Abstract
Recent studies have demonstrated that myelodysplastic syndromes (MDSs) arise from a small population of disease-initiating hematopoietic stem cells (HSCs) that persist and expand through conventional therapies and are major contributors to disease progression and relapse. MDS stem and progenitor cells are characterized by key founder and driver mutations and are enriched for cytogenetic alterations. Quantitative alterations in hematopoietic stem and progenitor cell (HSPC) numbers are also seen in a stage-specific manner in human MDS samples as well as in murine models of the disease. Overexpression of several markers such as interleukin-1 (IL-1) receptor accessory protein (IL1RAP), CD99, T-cell immunoglobulin mucin-3, and CD123 have begun to differentiate MDS HSPCs from healthy counterparts. Overactivation of innate immune components such as Toll-like receptors, IL-1 receptor–associated kinase/tumor necrosis factor receptor–associated factor-6, IL8/CXCR2, and IL1RAP signaling pathways has been demonstrated in MDS HSPCs and is being targeted therapeutically in preclinical and early clinical studies. Other dysregulated pathways such as signal transducer and activator of transcription 3, tyrosine kinase with immunoglobulinlike and EGF-like domains 1/angiopoietin-1, p21-activated kinase, microRNA 21, and transforming growth factor β are also being explored as therapeutic targets against MDS HSPCs. Taken together, these studies have demonstrated that MDS stem cells are functionally critical for the initiation, transformation, and relapse of disease and need to be targeted therapeutically for future curative strategies in MDSs.
Introduction
The myelodysplastic syndromes (MDSs) comprise a heterogeneous group of malignant hematopoietic stem cell (HSC) disorders that are characterized by disordered growth and differentiation of hematopoietic progenitors and a variable risk of transformation to acute myeloid leukemia (AML).1,2 Although approved agents such as 5-azacitidine and lenalidomide have resulted in clinical responses, relapse and refractory disease continue to occur in most patients. Whereas the stem cell origin of MDSs has long been discussed and theorized, it is only recently that precisely defined hematopoietic stem and progenitor cell (HSPC) alterations have been demonstrated. These aberrant HSPCs have been shown to persist during therapy and expand at the time of relapse.1,3,4 Aberrant stem and progenitor cell populations in MDSs and AML share many cellular features with healthy HSCs, such as sustained self-maintenance and proliferative capacity, and are not easily eliminated by conventional chemotherapies.5,6 Similar to AML, our understanding of clonal evolution in MDSs is developing from studies that include multiparameter fluorescence-activated cell sorter (FACS) analysis of primary samples, high throughput sequencing studies, and analysis of mouse models of these diseases.5,7-10 This review aims to highlight the important functional role of aberrant stem and progenitor cells in the pathogenesis and relapse of MDSs, the role of immune dysregulation, and emerging data on therapeutic targets aimed toward MDS HSPCs.
Stage-specific alterations are seen in stem and progenitor cells in MDS
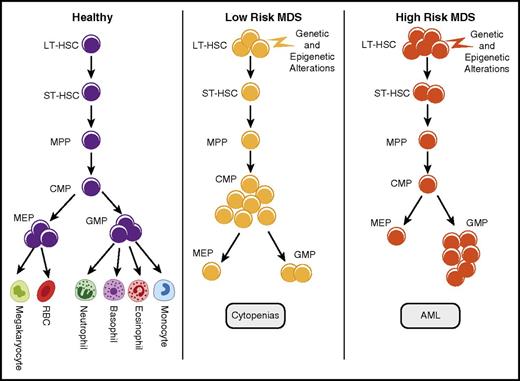
MDS is composed of different subtypes that are mainly distinguished on the basis of the risk of leukemic transformation. The International Prognostic Scoring System (IPSS) allows the disease to be divided into lower (Low and Intermediate-1 [Int-1]) and higher (Int-2 and High) risk categories; the higher-risk subtypes are associated with higher blast counts, increased risk of leukemic transformation, and shorter median overall survival.11 Analysis of HSPCs in primary MDSs shows distinct patterns of quantitative alterations in these MDS subtypes. Quantitative alterations were determined by FACS analysis using rigorous lineage depletion and analysis of phenotypic long-term HSCs (LT-HSCs; Lin−CD34+CD38−CD90+), short-term HSCs (Lin−CD34+CD38−CD90−), common myeloid progenitor (CMP; Lin−CD34+CD38+CD123+CD45RA−), megakaryocyte erythroid progenitor (MEP; Lin−CD34+CD38+CD123−CD45RA−), and granulocyte monocyte progenitor (GMP; Lin−CD34+CD38+CD123+CD45RA+) compartments on the basis of established surface marker characterization. We and others have observed that, although phenotypic HSCs are expanded in all MDS subtypes, a larger expansion of LT-HSCs is seen in the higher-risk cases.1,4,12 Higher-risk cases were also characterized by the expansion of the GMP compartment, whereas lower-risk MDSs were characterized by expansion of the CMPs4,13 (Figure 1). Another common abnormality seen was the striking decrease in MEPs in MDS, thus suggesting a relative differentiation block from CMPs to MEPs, although the exact molecular events that would lead to this differentiation arrest are not yet elucidated. This is pronounced in lower-risk cases and may explain, at least in part, the anemia and thrombocytopenia, hallmarks of patients with MDSs.4 The pattern of mutations frequently differs among CMP, GMP, and MEP compartments; accumulation of mutations in a linear fashion and persistence of a dominant subclone have been observed.14
Quantitative stem and progenitor alterations in MDS subgroups. Stem cells and various progenitor populations are shown in normal and myelodysplastic hematopoiesis. Although this is a simplified model of differentiation, recent studies have shown that multipotent progenitors (MPPs) and CMPs can take alternative paths toward differentiation. Expansion of phenotypic HSCs is seen in MDSs and is most pronounced in higher-risk subgroups. Lower-risk MDS samples are characterized by phenotypic CMP expansions and decreased MEPs. Higher-risk samples are associated with GMP expansions.1,4,12 RBC, red blood cell; ST-HSC, short-term hematopoietic stem cell.
Quantitative stem and progenitor alterations in MDS subgroups. Stem cells and various progenitor populations are shown in normal and myelodysplastic hematopoiesis. Although this is a simplified model of differentiation, recent studies have shown that multipotent progenitors (MPPs) and CMPs can take alternative paths toward differentiation. Expansion of phenotypic HSCs is seen in MDSs and is most pronounced in higher-risk subgroups. Lower-risk MDS samples are characterized by phenotypic CMP expansions and decreased MEPs. Higher-risk samples are associated with GMP expansions.1,4,12 RBC, red blood cell; ST-HSC, short-term hematopoietic stem cell.
Stem cell expansions seen in human cases are also observed in many murine models of myelodysplasia.10 Mutations in epigenetic modulator 10-11 translocation (TET2) are known to be pathogenic in MDSs and AML.15,16 Tet2 knockout murine models exhibit a chronic myelomonocytic leukemialike disease that is accompanied by an increase in the Lin−Sca-1+c-Kit+ stem cell pool in the bone marrow, with an accompanying loss of differentiation.13,17,18 Additional sex combslike1 (ASXL1) is a commonly mutated gene in MDSs, and conditional deletion of ASXL1 in a murine model leads to progressive, multilineage cytopenias with a corresponding increase in HSCs and changes in progenitors mimicking human MDSs.19 Concomitant deletion of TET2 and ASXL1 was furthermore shown to increase HSC self-renewal and accelerated progression of disease, thus demonstrating that combined effects of these two alterations leads to important functional effects on disease-initiating stem cells.19
Over the past 15 years, increasing evidence has shown the critical importance of the ETS family transcription factor PU.1 in MDSs and AML. PU.1 is frequently downregulated in patients with MDSs. Functionally critical decreases in PU.1 levels have been described in patients with MDSs with normal karyotype,10 as well as in specific molecular subtypes including RUNX1-mutant MDS,20 EVI1-mutant MDS,21 and GFI1B-mutant MDS.22 In addition, it was found that the anti-MDS effects of 5-azacitidine are, at least in part, mediated by epigenetic derepression of the PU.1 locus in patients with MDSs.23 Our own group has recently reported a new model of MDS development in the context of an elevated number of somatic aberrations, resembling the mutational spectrum acquired in aging human individuals and in patients with MDSs, by crossing mice with a heterozygous PU.1 enhancer deletion with mice deficient for the DNA mismatch repair factor Msh2. This model is characterized by functionally altered precancerous stem cells, which can be tracked over time and progress to MDS at a high frequency (∼80%), and later to AML.10 The mouse genetic models mentioned are based on known “preleukemic” or founder mutations, and other similar models of interest are the Ezh2-deleted model, the Tet2/Ezh2 knockout model, and the NUP19/HOXD13 model.24-26
In addition, mouse genetic models of AML have also shown GMP expansions and have demonstrated that GMPs contain leukemia-initiating capabilities in transplantation experiments.27-29 Taken together, these human and murine studies have revealed striking quantitative and qualitative alterations in stem and progenitor populations in MDSs that are stage specific. Although these murine models of stem cell dysfunction have advanced our knowledge, they continue to have considerable limitations that include variable rates of disease penetrance and mixed myeloproliferative disorder/MDS phenotypes.30 The ongoing advances in xenografting of patient-derived MDS HSPCs and the generation of induced pluripotent HSCs from patients with MDSs will help advance our understanding of the disease in future studies.31,32
New cell surface markers can potentially identify aberrant MDS stem cells
Because histological examination cannot differentiate between preleukemic and healthy HSCs, recent studies have attempted to uncover specific markers of aberrant HSPCs in MDSs. Use of parallel transcriptional profiling using multiple populations of FACS-sorted HSCs and progenitors from a large number of AML and MDS samples and age-matched healthy controls led to the discovery of interleukin-1 (IL-1) receptor accessory protein (IL1RAP) as a cell surface antigen that was overexpressed on HSCs and GMPs that were part of the malignant clone, including preleukemic stem cells and progenitors.33 IL1RAP-positive HSCs showed clonotypic cytogenetic aberrations, whereas IL1RAP-negative HSCs were negative for clonal markers such as deletions or losses of chromosome 7. Recent studies have shown that IL1RAP is a marker for aberrant stem cells in other myeloid malignancies, such as chronic myeloid leukemia, as well.34,35 Although the precise function and mechanisms of IL1RAP in aberrant stem cells in myeloid malignancies still need to be determined, these findings make IL1RAP an attractive candidate for the targeting of preleukemic and leukemic stem cells in MDS and AML.
CD99 is another cell surface marker that has been shown to be overexpressed on aberrant HSCs at diagnosis and relapse of AML. It correlates positively with survival in AML patient cohorts, and CD99-high cells have a CD34+CD38−CD90−CD45RA+ lymphoid-primed multipotent progenitorlike immunophenotype, which has been known to be enriched for leukemic stem cell (LSC) activity. CD99 may promote AML aggressiveness by improving transendothelial migration and mobilization.36,37 Although CD99 may be expressed in high-risk MDSs, evidence for CD99 expression in low-/intermediate-risk MDSs is lacking at this time.38
Recent studies have demonstrated that primary AML cells strongly expressed IL-3 receptor α chain (CD123) in the CD34+/CD38− cell population, whereas there was virtually no detectable CD123 antigen in normal bone marrow–derived CD34+/CD38− HSCs. Although CD123 is expressed on healthy progenitors, these studies suggest that leukemic stem and progenitors overexpress this antigen.39,40 Although initially discovered as an LSC-specific antigen in AML, CD123 is also overexpressed in MDS HSPCs.41,42
T-cell immunoglobulin mucin-3 (TIM3) is another unique AML stem cell marker that retains importance in MDSs. Similar to CD123, TIM3 appears to be more highly expressed on LSCs than on normal bone marrow HSCs. TIM3 expression was also observed to be significantly higher in AML associated with core binding factor translocations or mutations in CEBPA. TIM3 is usually expressed on innate immune cells and can mediate phagocytosis of the apoptotic cells by crosslinking with galactin 9 for Toll-like receptor (TLR) synergy and binding to phosphatidylserine.43,44
Although there is a significant overlap between MDS and AML surface markers, it must be noted that the “normal” hierarchy of hematopoiesis is largely preserved in MDSs,1,4 whereas both self-renewal and differentiation patterns are profoundly altered in AML, in which LSCs are largely unrestricted to the HSC population.33,45,46 Not only are these new markers valuable tools for identifying MDS HSPCs, prospective studies are now evaluating their prognostic importance in ongoing clinical trials.
Genetic alterations are seen in MDS HSCs
Cytogenetic abnormalities are enriched in MDS HSCs
Clonal cytogenetic abnormalities are detected in up to 50% of de novo MDS cases and 80% of therapy-related cases.47 Many studies utilizing a combination of FACS sorting and fluorescent in situ hybridization have demonstrated that the HSC compartment in MDSs is enriched for cytogenetically abnormal cells.1,3,48,49 The earliest studies were conducted in subsets of patients with MDSs with deletion of long arm of chromosome 5, known as del(5q) MDSs, and determined that the majority of the CD34+/CD38+ progenitors and CD34+/CD38− stem cells harbored this deletion at the time of diagnosis. It was also shown that a subset of these aberrant stem and progenitor cells persisted through treatment with lenalidomide and contributed to drug resistance and disease progression.3,48,49 Another study evaluated patients with MDSs who had combined del(5q−) and trisomy 8 (+8) alterations and demonstrated that del(5q) was the primary abnormality in MDS HSCs, whereas +8 was thought to have arisen as a secondary event in the progenitors.48 These results were confirmed in another study that observed that +8 was observed in myeloid progenitors (CD34+, CD33+), whereas the T, B, and natural killer cells were spared.50 More recently, our studies of both cytogenetic and mutational alterations in highly enriched Lin−CD34+CD38−CD90+CD45RA− cells found that such stringently defined MDS HSCs are particularly enriched for cytogenetically abnormal cells and have a significantly higher percentage of clonotypic cells compared with whole bone marrow aspirates. This enrichment was observed in cases of both higher- and lower-risk MDSs, including cases with loss of chromosome 7 and deletion of long arm of chromosome 20 (20q−).4 Dimitriou et al51 observed an expansion of the GMP and other CD45RA+ progenitor compartments in high-risk MDSs with isolated −7, similar to changes observed in AML, suggesting that high-risk MDSs resemble AML more than do low-risk MDSs.
Another study focused on low- and intermediate-risk MDS samples and found that in cases with isolated del(5q) MDS, the chromosome 5q deletion occurred prior to all of the recurrent driver mutations, and in up to 50% of the cases, no other recurrent driver mutation was identified, suggesting that del(5q) may be an initiating and propagating alteration in a portion of these cases.12 In contrast, Mossner et al52 recently demonstrated that del(5q) appears to be a potential founder event in only a minority of cases, even when its variant allele frequency is comparable to other key MDS founder mutations. These findings from multiple studies demonstrate that at the time of clinical presentation, a majority of phenotypic HSCs contain MDS-associated genetic alterations.
Distinct mutations can be seen in MDS HSPCs
MDSs are the result of sequential acquisition of somatic mutations in HSCs.6 Most sequencing studies have been conducted in whole bone marrow aspirate or blast cells and have used variant allele frequencies to bioinformatically estimate the frequencies of driver and passenger mutations in MDSs and AML. These studies assume that clonal mutations with higher allele frequencies are more likely to be present in the disease-initiating cells in MDSs. A study of targeted deep sequencing of 111 genes in 738 patients with MDSs and related conditions (MDS–myeloproliferative neoplasm, or chronic myelomonocytic leukemia) found that 78% of the patients had one or more pathogenic mutation. Typically, early driver mutations involved genes in RNA splicing and DNA methylation machinery. Specifically, mutations in IDH2, SF3B1, ZRSR2, DNMT3A, and U2AF1 were predicted to be the earliest mutations in MDSs. The number of driver mutations also correlated with median leukemia-free survival, and an increasing number of driver mutations decreased survival significantly.53 In another study, SF3B1 mutations were found to propagate from HSCs onto their myeloid progeny, providing evidence that these mutations were the driver mutations in MDSs with ringed sideroblasts.54 Another study found that more than 50% of patients carry at least one somatic mutation in unfractionated total bone marrow–derived cells in MDSs. A set of 18 genes are commonly mutated in MDSs, and mutations in TP53, EZH2, ETV6, RUNX1, and ASXL1 are independent predictors of poor survival in patients with MDSs.55 Furthermore, targeted sequencing performed on 401 MDS and MDS/AML bone marrow mononuclear cell patient samples just prior to allogeneic HSC transplantation demonstrated that certain driver mutations (ASXL1, RUNX1, and TP53) were independently associated with a higher relapse rate and decreased survival after allogeneic HSC transplantation in MDSs and MDS/AML.56
Recent studies have now started to directly examine the mutational makeup of sorted stem and progenitor cells in MDSs and AML. In a study of AML samples, the preleukemic HSCs were identified by differential expression of CD99 and TIM3 and sorted for further analysis. It was observed that these preleukemic HSCs harbored important driver mutations that initiate the process but lack the entire arsenal of mutations required for leukemogenesis. Using deep sequencing and single-cell analysis of preleukemic HSCs, these findings demonstrated the stepwise accumulation of genetic mutations in preleukemic stem cells in AML. This model dispelled the thought that later and genetically more altered clones are more dominant, given that it was observed that in some samples, earlier clones had a more expanded pool compared with later clones. Also, in particular, the FLT3-ITD mutation occurred as a secondary mutation in all of the samples that harbored this mutation.36 A further study of paired diagnosis-remission-relapse samples showed that epigenetic founding mutations remain in remission and are a critical cellular reservoir for later progression and relapse.57 Shlush et al58 reported conceptually very similar findings and also found that DNMT3A-mutated preleukemic HSCs had a functional repopulation advantage over wild-type HSCs, and this provided a clonal expansion advantage at the time of diagnosis. This expanded pool of aberrant HSCs and downstream progenitors is then likely to accumulate further mutations and lead to leukemogenesis. These observations are in line with findings on preleukemic stem and progenitor cell states and their stepwise progression in mouse genetic models of MDS and AML pathogenesis.8,10,19,59-61 The model derived from such studies proposes a leukemia-preceding evolution at the stem or progenitor cell level, from precancerous/pre-MDS stem cells, to MDS stem cells (MDS-initiating cells), to AML stem cells (AML-initiating cells). A conundrum of MDS pathophysiology is the contrast between clonal dominance and differentiation defect. In particular, the selective value of molecular alterations such as splice mutations that are mostly thought to alter differentiation without providing clonal advantage is unclear. An explanation for this is perhaps that the mutations that confer clonal dominance occur as an earlier event in HSCs and confer a selective advantage to mutations that can alter differentiation. It is also possible that initial events may not lead to overall clonal dominance but merely a “myeloid bias” of HSCs and multipotent progenitors, and such a bias could skew the accumulation of mutations toward cells of the myeloid lineage. An increased rate of apoptosis in more mature cells may be another explanation and has been observed to occur as a result of overactivation of the p38 MAPK pathway and the tumor necrosis factor α (TNF-α) and transforming growth factor β (TGF-β) pathways in previous studies.62-64 Although the existence and essentiality of preleukemic stem cells has been firmly demonstrated in mouse and human systems, almost nothing is known about the molecular mechanisms that lead to their formation and progression. This will be a key focus of ongoing and future research.
Dysregulated immune pathways regulate MDS HSCs
Regulators of inflammation and innate immunity have always been thought to play an important role in pathobiology of malignancies, but only recently have the specific immune effectors and their cell-intrinsic functional roles in MDS stem cell biology been elucidated.65-67 HSCs can be directly activated by pathogen recognition receptorlike TLRs or proinflammatory cytokine signals.68 Recent studies have shown that TLR4, TNF receptor–associated factor-6 (TRAF6), and Toll/IL-1 receptor domain–containing protein (TIRAP) are dysregulated in MDS HSCs.69-71 RNA expression analysis of a large cohort of patients with MDSs showed significant overexpression of TLRs 1, 2, and 6 in MDS bone marrow CD34+ cells.72 Mechanistically, TLR1 and TLR6 bind to TLR2 and form a TLR2-centered functional pathogen recognition receptor that recognizes bacterially derived, conserved molecular patterns.68,73,74 In fact, a study identified a TLR2-F217S polymorphism in 11% of the bone marrow mononuclear cells of patients with MDSs and suggested that this was responsible for a greater activation of nuclear factor κB (NF-κB), p38 MAPK, and IL-1 receptor–associated kinase (IRAK1) after stimulation by TLR2 agonists. TLR2 inhibition was shown to overcome the differentiation block seen in MDS HSPCs and promote erythroid differentiation from CD34+ cells.72
Activation of the innate immune pathways in MDS HSPCs with del(5q) is particularly pronounced and is driven by a microRNA (MiR) alteration.75 MiR-145 and miR-146a reside in the commonly deleted segment of chromosome 5q and are found to be deleted in 80% of all 5q− MDSs and AMLs.76-78 Knockout of MiR-146a in murine models results in increased levels of TRAF6, a lysine (K)–63 E3 ubiquitin ligase, and IRAK1, a serine/threonine kinase, that are interacting proteins and downstream mediators of TLRs and IL-1 receptors (IL1Rs). Activation of TLR and IL1R results in IRAK1 phosphorylation, which in turn leads to TRAF6 and downstream NF-κB activation.79-81 IRAK1 has been shown to be significantly overexpressed in MDS CD34+ cells when compared with normal mononuclear cells and cord blood CD34+ cells. Conversely, an IRAK inhibitor was able to decrease phosphorylated IRAK1, inhibit TRAF6 and NF-κB activation, and reverse the MDS phenotype in a murine model. Combining IRAK1 inhibition with BCL-2 inhibition resulted in enhanced cytotoxicity against malignant cells. Hence, IRAK1 has emerged as a promising therapeutic target for MDSs.76 Furthermore, transgenic TRAF6 overexpression in murine HSPCs leads to an MDS-like phenotype in vivo, further underscoring the critical role of these pathways in the pathobiology of MDSs.65
IL-8 is an important component of innate immune signaling, and IL-8 and its G-protein–coupled receptor CXCR2 were found to be overexpressed in MDS and AML stem and progenitor compartments and associated with worse clinical outcomes.82 LT-HSCs, short-term HSCs, and GMPs from MDS samples were found to significantly overexpress IL-8 when compared with healthy counterparts, suggesting an autocrine loop regulating IL8/CXCR2 in aberrant HSPCs. CXCR2 inhibition/downregulation selectively led to decreased viability and clonogenic capacity of AML/MDS CD34+/CD38− cells via cell cycle arrest and decreased signaling of downstream oncogenic MAPK pathways.82
It was recently demonstrated that Lin−HLA−DR−CD33+ myeloid-derived suppressor cells accumulate specifically in MDS bone marrows and associate with the danger-associated molecular pattern heterodimer S100A8/S100A9 proteins.65 Danger-associated molecular pattern molecules have been increasingly associated with perpetuating noninfectious inflammatory responses.83,84 CD33 binding to S100A9 causes expansion of the myeloid-derived suppressor cell compartment, producing a variety of effects that include direct suppression of erythroid and myeloid progenitors and overproduction of inflammatory markers such as IL-10, TGF-β, nitric oxide, and arginase. These effects were validated in vivo in a murine model in which overexpression of S100A9 produced ineffective and dysplastic hematopoiesis and mimicked human MDSs.65
Another component of innate immune signaling pathways, IL1RAP, was found to be one of the top differentially expressed genes in the stem and progenitor compartment of patients with high-risk (−7/7q−) AML, high-risk MDSs, and normal-karyotype AML. It was also correlated with poor survival in three independent clinical cohorts, suggesting a pervasive role of this protein in disease pathogenesis. IL1RAP mediates the response to IL-1, IL-33, and IL-36 and has been shown to regulate the inflammatory response and activation of mast cells and T lymphocytes.33-35 These emerging data highlight the important role of innate immune signaling pathways in MDS HSPCs and suggest that activation of these pathways is critical for survival and growth of aberrant stem and progenitor cells and can persist through treatment.
The role of MDS HSPCs in disease relapse
With better characterization of the MDS HSPCs, recent studies have begun to evaluate the role of these cells as treatment-resistant cellular reservoirs that play a key role in disease relapse. One of the earliest studies exploring the role of the MDS HSPCs in relapse demonstrated that a majority of the CD34+CD38−/lowCD90+ stem cells and CD34+CD38+ progenitors had the chromosome 5q deletion prior to lenalidomide therapy. The subset of patients with MDS with 5q− exhibit a high response rate to lenalidomide; however, a majority of these patients relapse over time.85 In this study, it was observed that the progenitor fraction showed a much greater molecular response to lenalidomide therapy in comparison with the stem cell fraction. The aberrant stem cells with 5q− phenotype eventually expanded and were associated with clinical and cytogenetic progression.3 Another study evaluated the molecular characteristics of a patient with classic 5q− syndrome with a complete cytogenetic response to lenalidomide and found that his pretreatment marrow had a TP53-mutated progenitor population, which remained stable during treatment and expanded afterward.86 Treatment with 5-azacitidine, a DNA methyltransferase inhibitor, leads to increased overall survival in high-risk MDSs.87 Work from our group demonstrated that karyotypically abnormal HSCs containing deletion of chromosome 7 persist during clinical remissions induced by 5-azacitidine and vorinostat therapy and expand just prior to relapse.4 The inability of 5-azacitidine to eliminate clonally abnormal HSPCs is thought to be the reason for relapse. A larger study evaluated the abnormal stem and progenitor compartment of 79 patients with high-risk MDSs and AML treated with 5-azacitidine and sodium valproate and found that in the responders, there was a reduction in the abnormal compartment, but the aberrant pool of HSCs and progenitors was never completely eradicated, and expansion of this compartment preceded clinical relapse. They also noted no substantial reduction in the abnormal HSC and progenitor compartment of the nonresponders.88 Similar findings have been reported for lenalidomide-treated MDSs without del(5q), for which it was noted that responders had a transient decrease in the variant allele frequency of major mutations and a decrease in the size of the dominant subclones, which was then followed by reemergence of the same at relapse.14 Whole-genome sequencing of MDS and secondary AML samples has shown that most of the bone marrow cells are clonally derived with a small degree of residual normal hematopoiesis.2
Quantification of the aberrant MDS HSC compartment in measuring response to treatment, predicting relapse, and guiding future therapies is gaining significant traction. A recent study evaluating the response of the HSPC compartment has demonstrated the persistence and rapid growth of disease-initiating clones after initial clinical response.52 This finding was further corroborated by Ho et al,89 who assessed LSC populations from patients with AML by limiting dilution analysis and demonstrated a ninefold to 90-fold increase in LSC frequency between diagnosis and relapse.
Therapeutic targeting of MDS HSCs
Numerous studies have determined that MDS HSPCs are key players in the initiation and propagation of disease and also significantly contribute to disease relapse (Figure 2). The logical next step is to rationally design therapies that target the aberrant MDS HSPC compartment. The challenge of targeting MDS stem cells is that they share many pathways with healthy stem cells; therefore, the therapeutic index is a critical consideration when designing therapies against these disease-initiating cells.
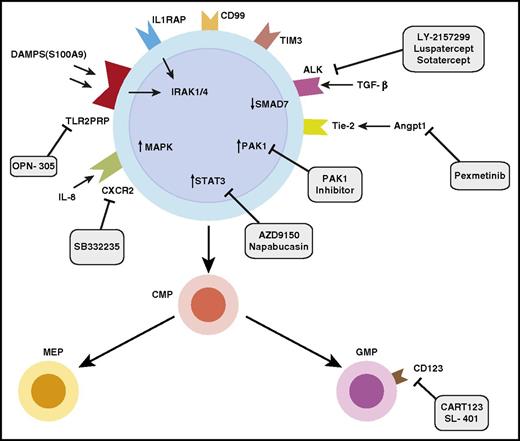
Identification and therapeutic targeting of aberrant stem and progenitor cells in MDSs. Diagram of MDS stem and progenitor cells demonstrates overexpressed markers such as IL1RAP, CD99, and TIM3. Overexpressed pathways include innate immune signaling pathways, Pak1, STAT3, and TGF-β signaling pathways. Various therapeutic approaches in development targeting these pathways are shown. ALK, anaplastic lymphoma kinase; CART123, chimeric antigen receptor T-cell 123; DAMPS: damage associated molecular patterns; TLR2PRR: TLR2-centered pathogen recognition receptor.
Identification and therapeutic targeting of aberrant stem and progenitor cells in MDSs. Diagram of MDS stem and progenitor cells demonstrates overexpressed markers such as IL1RAP, CD99, and TIM3. Overexpressed pathways include innate immune signaling pathways, Pak1, STAT3, and TGF-β signaling pathways. Various therapeutic approaches in development targeting these pathways are shown. ALK, anaplastic lymphoma kinase; CART123, chimeric antigen receptor T-cell 123; DAMPS: damage associated molecular patterns; TLR2PRR: TLR2-centered pathogen recognition receptor.
We have demonstrated that signal transducer and activator of transcription 3 (STAT3), a transcription factor that belongs to the STAT family, is overexpressed and is accompanied by promoter demethylation in MDS HSCs. We further validated this finding by observing significantly elevated STAT3 levels in CD34+ cells in an independent large cohort of patients with MDSs4 and observed that small-molecule inhibitors of STAT3 dimerization led to selective activity against MDS HSCs.4,90 Various inhibitors of STAT3 are in different stages of clinical development.91,92 These include AZD9150, an optimized antisense oligonucleotide and specific inhibitor of STAT3 that has recently demonstrated single-agent antitumor activity in patients with highly treatment-refractory non–small cell lung cancer in a phase 1 dose-escalation study.93 The rationale for testing of STAT3 inhibitors in MDS/AML models is strengthened by the recently demonstrated therapeutic potential of this approach in myeloproliferative disease models, in which it was shown that STAT3 inhibition interrupts cytokine signaling pathways that drive malignant cell proliferation.94
A pathway that has come to recent attention is the angiopoietin-1 (Angpt-1)/tyrosine kinase with immunoglobulin-like and endothelial growth factorlike domains 1 (Tie2) pathway that is dysregulated in MDSs. Angpt-1 is a cytokine that is implicated in vascular development and angiogenesis. It binds to the receptor tyrosine kinase TIE2, which supports quiescence and self-renewal in HSCs.95 A recent study from our own group demonstrated that ANGPT-1 was overexpressed in MDS HSPCs and associated with worse prognosis in MDS and AML cohorts. We showed that TIE2 knockdown inhibits leukemic proliferation and enhances hematopoietic differentiation of MDS HSPCs.96 Another important kinase, the p38 MAPK, is an evolutionarily conserved serine-threonine kinase and has been shown to regulate cytokine-dependent suppression of healthy hematopoietic stem cells in MDSs.97 We found that a small-molecule, dual inhibitor of TIE2 and p38 MAPK, pexmetinib (ARRY-614), was highly efficacious in preclinical MDS models.96 Early-phase clinical studies of pexmetinib showed good tolerability and revealed efficacy in patients with platelet-refractory MDSs who failed to respond to treatment with 5-azacitidine.98
Small noncoding RNAs such as miRs have emerged as important regulators of hematopoiesis and have been reported to be overexpressed in MDSs.99 We have previously demonstrated that miR-21 is upregulated in MDSs and binds directly to the 3′ untranslated region of SMAD 7, a negative regulator of the TGF-β pathway. SMAD 7 was noted to be markedly decreased in MDSs, and this in turn led to an overactive TGF-β pathway, which has previously been shown to cause HSC dysregulation in MDSs.66,100 MiR-21 is also frequently upregulated in AML, and its inhibition has been shown to specifically interfere with homeobox-driven leukemia cell growth in vitro and in vivo.101 Multiple investigators have demonstrated that chemically modified locked nucleotide antisense inhibitors of miR-21 are efficacious in preclinical MDS and AML models and support testing of clinically available inhibitors in development.100-102
As discussed above, innate immune signaling pathways are overactivated and functionally important in MDS stem and progenitor cells and can serve as therapeutic targets. The immune-modulating kinase IRAK1 is overexpressed and hyperactive in MDS HSPCs. A small-molecule inhibitor of IRAK1, which was initially developed for the treatment of autoimmune disease, was able to inhibit the growth of MDS/AML cell lines, prevent the clonal expansion of MDS progenitor cells, and delay disease formation in a human xenograft model. Combined BCL-2 and IRAK1 inhibition can also delay MDS-like disease in a murine model.76 IRAK1 is an exciting new therapeutic target in MDSs and in several other cancers, and IRAK inhibitors are now in preclinical and early clinical development.103-107 Likewise, the above-discussed IL8/CXCR2 axis is aberrantly upregulated in MDS HSCs in comparison with normal HSCs and represents an attractive therapeutic target. We demonstrated that a small-molecule inhibitor of CXCR2, SB332235, led to dose-dependent decreases in proliferation of AML and high-risk MDS cell lines in primary patient samples without significant effects on healthy controls. Several small-molecule inhibitors of CXCR2 are currently in clinical development, some of which have shown efficacy in solid tumor models and could potentially be tested in MDSs.108-110 TLR2 is upregulated in MDS HSCs and is a novel therapeutic target in this disease.70 OPN-305 is a humanized immunoglobulin G4 monoclonal antibody against TLR2 that is being tested in an ongoing phase I/II study for low- and Intermediate-1–risk MDSs.111,112
p21-activated kinase (PAK1) is another relevant target in myeloid malignancies. PAK1 is a serine/threonine kinase and a downstream effector molecule of H2.O-like homeobox (HLX), a gene that is upregulated in preleukemic HSCs and found to be functionally relevant for pathogenesis of myeloid malignancies including MDSs and AML.113,114 Inhibition of PAK1 chemically and by using a short-hairpin RNA leads to monocytic differentiation and apoptosis as a result of repression of the oncoprotein c-MYC. Human AML and MDS HSCs were observed to be preferentially reliant on PAK1 function, suggesting a potential role for PAK1 inhibitors in targeting of aberrant MDS stem cells.113 Efforts to develop PAK1-specific inhibitors are ongoing.
IL-3 receptor α chain, or CD123, is also selectively upregulated on LSCs and is being targeted by a variety of pharmacologic approaches. SL-401, a recombinant fusion protein composed of the catalytic and translocation domains of diphtheria toxin fused via a Met-His linker to IL-3, is in early clinical development and selectively targets the CD123+ LSC population. SL-401 has demonstrated promising single-agent activity in AML in early trials.115,116 CD123-specific chimeric antigen receptor (CAR) T cells have also been developed and are able to eliminate leukemic HSCs in vivo. CD123-specific CARs with CD28 and CD137 costimulatory domains prolonged survival in AML and ALL preclinical in vivo models, respectively.117
In addition to therapeutic targeting, it is also important to note that the quantitative estimation of the aberrant HSC compartment, as mentioned above, will continue to be developed as a clinical biomarker for monitoring response to therapy at the stem cell level, and as an early indicator of disease relapse. However, quantitative HSC compartment estimation continues to present significant challenges with respect to technical implementation; also challenging is the emerging knowledge that mutational hierarchies are fluid and may not follow a prespecified pattern.52
Conclusions
Our understanding of the role played by disease-initiating stem and progenitor cells in MDSs has exponentially increased in the past five years. We now know that MDS HSPCs carry clonotypic abnormalities and are involved in disease initiation, persistence, and relapse. Singificant progress has been made in recent years in the development of preclinical models to study MDS pathogenesis and pathway targeting at the stem cell level. We anticipate that future preclinical drug development and clinical trials will target MDS HSPCs and aim for a lasting cure for this disease. We also predict that quantitative measurements of the HSC compartment in patients with MDSs will be increasingly used in future studies, and provide patients and clinicians with a powerful tool to monitor the disease at an unprecedented resolution and sensitivity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tatyana Harris for her assistance with the graphics included in the paper.
This work was supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01DK103961), and grants from the Leukemia and Lymphoma Society and the Edward P. Evans Foundation.
Authorship
Contribution: A.S. and A.V. developed the study concept and designed the study; A.S., U.S., and A.V. drafted the manuscript; A.S., B.W., U.S., and A.V. critically revised the manuscript and contributed important intellectual content; and A.S. and A.V. provided administrative, technical, and material support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amit Verma, Department of Oncology, Molecular and Developmental Biology, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: amit.verma@einstein.yu.edu; and Ulrich Steidl, Department of Oncology and Cellular Biology, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: ulrich.steidl@einstein.yu.edu.