activation can maintain erythroid precursors in a proliferating, undifferentiated state, and GATA-1-mediated repression of exosome complex subunits decreases c-Kit expression and attenuates c-Kit signaling.148 Moreover, GATA-1 directly represses Kit transcription.149 Second, GATA-1 is implicated in activating expression of the gene encoding EpoR, which generates prodifferentiation signals.150 In addition to sustaining c-Kit signaling, the exosome complex suppresses EpoR signaling.148 GATA-1-mediated transcriptional repression of exosome complex subunit genes and Kit, and exosome complex-mediated c-Kit expression conforms to a type II coherent feed-forward loop143 (Figure 3D). GATA-1-mediated repression of exosome complex subunits and exosome complex-mediated suppression of EpoR signaling conforms to a type IV coherent feed-forward loop143 (Figure 3D). The GATA-1-exosome complex circuit balances erythroid precursor proliferation vs differentiation. It is instructive to consider the interconnectivity of pro- and antidifferentiation circuits in various biological systems, and further probing into this problem will almost certainly yield new physiological and pathological insights.
Human disease mutations inform GATA factor mechanisms
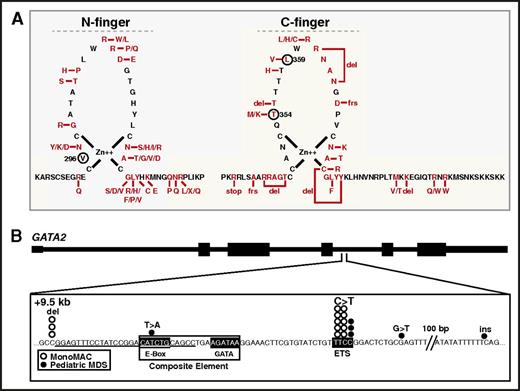
Mechanisms governing GATA factor function have been informed by disease-causing mutations in humans with hematologic disorders. As GATA-1 dysregulation has been reviewed,151,152 our focus will be on mechanistic insights emerging from human GATA-2 mutations. Although the preponderance of known GATA2 mutations occur within the coding region,153-156 a minority reside in noncoding sequences.35,157 A GATA2-AML link emerged when GATA2 heterozygous mutations were detected in the clinical syndromes MonoMAC and DCML (dendritic cell, monocyte, B and NK lymphoid deficiency) primary immunodeficiency, familial MDS/AML and Emberger syndrome.153-156 Most missense mutations reside in or near the DNA binding GATA-2 C-finger, although function-disrupting frameshift and nonsense mutations also exist158 (Figure 4A). The GATA-2 T354M mutant detected in MonoMAC and familial MDS/AML, exhibited reduced chromatin occupancy and failed to activate the GATA-2 target gene Hdc.122 Reporter and DNA binding assays identified loss-of-function GATA2 mutations in MonoMAC.153,156 In addition to AML, GATA2 L359V mutation was detected in chronic myeloid leukemia patients in blast crisis.159 In AML patients with biallelic CEBPA mutations, GATA2 mutations are present with frequencies of ∼30% of patients, with most mutations being N-finger missense.160-162 N-finger mutations were described in 22% of patients with acute erythroid leukemia.163 Although N-finger mutations are known to disrupt GATA-1 function in humans164 and experimental models,165,166 how N-fingers mutations impact GATA-2 function is unknown (Figure 4A).
GATA-2 mutations in human hematologic disorders inform GATA factor mechanisms. (A) Left, GATA-2 N-finger mutations in human AML patients with biallelic CEBPA mutations.160-163,174,175 V296 corresponds to GATA-1 V205, which enhances GATA-1 and FOG-1 binding. Right, C-finger mutations identified in AML-associated diseases.153-156,158,176-178 T354M is a loss-of-function mutation that inhibits chromatin occupancy and target gene activation.122,123 L359V was identified in chronic myeloid leukemia.159 (B) Mutations at and near the +9.5 GATA switch site enhancer in pediatric MDS169 and MonoMAC syndrome.35,157,158 del, deletion; ins, insertion.
