Abstract
Similar to their healthy counterpart, malignant hematopoietic stem cells in myeloid malignancies, such as myeloproliferative neoplasms, myelodysplastic syndromes, and acute myeloid leukemia, reside in a highly complex and dynamic cellular microenvironment in the bone marrow. This environment provides key regulatory signals for and tightly controls cardinal features of hematopoietic stem cells (HSCs), including self-renewal, quiescence, differentiation, and migration. These features are essential to maintaining cellular homeostasis and blood regeneration throughout life. A large number of studies have extensively addressed the composition of the bone marrow niche in mouse models, as well as the cellular and molecular communication modalities at play under both normal and pathogenic situations. Although instrumental to interrogating the complex composition of the HSC niche and dissecting the niche remodeling processes that appear to actively contribute to leukemogenesis, these models may not fully recapitulate the human system due to immunophenotypic, architectural, and functional inter-species variability. This review summarizes several aspects related to the human hematopoietic niche: (1) its anatomical structure, composition, and function in normal hematopoiesis; (2) its alteration and functional relevance in the context of chronic and acute myeloid malignancies; (3) age-related niche changes and their suspected impact on hematopoiesis; (4) ongoing efforts to develop new models to study niche-leukemic cell interaction in human myeloid malignancies; and finally, (5) how the knowledge gained into leukemic stem cell (LSC) niche dependencies might be exploited to devise novel therapeutic strategies that aim at disrupting essential niche-LSC interactions or improve the regenerative ability of the disease-associated hematopoietic niche.
Introduction
Hematopoietic stem cells (HSCs) are primitive multipotent tissue stem cells that reside in the bone marrow (BM) and ensure life-long regeneration of the blood system. This vital process is controlled by both cell-intrinsic features of HSCs and extrinsic signals, emanating from the BM microenvironment.
Perturbations of the BM niche inevitably affect normal hematopoiesis and are thought to contribute to the emergence of hematopoietic malignancies in both mice and humans. Therefore, the cellular components of the BM niche and the means by which they modulate normal and malignant hematopoiesis have been extensively interrogated in the past decade. Taking advantage of new technologies, such as intravital imaging and genetic mouse models, that allow for marking or deleting specific cell types, the field has recognized that the BM niche is a complex structure that contains a number of different cell types: multipotent mesenchymal stem cells (MSCs) and their progeny, a complex vascular network, nerve fibers, mature blood cells, and others. These cell types have all been shown to modulate the function of HSCs in mice, and be perturbed in the context of malignancies. A new picture has emerged in which normal and malignant cells engage in bidirectional cross talk with their surrounding niche cells and establish specific interdependencies. These cellular interactions are thought to contribute to the emergence of hematopoietic malignancies by modulating important failsafe mechanisms related to self-renewal, proliferation, survival, and immune evasion. These mouse studies have recently been reviewed elsewhere1,2 and will not be discussed here.
This review puts emphasis on our current knowledge of the human HSC niche and its active involvement in the pathogenesis of human myeloid malignancies, namely myeloproliferative neoplasms (MPN, including chronic myeloid leukemia [CML]), myelodysplastic syndromes (MDS), and acute myeloid leukemia (AML). Moreover, this review provides an overview of emerging strategies being used to improve current models to study human myeloid malignancies, including integrating the human niche component. Importantly, we discuss how this new area of research is paving the way to the development of novel therapeutic strategies.
Anatomical structure and key functional players in the human hematopoietic niche
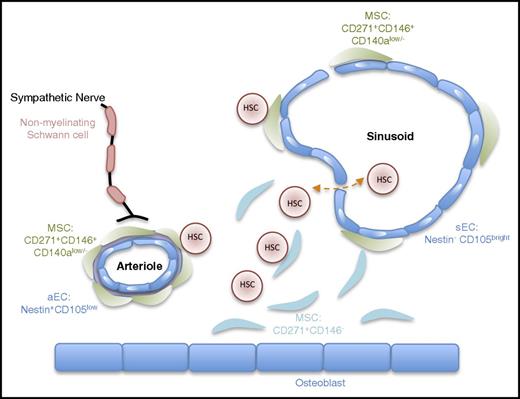
Compared with the mouse system, much less detailed knowledge exists regarding the architecture of the human BM and the function of its different cellular constituents. Using a comprehensive set of descriptive (flow cytometry, immunohistochemistry of BM trephine) and functional analysis in xenografts, Guezguez and colleagues3 demonstrated that human HSCs (lin–CD34+CD38–CD45RA–CD49f+)4 preferentially locate to trabecular areas of the BM.3 In both humans and mice, these areas contain various types of stromal cells, endothelial cells (ECs), and nerve fibers; the latter are arranged mainly around medium-sized arteries and capillaries,5,6 also pointing to a conserved BM innervation by the sympathetic nervous system (Figure 1).7,8
Schematic view of the anatomical structure of the human hematopoietic niche. This schema provides a simplified view of the cellular architecture of the human BM niche. It reflects the idea that hHSCs are mainly found in close proximity to the vasculature in direct contact with CD271+ MSCs.3,9 Similar to the mouse BM niche, arterioles and capillaries near the endosteum are ensheated by sympathetic nerve fibers.10 Surface markers distinguishing different cellular subsets are also indicated. CD146+ MSCs are close to the vasculature.11 Nestin and endoglin (CD105) have been proposed to mark arteriolar and sinusoidal ECs, respectively.5,20
Schematic view of the anatomical structure of the human hematopoietic niche. This schema provides a simplified view of the cellular architecture of the human BM niche. It reflects the idea that hHSCs are mainly found in close proximity to the vasculature in direct contact with CD271+ MSCs.3,9 Similar to the mouse BM niche, arterioles and capillaries near the endosteum are ensheated by sympathetic nerve fibers.10 Surface markers distinguishing different cellular subsets are also indicated. CD146+ MSCs are close to the vasculature.11 Nestin and endoglin (CD105) have been proposed to mark arteriolar and sinusoidal ECs, respectively.5,20
Sympathetic nerve fibers
In mice, HSCs’ egress from their quiescence-enforcing niches follows a circadian rhythm imposed by the cyclic expression of Cxcl12 on MSCs and its receptor, Cxcr4, on HSCs, which are under the control of β3-adrenergic signals and the clock gene (Bmal1), respectively.8,12 Although inverted, timing-wise, human HSCs’ (hHSCs) egress also fluctuates in a circadian manner.12 Notably, β2-adrenergic receptor (β2-AR) signaling directly modulates the motility and proliferation of immature CD34+ human hematopoietic stem/progenitor cells (hHSPCs), and the expression of ARs is induced by granulocyte colony-stimulating factor (G-CSF), a potent HSPC mobilizing agent.13 These data point to an essential role of the sympathetic nervous system in the human HSC niche. Whether the molecular mechanisms at play in humans and mice are similar remains to be tested.
Endothelial cells
Comparable to mice,14-18 hHSCs mainly reside within a few cell diameters of the BM vasculature in direct contact with CD271+ MSCs.3,5,12 In mice, the vascular network is composed of fenestrated sinusoidal vessels and endosteal arterioles, which are proposed to differentially harbor active versus dormant HSCs, a topic that is still a matter of debate.16-19 Nevertheless, a similar architecture exists in human BM, where endoglin (CD105) and nestin expression has been proposed to preferentially identify sinusoidal vessels and arterioles/capillaries, respectively.5,20 Although enriched in the arterioles containing trabecular areas of the human BM, CD34+ hHSPCs have been reported to also localize close to sinusoidal vascular cells in contact with CD271+ MSCs.3 Functionally, human BM-derived ECs transformed with an AKT-activating adenoviral gene21 support the in vitro expansion of lin–CD45+CD34+CD45RA–CD49f+ hHSCs capable of multilineage reconstitution in immune-deficient mice,22 thereby arguing in favor of a direct effect on hHSC maintenance.
Multipotent MSCs
Human MSCs (hMSCs) are functionally defined by their clonogenic potential (colony-forming unit-fibroblasts) and ability to assemble a functional BM niche in vivo. These cells are proposed to reside within the lin–CD45–CD271+CD140alow/– fraction.23-25 CD271+ hMSCs can further be subdivided by CD146 expression, which marks MSCs that locate close to the vasculature14 and are reported to express high levels of well-characterized HSC-supportive genes (IGF2, WNT3A, JAG1, CXCL12, KITLG, ANGPTL1).26,27 Similar to mice,28 these cells also express LEPR,25 CD105, and CD90.29,30 However, mouse and fetal MSCs are rather enriched in the PDGFRa+ (CD140a+) fraction,25,31 which appears to be a developmentally regulated marker in humans. Nestin expression, however, is restricted to a subset of hMSCs, as suggested by analysis of liquid biopsies32,33 versus in situ core biopsies (BM trephine), where high nestin expression is rather proposed to mark ECs.5 Functionally, however, both fetal and adult BM nestin+ MSCs were shown to enhance the long-term multilineage reconstitution activity of HSPCs.25,29,31 Recently, more restricted osteolineage progenitors were shown to promote, ex vivo, the expansion of hHSCs capable of hematopoietic reconstitution through the secretion of microRNA-containing extracellular vesicles.34
Although further studies are required, these data provide compelling evidence that the anatomical structure of the human and mouse BM niche are highly conserved, which hints toward potentially conserved non–cell autonomous mechanisms of HSC maintenance.
LSCs in myeloid malignancies
Genetic events are well established as main intrinsic drivers of leukemogenesis. These transformation events are thought to primarily occur in self-renewing HSCs that gain a competitive advantage over normal HSCs and give rise to the disease-propagating leukemic cells, also referred to as leukemic stem cells (LSCs).35-39 Nevertheless, evidence also exists to support transformation of early progenitors, such as lymphoid-primed multipotent progenitors and granulocyte macrophage progenitors in AML,40,41 a granulocyte macrophage progenitor-like subset in blast crisis CML,42 and multipotent progenitors in sporadic MDS cases.43,44 Whether the cell of origin defines the specific interdependencies with niche cells is largely unknown. More broadly, the issue of spatial distribution of LSCs in the human BM and their niche dependency remains highly underexplored. Studies using in situ localization and functional repopulation assays showed that, in xenografts, AML LSC-enriched populations exhibit a spatial and functional distribution similar to that of normal HSCs.45,46 This localization to physiological HSC niches appears to be different in T-cell acute lymphoblastic leukemia.47 Nevertheless, these findings add to the emerging importance of the BM niche, which can either instigate hematopoietic changes and myeloproliferation or, more frequently, act as a driver of disease maintenance/progression.
Evidence pointing to the contribution of niche cells in the initiation of human myeloid malignancies
The concept of niche-induced disease initiation in vivo has been demonstrated using animal models of MNP,51-55 MDS,56,57 and AML,58,59 a topic that was extensively reviewed elsewhere.1,60 Evidence to support this in humans is mainly based on the occurrence of rare but notable cases of donor-derived leukemia, observed in patients receiving BM transplant, where preexisting niche changes in the host are thought to initiate leukemogenesis.61 This idea of microenvironment-driven disease postulates that in the context of a healthy donor microenvironment, a genetically abnormal “primed” HSC clone would be kept in check, while possibly gaining competitive advantage and undergoing further clonal evolution in the disease-primed microenvironment of the recipient.
Along this line, analysis of ex vivo expanded mesenchymal stromal niche cells from patients with MDS and AML are reported to exhibit a wide range of functional and molecular alterations, including chromosomal aberrations,62-66 transcriptional,67 and epigenetic changes68 as well as functional alterations in their differentiation potential and HSC-supportive activities69,70 (reviewed in Pleyer et al71 ). Genetic and transcriptomic alterations were also reported in unmanipulated CD271+ MSCs directly isolated from MDS patients by array comparative hybridization and transcriptomic profiling.26,72,73 Most notably, some of the events shown to initiate malignant transformation in mice are also reported in patient-derived mesenchymal cells, including downregulation of DICER and SBDS57,70,73 and activation of β-catenin in osteoblastic cells.58 More recently, Dong et al55 reported that the expression of a PTPN11 mutant found in juvenile myelomonocytic leukemia (JMML) cases (an aggressive form of childhood MPN) associated with Noonan syndrome recapitulates JMML in nestin+ cells in mice, strongly arguing for its likely involvement as an initiating event in the pathogenesis of congenital JMML.
In addition to these studies addressing the contribution of mesenchymal niche cells, emerging evidence also points to a potential role for ECs in disease initiation. JAK2V617F+ ECs have been reported in a subpopulation of MPN patients74 and recently demonstrated to actively contribute to the emergence of MPN in mice by favoring the expansion of Jak2V617F HSC over the Jak2wt counterpart.75 These effects appear to be mediated through upregulation of Cxcl12 and Scf by the vascular niche cells. Likewise, another study presented at the 58th annual meeting of the American Society of Hematology reported that EC-specific KRasG12D expression causes an MPD phenotype and had a detrimental effect on HSC function.76
Niche remodeling as a contributing factor to the maintenance/progression of human myeloid malignancies
In mice, evidence indicates that malignant cells actively shape their microenvironment to reinforce disease progression at the expense of normal hematopoiesis (reviewed in Hoggat et al1 and Schepers et al60 ). Remodeling of the mesenchymal, endothelial, and nerve components appear to play a central role in this process. Evidence pointing to the existence of conserved mechanisms in humans is progressively becoming available. However, experimental data remain scarce.
Deregulated CXCL12/CXCR4 signaling
Deregulated expression of CXCL12 by niche cells appears to be a unifying feature in myeloid malignancies. CXCL12 binding to its receptor, CXCR4, enhances the affinity of integrins that mediate HSC adhesion to their niches.77-79 Integrin-mediated cellular adhesion and lodging of normal human HSCs into supportive niches was recently demonstrated and visualized using human HSCs xenograft models and intravital imaging.80 The critical role of this axis in normal HSC function is further underscored by the use of CXCR4 receptor antagonist, such as AMD3100, a potent HSC mobilizing compound.81,82 Therefore, altered expression/distribution of CXCL12 or integrins by disease-associated stromal cells is likely to impinge on the ability of normal HSCs to find and reside in their quiescence maintaining niches, thereby possibly promoting their exhaustion and the progressive clonal dominance of the malignant HSCs observed in myeloid malignancies. In a recent study, Agarwal and colleagues83 showed increased expression of Cxcl12 by ECs and decreased expression by MSCs in a mouse model of CML. The study reported that loss of MSC-derived CXCL12 favors the loss of normal HSCs, whereas its increased expression by ECs favors the expansion of malignant cells. These findings suggest that the niche function of CXCL12 appears to be dependent on its cellular origin.83 Similar to mice, CXCL12 is expressed by both mesenchymal cells and ECs in the human BM.9 This data adds yet another layer of complexity to the niche; however, its relevance to the human system remains unexplored.
Sympathetic neuropathy
Neuropathy, which is characterized by decreased sympathetic nerve fibers and ensheathing Schwann cells, is readily observed in the BM of newly diagnosed MPN and AML patients.10,84 Moreover, denervated mice that were transplanted with primary human AML exhibited higher levels of BM infiltration compared with controls,85 suggesting that neuropathy exacerbates the AML phenotype and/or improves homing/engraftment of the AML cells. In a mouse model of MPN, the overproduction of interleukin-1β (IL-1β) by mutant progenitors was shown to damage the Schwann cells, which ensheath and protect the ending of the sympathetic nerve, leading to neuropathy, increased MSC apoptosis, and decreased expression of HSC retention factors/adhesion molecules by MSCs (Vcam1, Cxcl12, Kitl). This ultimately enforces mobilization of normal HSCs to the periphery.84,85 Importantly, high levels of IL1-β are readily detected in human MPN, possibly pointing to a conserved mechanism of MPN-induced neuropathy in mice and humans.84 Of note, a chronic increase in IL-1β is also anticipated to deplete normal HSCs through cell-intrinsic effects86 while concomitantly expanding malignant cells through binding to IL1RAP, a newly identified putative LSC marker and therapeutic target in MDS, AML, and CML.87-91
Although sympathetic nerve degeneration is observed both in MPN and AML, experimental modulation in mice demonstrated that defective β3-adrenergic signals are responsible for the phenotypes observed in MPN, whereas β2-adrenergic signals seem to be the main mediators in AML. Although β3-AR agonist or IL-1β antagonist appear to be promising therapeutic strategies, the use of β2-AR agonists is mitigated by the risk of promoting the growth of the AML LSCs, which also express β2-AR.
Notably, the relevance of sympathetic neuropathy in the emergence or progression of other myeloid neoplasms, such as MDS or CML, remains to be tested.
Mesenchymal niche remodeling
BM fibrosis, a consequence of extensive mesenchymal niche remodeling, is observed, to various degrees, across the full spectrum of myeloid malignancies, where it often correlates with poor outcome.92-94
The concept of leukemia-induced fibrosis was elegantly exemplified by the landmark study from the Passegué laboratory,95 demonstrating that mouse CML cells are able, through a combination of direct cell-cell contact and soluble factors (TPO and CCL3), to stimulate the overproduction of osteoblastic cells by MSCs, thereby leading to fibrosis. Likewise, our group recently demonstrated that, in a coculture model, human primary MDS cells were sufficient to instruct stromal cells from healthy volunteers to adopt MDS-like features, such as high expression of proangiogenic factors (VEGFA, IGFs, and EGFs) and mediators of fibrosis (LOXL, TGF-β, and LIF) (Medyouf et al67 and unpublished data). The functional relevance of these changes is supported by experimental data demonstrating that MDS diseased stem cells specifically rely on their disease-associated MSCs for disease propagation in vivo.67 Lastly, preliminary data from a phase II clinical trial indicate that treatment of JAK2V617F-positive MPN patients with a β3-AR agonist (mirabergon) slightly improves the fibrotic manifestations associated with MPN, possibly pointing to a conserved mechanism of MPN-induced fibrosis in humans and mice.84,96
Reduced expression of essential HSC niche factors (CXCL12, ANGPT1, and KITL) and increased expression of proinflammatory factors (TNF, IL-6, IL-8, CCL3, and S100A8/A9) has also been documented in unmanipulated CD271+ MSCs.26,73 For instance, in mice, damage-associated molecular pattern (DAMP) molecules S100A8/A9 DAMPs, are shown to activate TLR4 signaling in HSPCs and induce genotoxic stress, a mechanism by which niche cells may cooperate with aberrant HSPCs toward leukemogenic transformation. Notably, S100A8/A9 signaling in mesenchymal niche cells appears to be an independent predictor of disease outcome in human MDS.73 Moreover, S100A9 binding to CD33 is also reported to expand myeloid-derived suppressor cells, which contribute to the development of myelodysplastic phenotypes in mice.97 Importantly, human myeloid-derived suppressor cells also express CD3397 and their number correlates with the number of regulatory T cells and disease progression in MDS patients.98 Taken together, these data argue in favor of a critical role of niche-derived S100A8/A9 in promoting disease evolution through both direct induction of genotoxic stress on hematopoietic cells and indirect mechanisms linked to increased immune suppression.
Vascular remodeling
Vascular remodeling is yet another unifying feature in myeloid malignancies, which likely contributes to disease progression (reviewed in Testa et al99 ). As such, increased microvessel density is predictive of a poor outcome in most myeloid malignancies.100-105 The activation of a leukemia-associated neoangiogenic program is instructed by proangiogenic factors released by the neoplastic cells, including VEGF, HGF, bFGF, and TNFα.106-109 Cytokine contribution to angiogenesis in non–BCR-ABL MPN has recently been reviewed elsewhere.110 VEGF, for instance, is highly expressed by megakaryocytes, myeloid progenitors, and granulocytes in chronic and blast crisis CML,111 with increased levels also observed in human66,107 and mouse models of MDS,112 where it appears to be equally secreted by malignant and stromal niche cells. EC activation by proinflammatory cytokines secreted by malignant cells (eg, TNFα, IL-6, and IL-1β) increases EC proliferation, EC expression of myeloid-promoting cytokines (G-CSF, granulocyte-macrophage–CSF [GM-CSF]) and might compromise vascular integrity. In mice, experimental alterations of endothelial barrier integrity trigger profound negative effects both on HSCs and other nonhematopoietic niche components,16 pointing to a potential mechanism by which vascular niche remodeling may contribute to malignant progression. Along these lines, recent evidence from the Bonnet laboratory113 show that human AML cells induce increased vascular permeability in PDX models, a phenomenon that appears to be mediated by increased nitric oxide (NO) production. Most notably, the study shows that inhibition of NO production reverts vascular permeability, potentiates normal HSC function, and improves treatment response in PDX.113 Evaluation of this niche-therapeutic approach in other myeloid malignancies may have tremendous therapeutic implications. Lastly, IL-33–expressing ECs are reported to be increased in BM biopsies of BCR-ABL– MPN patients114 and might also contribute to malignant progression. Indeed, IL-33 stimulates the expression of myeloid cytokines (IL-6 and GM-CSF) by myeloid and nonhematopoietic niche cells. It specifically promotes the proliferation and colony formation of human primary CD34+ MPN, but not of their normal counterpart.114 Of note, IL-33 is also a ligand for IL1RAP, which has emerged as a putative selective LSC marker and therapeutic target in higher-risk MDS, AML, and CML.87-89
In summary, pharmacological approaches aiming at reverting niche changes are increasingly recognized to be of therapeutic interest. These include, but are not limited to, reverting vascular leakiness (eg, NO synthase inhibitors), sympathetic neuropathy (eg, neuroprotective agents), and stromal remodeling (eg, β3-AR agonists and CCL3 antagonists). Future work, aiming at identifying the instructive signals that alter the function of the HSC niche, will likely identify new potential therapeutic targets that could be used to prevent the acquisition of disease-promoting niche features and contribute to improving patient outcomes (Figure 2).
BM alterations and emerging niche-targeted therapeutic strategies in myeloid malignancies. This schema summarizes the most recurrent niche changes observed in human myeloid malignancies and the pharmacological approaches being explored to target niche-leukemia interdependencies. Depicted niche changes reflect mesenchymal, neurological, and endothelial niche remodeling. Pharmacological approaches include strategies to revert vascular leakiness (eg, NO synthase inhibitors)113 and prevent nerve damage (eg, IL-1 antagonists, and neuroprotective agents such as 4-methylcatechol [4-MC]) or its impact on MSCs (β3-AR agonists, such as mirabegron in phase II clinical trial in MPN [identifier NCT02311569]).84,96 Approaches to prevent or overcome therapeutic resistance include the inhibition of the FAO by blocking the function of the rate-limiting enzyme CPT-1 (eg, etomoxir, ranolazine),115 or interfering with mitochondrial function using tigecycline.
BM alterations and emerging niche-targeted therapeutic strategies in myeloid malignancies. This schema summarizes the most recurrent niche changes observed in human myeloid malignancies and the pharmacological approaches being explored to target niche-leukemia interdependencies. Depicted niche changes reflect mesenchymal, neurological, and endothelial niche remodeling. Pharmacological approaches include strategies to revert vascular leakiness (eg, NO synthase inhibitors)113 and prevent nerve damage (eg, IL-1 antagonists, and neuroprotective agents such as 4-methylcatechol [4-MC]) or its impact on MSCs (β3-AR agonists, such as mirabegron in phase II clinical trial in MPN [identifier NCT02311569]).84,96 Approaches to prevent or overcome therapeutic resistance include the inhibition of the FAO by blocking the function of the rate-limiting enzyme CPT-1 (eg, etomoxir, ranolazine),115 or interfering with mitochondrial function using tigecycline.
Niche-LSC cross talk in therapeutic resistance: translational implications
The role of the niche in therapeutic resistance has recently been discussed.2 However, novel emerging means of niche-mediated therapeutic resistance will be discussed in this section. In particular, new studies pointing to heterocellular transfer of material as well as metabolic adaptation allowing LSCs to strive in novel or harsh environments will be discussed.
Heterocellular transfer of material
Moschoi and colleagues116 demonstrated the existence of a contact-dependent transfer of functional mitochondria from MSCs to AML cells in vivo, leading to a 1.5-fold increase in energy production and significantly better survival rate upon chemotherapy. Mitochondrial transfer was increased under chemotherapy conditions, suggesting the existence of specific, but yet to be identified, “feed me signals” from the leukemic cells that instructed the transfer.116 A similar study reported transfer of mitochondria from primary MSCs to AML blasts ex vivo and in vivo (in PDX) and provided additional evidence that this unidirectional transfer was sensitive to cytochalasin treatment, highlighting the possible involvement of tunneling nanotubes.117 A study from the Lapidot laboratory118 also supports this mechanism. However, in this case, the mitochondria transfer was primarily directed from the AML blasts toward the stromal cells, suggesting the existence of bidirectional mitochondrial transfer between leukemic HSCs and their BM stromal cells. In line with these AML data, tyrosine kinase inhibitor–resistant CML stem cells appear to be dependent on mitochondrial oxidative metabolism for their survival.119 Consequently, CML LSCs could be efficiently targeted in vivo by a Food and Drug Administration–approved pharmacological inhibitor of mitochondrial protein synthesis, namely tigecycline used in combination with imatinib (Figure 2).
In summary, although the functional significance of this transfer is in its infancy, targeting mitochondrial transfer or function could possibly emerge as a new means to sensitize leukemic cells or overcome therapeutic resistance.
Metabolic adaptation
Another means of resistance has recently been reported by the Jordan laboratory,115 which identified a specific subset of LSCs, in blast crisis CML and AML, that were able to highjack the lipolytic process in gonadal adipose tissue to fuel their metabolic needs and evade chemotherapy-induced apoptosis. These cells expressed the fatty acid transporter, CD36. Interestingly, high expression of CD36 in AML was previously shown to be associated with a poor prognosis.120 Moreover, the fatty acid oxidation (FAO) pathway has been suggested as an alternative carbon-producing anabolic process used by cancer cells, but its most notable effect in leukemia could rely on its ability to interfere with the oligomerization of BAX and BAK in response to apoptotic stimuli.121,122 Consequently, carnitine O-palmitoyltransferase 1 (CPT-1), a protein involved in FAO, has been shown to have antiapoptotic functions in myeloid malignancies.123 A CPT-1 inhibitor, etomoxir,124 inhibits FAO and sensitizes leukemic cells to therapeutic challenge (Figure 2). These data provide evidence for yet another mechanism by which specific niches might shelter defined LSC subsets and promote their therapeutic resistance, thereby providing new opportunities for therapeutic intervention.
Relevance of age-related niche changes to myeloid malignancies
The cellular and molecular mechanisms that contribute to the age-associated increase in myeloid malignancies remain poorly defined. Beyond the well-recognized cell-intrinsic changes affecting aged HSCs,125-130 emerging studies in mice point to the importance of a complex remodeling and intrinsic modifications in HSC-supportive niche cells that are detrimental to HSC function. These include nerve damage and dramatic reduction of HSC niche-forming vessels, which lead to the acquisition of niche features that are reminiscent of the patient’s situation, such as increased vascular density and expanded MSCs that exhibit decreased stem cell potential and reduced expression of HSC maintenance factors (CXCL12, SCF, and ANGPT1).18,131 In 1 study, these complex phenotypes could be reverted by the EC-specific activation of Notch signaling,18 whereas, in the second study, nerve damage–mediated niche aging and HSC functional decline could be rescued by β3-AR agonists.131 Functionally, transplantation of young HSCs to aged recipients results in reduced homing and engraftment, increased myeloid output, and a shift toward reduced clonality.132,133 Conversely, exposure of aged HSCs to a young environment reduces the myeloid skewing.134 These experimental data support the hypothesis that an aged environment could contribute to the emergence of a myeloid skewing phenotype and possibly promote myeloid malignancies. In support of this, a mouse study showed that aged animals transplanted with AML1-ETO–positive HSCs exhibited more severe myeloproliferative phenotypes and increased immature myeloid load than young recipients.133 Likewise, aged MSCs exhibit decreased colony-forming unit fibroblast potential, increased adipogeneic output, and increased senescence both in mice and human.135,136 These changes are associated with reduced hematopoietic support.137,138
In summary, these studies argue in favor of a prominent role of niche-related extrinsic changes in the pathogenesis of myeloid malignancies, in particular as potential mediators of normal HSC loss, which consequently would provide a selective advantage to mutant HSCs. It would thus be of great interest to reevaluate the impact of hematopoietic-specific mutations when introduced in aged HSCs and/or exposed to an aged environment. This could lead to the development of therapeutic approaches to improve the regenerative potential of the niche, which is critical in the context of a BM stem cell transplant setting, while possibly also interfering with detrimental phenotypes that actively promote disease progression (eg, β3-AR agonists MPN).
Emerging strategies to improving the modeling of human myeloid malignancies and evaluating the impact of LSC-niche interactions
In addition to genetic mouse models, the development of highly immune-compromised model systems, such as the NOD-SCIDPrkdcIL2rγ−/− (NSG) mouse strain,139 which allow for the in vivo propagation of human cancers in a relevant environment, is a matter of intense investigation. Notably, propagation of human myeloid leukemia in vivo remains challenging, even for a subset of AML, a fully penetrant and deadly disease in patients. Besides the notoriously poor engraftment of MDS37,140-142 and MPNs,143,144 ∼50% of AMLs fail to engraft immune-compromised hosts.145 This fraction of “engrafter” cases could be further increased in AML by using new mouse lines that express a number of human cytokines that are poorly or not at all cross-reactive between mice and humans.146 Some of these mouse lines have been discussed in an excellent review by Goyama and colleagues,147 whereas new models are detailed in Table 1 and are briefly discussed below. The NSGS model has been reported to improve engraftment of AML148 as well as a very limited number of chronic-phase CML cases.88 However, in the context of MDS PDX, excessive myeloid stimulatory signals in this mouse line was reported to lead to the rapid exhaustion of malignant stem cells (CD34+)67 or engraftment of healthy HSPCs.43 New, improved models expressing human cytokines that are knocked-in to the endogenous mouse loci, namely MITRG or MISTRG (Table 1), have demonstrated engraftment of “favorable risk “AML cases149 that are notoriously difficult to propagate in vivo and were used to demonstrate the specific dependency of inv16 AMLs on exogenous niche-derived macrophage-CSF signals.149 The ability of such models to support other myeloid malignancies or AML subtypes remains to be tested. Last but not least, NSG models expressing a hypomorphic Kit receptor variant (NBSGW[Kitw41],150 NSGKitWv, or NSGKitw41151 ) have recently been generated. These kit mutants weaken the interaction of endogenous HSCs with kit ligand (SCF)–expressing niche cells, thereby providing a competitive advantage for exogenous HSCs expressing wild-type Kit variant. These mice are of particular interest when investigating niche-HSC or niche-LSC interactions because they do not require preconditioning and therefore would allow modeling in an intact (nondamaged) niche. In addition to improving the mouse models per se, experimental models in which malignant HSC engraftment was improved by coinjection of MSC cell lines or patient-derived MSCs64,152 strongly emphasize the importance of a humanized niche. Importantly, initial studies show that PDX models can, at least in part, preserve intraindividual heterogeneity, thereby partially recapitulating the disease complexity seen in patients.39,44,67
Advances in immune-deficient mice for xenograft studies
| . | Genetic background . | Rag . | IL-2rγ . | Sirpα . | c-Kit . | Human cytokines . | WT HSC . | Leukemia models . |
|---|---|---|---|---|---|---|---|---|
| NSG | NOD | WT | Null | NOD Sirpα | WT | None | 139 | Widely used |
| NSGS | NOD | WT | Null | NOD Sirpα | WT | Transgenic hIL3/hGM-CSF/hSCF | — | AML148 ; MDS43,67 ; CML88 |
| NSGW41(Wv) | NOD | WT | Null | NOD Sirpα | W41/W41 | None | 151 | — |
| NBSGW | NOD | WT | Null | NOD Sirpα | W41/W41 | None | 150 | — |
| C57BL/6 | ||||||||
| MITRG | BALB/c | Null | Null | BALB/c Sirpα | WT | Knock-in:hIL3/ hMCSF/hTPO/hGM-CSF | 158 | Inv16/NPM1 AMLs149 |
| MISTRG | BALB/c | Null | Null | Transgenic hSIRPa | WT | Knock-in:hIL3/ hMCSF/hTPO/hGM-CSF | 158 | Inv16/NPM1 AMLs149 |
| . | Genetic background . | Rag . | IL-2rγ . | Sirpα . | c-Kit . | Human cytokines . | WT HSC . | Leukemia models . |
|---|---|---|---|---|---|---|---|---|
| NSG | NOD | WT | Null | NOD Sirpα | WT | None | 139 | Widely used |
| NSGS | NOD | WT | Null | NOD Sirpα | WT | Transgenic hIL3/hGM-CSF/hSCF | — | AML148 ; MDS43,67 ; CML88 |
| NSGW41(Wv) | NOD | WT | Null | NOD Sirpα | W41/W41 | None | 151 | — |
| NBSGW | NOD | WT | Null | NOD Sirpα | W41/W41 | None | 150 | — |
| C57BL/6 | ||||||||
| MITRG | BALB/c | Null | Null | BALB/c Sirpα | WT | Knock-in:hIL3/ hMCSF/hTPO/hGM-CSF | 158 | Inv16/NPM1 AMLs149 |
| MISTRG | BALB/c | Null | Null | Transgenic hSIRPa | WT | Knock-in:hIL3/ hMCSF/hTPO/hGM-CSF | 158 | Inv16/NPM1 AMLs149 |
The table provides a brief overview of the recent mouse models that have significant potential to improve the engraftment of human myeloid malignancies. Previous models are detailed in a recent review by Goyama and colleagues.147 Of note, due to high levels of cytokines, the NSGS model does not support long-term engraftment of normal hHSCs (H.M. and A. Trumpp, unpublished data). Note that both NBSGW and NSGW (W41 or Wv) lines do not require preconditioning prior to human HSC/leukemia transplantation. The NBSGW line has a mixed genetic background. MISTRG/MITRG have the advantage of expressing endogenous levels of human cytokines and are reported to engraft favorable-risk AMLs.
— , Not determined; NSG, NOD-SCIDPrkdcIL2rγ−/−; WT, wild-type.
Finally, to overcome the limitation imposed by the limited availability of patient material, several laboratories have embarked on experimental approaches that allow for the genetic engineering of human stem/progenitor cells to interrogate the effects of individual or combinatorial occurrence of specific somatic events (reviewed in Goyama et al147 ), which are being further developed thanks to the multiplexing power offered by CRISPR-Cas technology.153 Likewise, several laboratories are developing 3-dimensional (3D) niche models that mimic human bone structure and composition to propagate primary specimens both in vitro and in vivo upon implantation to mice. Such models were successfully used to engraft AMLs, blast crisis CML,154-156 and a limited number of MPNs (myelofibrosis).157
These emerging models represent invaluable and cost-effective platforms for preclinical studies. However, propagation of chronic diseases, such as chronic phase CML, MDS, and a majority of MPNs, remains a challenge and may reflect their dependency on a more complex environment that has coevolved with the diseased cells, as was recently demonstrated in our niche-dependent PDX model of MDS.67 Of note, current 3D scaffolds rely on MSCs isolated from healthy volunteers, which are molecularly and functionally distinct from disease-“educated” ones. Moreover, the vasculature is exclusively of mouse origin or supported by transformed human ECs. The use of patient-derived niche components and biologically relevant scaffold materials may further improve these models and help interrogate the role of intercellular communication in disease-relevant accessible systems that are amenable to experimental manipulation.
Concluding remarks and future directions
Our understanding of niche contributions to hematological malignancies has tremendously increased over the past decade; however, many open questions remain, in particular, as to whether the observed phenotypes and molecular mechanisms identified in mouse models are conserved and therapeutically relevant in the human situation. This inevitably relies on a better understanding of the architecture and the cellular interactions at play in the human BM and how these are modulated during aging. Such studies will without doubt benefit from new technologies, such as high performance in vivo imaging and in situ analysis of core biopsies. Prospective studies linking age-associated bone phenotypes and hematological malignancies may also help address some of these pending questions.
More work is also required to interrogate the “functional” niche dependency of human LSCs as well as their in situ localization in the human BM. Are these features influenced by disease subtype, disease stage, and/or even genetic make-up? Can we devise niche-targeting strategies that hamper the growth of diseased cells, while concomitantly improving the fitness of residual normal HSCs (or at the very least preleukemic subclones)? Importantly, such niche-directed treatment strategies could possibly be applicable to a wide range of patients, across different disease subtypes, if conserved mechanisms are at play. In addition, resistance is less likely to emerge because these therapies will target normal cells with more limited cellular plasticity.
Functional assessment of niche contribution requires the establishment of disease-relevant model systems. Beyond PDX models, bone tissue engineering approaches are being used to build 3D model systems that aim to closely recapitulate the human BM niche complexity. Whether such systems maintain the complex genetic diversity seen in individual patients remains to be seen. Can they be used to interrogate the role of age-associated niche changes on disease initiation? Can they test whether age- or leukemia-induced niche changes are reversible? Can we use them to assess the (clone-specific) response to therapeutic challenge or to explore mechanisms of niche-mediated resistance and/or mimic minimal residual disease or relapse?
Finally, the applicability of easy-to-use genome editing technologies to human cells, such as CRISPR/Cas, opens new possibilities for modeling genetic complexity and moving forward with functional investigations using primary human cells instead of ill-defined cell lines that often do not recapitulate the outcomes observed with primary patient material.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The author thanks Henner Farin for critical reading of the manuscript. The author apologizes to the authors of the many excellent studies that are not included in this concise review.
H.M. is supported by the European Research Council (ERC grant no. 639795) and the German José Carreras Leukemia Foundation (award no. DJCLS A 14/01).
Authorship
Contribution: H.M. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Hind Medyouf, Georg-Speyer-Haus Institute for Tumor Biology and Experimental Therapy, Paul-Ehrlich-Str, 42-44, 60596 Frankfurt, Germany; e-mail: hind.medyouf@medyouflab.com and medyouf@gsh.uni-frankfurt.de.

![Figure 2. BM alterations and emerging niche-targeted therapeutic strategies in myeloid malignancies. This schema summarizes the most recurrent niche changes observed in human myeloid malignancies and the pharmacological approaches being explored to target niche-leukemia interdependencies. Depicted niche changes reflect mesenchymal, neurological, and endothelial niche remodeling. Pharmacological approaches include strategies to revert vascular leakiness (eg, NO synthase inhibitors)113 and prevent nerve damage (eg, IL-1 antagonists, and neuroprotective agents such as 4-methylcatechol [4-MC]) or its impact on MSCs (β3-AR agonists, such as mirabegron in phase II clinical trial in MPN [identifier NCT02311569]).84,96 Approaches to prevent or overcome therapeutic resistance include the inhibition of the FAO by blocking the function of the rate-limiting enzyme CPT-1 (eg, etomoxir, ranolazine),115 or interfering with mitochondrial function using tigecycline.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/12/10.1182_blood-2016-11-696070/4/m_blood696070f2.jpeg?Expires=1769092474&Signature=hfLUUlMzz8uoX7w9MrM-TgZdvxhlVeneSP40wVChBcmEOV1P1komiC0P80-2UIAJDdxcGudzacAF2UpYZTioT~-NhcuwaE6mT-izBfW-3fR7zaoiBCFkALMJD4z-YLdx71F5YhNu5XUsg1tZMwgEPeCaNhUOi8bkkJuqw8gprdwyvJPykiX-xvxNJnEOLbC2y0nBK2Fxkieo38tmMbitgwS7g2RuIqfZ5Chzty-FcYuYiO-XS2Go5Pd~rxaNCezi-nD7Cx-jy0-vX04Ge15cIv-JHusrEkUzex8JYIX3Rf5rQ1i3nB46gDJZeGBXaaViEdcuvecN2RKJmyjhIC8Bgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)