GATA-2 mutations in human hematologic disorders inform GATA factor mechanisms. (A) Left, GATA-2 N-finger mutations in human AML patients with biallelic CEBPA mutations.160-163,174,175 V296 corresponds to GATA-1 V205, which enhances GATA-1 and FOG-1 binding. Right, C-finger mutations identified in AML-associated diseases.153-156,158,176-178 T354M is a loss-of-function mutation that inhibits chromatin occupancy and target gene activation.122,123 L359V was identified in chronic myeloid leukemia.159 (B) Mutations at and near the +9.5 GATA switch site enhancer in pediatric MDS169 and MonoMAC syndrome.35,157,158 del, deletion; ins, insertion.
A subset of acute AML is characterized by chromosomal rearrangements involving 3q21 and 3q26, which result in overexpression of MECOM encoding the proto-oncogene EVI1.167 Chromosomal rearrangements translocate the GATA2 −77 kb GATA switch site enhancer ∼4 megabases away into proximity of MECOM.167,168 As the −77 confers Gata2 expression to confer differentiation potential in mouse myeloid progenitors,40 its removal from human GATA2 likely decreases GATA-2 levels, while elevating EVI1 levels. Thus, co-opting a hematopoietic GATA switch site enhancer deregulates a proto-oncogene and underlies 3q21:q26 AML. These studies provided strong evidence that integrity of the −77 enhancer is crucial to suppress the development of a human hematologic malignancy.
Disruption of the GATA2 +9.5 GATA switch site enhancer can also cause MonoMAC.35 Typically, MonoMAC patients have mutations within the DNA binding region of one GATA2 allele, leading to haploinsufficiency. However, a MonoMAC patient with both GATA2 genes intact harbored a heterozygous deletion within the +9.5 site, which eliminated the E-box and several base pairs of upstream sequence, while preserving the GATA motif.35 Additional MonoMAC patients have +9.5 kb mutations in a neighboring E-twenty six (ETS) motif157 (Figure 4B). The +9.5 kb site mutations have also been detected in juvenile MDS patients.169 These studies indicate that multiple modules within the +9.5 composite element, which normally triggers the mouse HSC generator, control enhancer activity and hematopoiesis in humans. We expect additional GATA2 coding and noncoding mutations will provide vital mechanistic insights, and these insights may transform our understanding of how GATA factors are regulated, how they function physiologically, and how mechanistic corruption instigates pathologies including malignancy and anemia.
Acknowledgments
E.H.B. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grants DK50107 and DK68634), the National Heart, Lung, and Blood Institute (grant HL116365), and the National Cancer Institute (Cancer Center Support Grant P30 CA014520), National Institutes of Health. Skye C. McIver and Kyle J. Hewitt were supported by American Heart Association postdoctoral fellowships. Alexandra A. Soukup was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (T32 HL07899 Training Grant in Hematology).
Authorship
Contribution: This review was generated by a multidisciplinary team under the leadership of E.H.B.; and all authors contributed actively and significantly to the conceptualization and writing of the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of additional members of the GATA Factor Mechanisms Group appears in “Appendix.”
Correspondence: Emery H. Bresnick, Department of Cell and Regenerative Biology, University of Wisconsin School of Medicine and Public Health, 4009 WIMR, 1111 Highland Ave, Madison, WI 53705; e-mail: ehbresni@wisc.edu.
Appendix: study group members
Additional members of the GATA Factor Mechanisms Group are: Xin Gao (University of Wisconsin School of Medicine and Public Health), Kyle J. Hewitt (University of Wisconsin School of Medicine and Public Health), Daniel R. Matson (University of Wisconsin School of Medicine and Public Health), Skye C. McIver (University of Wisconsin School of Medicine and Public Health), Charu Mehta (University of Wisconsin School of Medicine and Public Health), Alexandra A. Soukup (University of Wisconsin School of Medicine and Public Health), Nobuyuki Tanimura (University of Wisconsin School of Medicine and Public Health), Lihong Shi (State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College), and Kirby D. Johnson (University of Wisconsin School of Medicine and Public Health).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal