Key Points
Low-dose IL-2 is efficacious in steroid-refractory cGVHD, with objective responses in >50% of patients, and durable disease control.
IL-2 initiation earlier after cGVHD onset, prior to severe impairment of Treg:Tcon ratios, improves likelihood of clinical response.
Abstract
Chronic graft-versus-host disease (cGVHD) is associated with inadequate reconstitution of tolerogenic CD4+CD25+FOXP3+ regulatory T cells (Tregs). Previous phase 1 studies identified a low daily dose of interleukin-2 (IL-2) that was well tolerated, did not exacerbate alloimmunity, augmented Treg in vivo, and was associated with improvement of active cGVHD. In the current phase 2 study, 35 adults with steroid-refractory cGVHD received daily IL-2 (1 × 106 IU/m2) for 12 weeks. Median time from transplantation and cGVHD onset was 616 days (range, 270-2145 days) and 317 days (range, 28-1880 days), respectively. Two patients withdrew and 5 required IL-2 dose reductions due to side effects. Twenty of 33 evaluable patients (61%) had clinical responses at multiple cGVHD sites (liver, skin, gastrointestinal tract, lung, joint/muscle/fascia). Three patients (9%) had progressive cGVHD. Compared with pretreatment levels, Treg and natural killer cell counts rose >fivefold (P < .001) and >fourfold (P < .001), respectively, without significant change in conventional CD4 T cells (Tcons) or CD8 T cells. The Treg:Tcon ratio rose >fivefold (P < .001). Clinical responders initiated IL-2 earlier (508 vs 917 days after transplantation, P = .005; 249 vs 461 days after cGVHD onset; P = .03). Treg:Tcon ratios ≥0.07 at baseline and ≥0.2 at week 1 also predicted clinical response (P = .003; P = .0003, respectively). After a 4-week treatment hiatus, clinical responders were eligible to continue IL-2 therapy indefinitely. During 2 years of extended IL-2 therapy, clinical and Treg immune responses persisted, while Tcon count and Treg:Tcon ratio gradually normalized. Low-dose IL-2 provides durable clinical improvement in active cGVHD and extended therapy is well-tolerated.
Introduction
Chronic graft-versus-host-disease (cGVHD) is a common cause of morbidity and mortality in patients undergoing allogeneic HSCT.1 Previous studies have shown that cGVHD is a systemic inflammatory disorder that involves effector T- and B-cell immune responses to both allogeneic and autologous antigens.2 Corticosteroids are the only accepted therapy for cGVHD, but have limited efficacy and substantial long-term toxicity. There is no established second-line therapy for cGVHD.3
CD4+CD25+Foxp3+ Tregs comprise ∼5% to 10% of circulating CD4+ T cells, suppress autoreactivity, and control innate and adaptive immune responses.4-11 Treg impairment is associated with loss of tolerance, autoimmunity, and cGVHD.12-14 Adoptive transfer of Tregs can ameliorate GVHD in preclinical models, but Good Manufacturing Practice–grade ex vivo Treg expansion is challenging.15-17 We undertook an alternative strategy to augment Tregs in vivo. At low physiologic concentrations, interleukin-2 (IL-2) is critical for normal Treg development, expansion, activity, and survival.18,19 In a phase 1 trial, we established that low-dose IL-2 (1 × 106 IU/m2 per day) was safe, well tolerated, and preferentially enhanced CD4+ Tregs in vivo, with clinical responses in 52% of patients with steroid-refractory cGVHD.20,21 Utilizing the dose and daily regimen established in our first study, we now report results of a phase 2 trial undertaken to establish the clinical efficacy of low-dose IL-2 therapy in a larger cohort of 35 adult patients with steroid-refractory cGVHD. Combining results of both studies, we are also able to evaluate predictors of clinical response and to assess the long-term clinical and immunologic effects of low-dose IL-2.
Methods
Phase 2 clinical protocol
Patients with steroid-refractory cGVHD were enrolled in a phase 2 study of daily subcutaneous (SC) IL-2 at 1 × 106 IU/m2 (aldesleukin) for 12 weeks. The initial treatment period was followed by a mandatory 4-week hiatus. Thereafter, improved participants could resume daily IL-2 at the same dose and continue treatment indefinitely. A 50% dose reduction was allowed for the development of severe hematologic or grade 3 nonhematologic toxicities. Eligibility criteria included active cGVHD despite ≥4 weeks of immunosuppressive therapy with ≥0.25 mg/kg per day prednisone (or equivalent) in the prior 12 months, up to 2 prior lines of cGVHD therapy with stable concurrent immunosuppression in the prior 4 weeks, no active infection or malignancy, age ≥18 years, Eastern Cooperative Oncology Group–Performance Status (ECOG-PS) 0 to 2, and normal renal function. Systemic sirolimus plus calcineurin inhibitor use were not eligible owing to concern for thrombotic microangiopathy (TMA) with this combination in the phase 1 study. Dose modification of corticosteroids and other immunosuppressive medications were not allowed during the initial 6 weeks but were permitted thereafter. Patients were evaluable for toxicity at any time during the 12-week treatment period and for response after at least 6 weeks of IL-2. All participants provided informed consent. The trial was approved by the Dana-Farber/Harvard Cancer Center institutional review board.
Clinical assessments
cGVHD assessments using 2005 National Institutes of Health (NIH) consensus criteria were undertaken at baseline, after 6 and 12 weeks of IL-2 therapy, and 4 weeks after stopping IL-2.22 Complete response (CR) is resolution of all reversible cGVHD-associated manifestations; partial response (PR) is ≥50% improvement in organ-specific cGVHD scale without progression at other organ/site(s); progressive disease (PD) is ≥25% increase in organ-specific cGVHD scale; and stable disease (SD) encompasses the remainder, including minor response (MR) not meeting PR criteria.23 Per protocol, responses were not scored for ocular or oral cGVHD, where additional topical therapy was permitted. Stabilization of pulmonary cGVHD is a PR by NIH criteria, but improvement was required in this study. “Trivial” intestinal increase (score 0→1) was not deemed progression, per the 2014 NIH consensus update.24 We assessed patient-reported outcomes (PRO) using the Lee cGVHD Symptom Score.25
Flow cytometry analysis
Protocol-specified immunophenotypic analyses were obtained at: baseline; 1, 2, 4, 6, 8, 12 weeks during IL-2; 4 weeks after stopping treatment; and every 8 weeks while receiving extended-duration IL-2. Tregs were defined as CD3+CD4+CD25medium-highCD127low; conventional CD4 T cells (Tcons) as CD3+CD4+CD25negative-lowCD127medium-high; natural killer (NK) cells as CD56+CD3−; NKT cells as CD56+CD3+; and B cells as CD3−CD19+. Fifty microliters of whole blood (15% EDTA) was incubated with fluorophore-conjugated monoclonal antibodies: anti-CD3 V450 (clone UCHT1; BD Biosciences), anti-CD4 allophycocyanin (APC)-H7 (clone RPA-T4; BD Pharmingen), anti-CD8 Pacific Orange (clone RPA-T8; BioLegend), anti-CD25 phycoerythrin (PE)-Cy7 (clone M-A251; BD Pharmingen), anti-CD127 PE-Cy5 (clone eBioRDR5; eBioscience), anti-CD45RO fluorescein isothiocyanate (clone UCHL1; BD Pharmingen), and anti-CD62L APC (clone DREG-56; BD Pharmingen) for T-cell subsets; anti-CD56 PE (clone B159; BD Pharmingen), anti-CD3 V450 (clone UCHT1; BD Horizon) for NK/NKT cells; anti-CD19 APC (clone HIB19; BD Pharmingen) for B cells. Red blood cell (RBC) lysis with 500 μL of 1× BD PharmLyse followed. Cell analysis used FACSCanto-II (BD Biosciences) and FACSDiva software (BD Biosciences).
Plasma cytokine measurements
Plasma samples obtained during IL-2 therapy were cryopreserved before being analyzed. Plasma IL-2 concentrations were measured by high-sensitivity enzyme-linked immunosorbent assay (eBioscience). Soluble IL-2 receptor α (sIL-2R) concentrations were measured by Quantikine enzyme-linked immunosorbent assay per the manufacturer’s instructions (R&D Systems).
Statistical analysis
This was a 1-stage phase 2 trial of 12-week low-dose IL-2 in steroid-refractory cGVHD. The primary end point was overall response rate per NIH criteria. The study was powered to detect at least 20% improvement in overall response rate basing the response rate from steroid-alone treatment as a null hypothesis (Ho: Po ≤ 0.2). Target accrual was 31 evaluable patients, with up to 41 enrolled to allow for premature participant dropout. With 31 evaluable patients who receive at least 6 weeks of treatment, the study had 86% power with a type I error rate of 7%, based on an exact binomial distribution.
Baseline characteristics were reported descriptively. The Fisher exact test or a χ2 test was used for group comparison of categorical variables. The Wilcoxon-rank sum test was used for group comparison of continuous variables. Immunologic parameters and PRO assessments were analyzed descriptively and compared using the exact Wilcoxon-rank-sum test for group comparison and Wilcoxon-(signed)-rank test for paired group comparison. To explore threshold Treg:Tcon ratios at baseline and week 1 of treatment, receiver-operating-characteristic analysis was performed. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method; cumulative incidences of relapse and nonrelapse mortality (NRM) were estimated in the competing risks framework treating NRM and relapse as a competing event, respectively. Time to events was measured from study enrollment to death (OS) or death/disease progression (PFS, NRM, relapse), whichever occurred first. OS and PFS were compared using the log-rank test and cumulative incidences were compared using the Gray test.26 Landmark analysis at week 12 was performed for comparison of OS, PFS, NRM, and relapse between responders and nonresponders to avoid a lead-time bias. However, all patients, including 2 unevaluable subjects, were alive and relapse-free at 12 weeks. To identify predictors for clinical response, we first performed univariable logistic regression analysis for variables listed in Table 1 and Treg:Tcon ratio at baseline and at 1 week after treatment. Variables with P < .1 from univariable analysis were considered for multivariable analysis. These variables are age, time from HSCT, time from cGVHD, Treg:Tcon ratio at baseline and at 1 week after study entry. Since time from HSCT and time from cGVHD are highly collinear, these variables were analyzed separately. All testing was 2-sided at the significance level of 0.05. Multiple comparisons were not adjusted. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), and R v2.13.2 (the CRAN Project).
Baseline characteristics
| . | N . | % . |
|---|---|---|
| Total | 35 | 100 |
| Median age, y (range) | 51 (22, 72) | |
| Patient sex | ||
| F | 17 | 48.6 |
| M | 18 | 51.4 |
| Patient-donor sex match | ||
| FF | 9 | 25.7 |
| FM | 7 | 20 |
| MF | 8 | 22.9 |
| MM | 11 | 31.4 |
| HLA typing (A, B, C, DRB1) at HSCT | ||
| Matched unrelated | 18 | 51.4 |
| Matched related | 14 | 40 |
| Mismatched related | 3 | 8.6 |
| Conditioning intensity at HSCT | ||
| MAC | 18 | 51.4 |
| RIC | 17 | 48.6 |
| Primary disease | ||
| Acute lymphoid leukemia | 3 | 8.6 |
| Acute myeloid leukemia | 9 | 25.7 |
| CLL/SLL/PLL | 4 | 11.4 |
| Chronic myeloid leukemia | 1 | 2.9 |
| Hodgkin lymphoma | 1 | 2.9 |
| Myelodyplastic syndromes (MDS) | 7 | 20 |
| MM/PCD | 1 | 2.9 |
| Myeloproliferative disorder (MPD) | 1 | 2.9 |
| Mixed MDS/MPD | 1 | 2.9 |
| Non-Hodgkin lymphoma | 6 | 17.1 |
| Other acute leukemia | 1 | 2.9 |
| Disease Risk Index (DRI) | ||
| Low | 7 | 20 |
| Intermediate | 17 | 48.6 |
| High | 10 | 28.6 |
| Very high | 1 | 2.9 |
| Graft source at HSCT | ||
| Bone marrow (BM) | 1 | 2.9 |
| Peripheral blood stem cell (PBSC) | 32 | 94.1 |
| BM + PBSC | 1 | 2.9 |
| Median no. of cGVHD sites (range) | 4 (1, 7) | |
| Global cGVHD severity (per NIH criteria) | ||
| Mild | 6 | 17.1 |
| Moderate | 22 | 62.7 |
| Severe | 7 | 20 |
| Median prednisone dose at baseline (range) | 20 (2.5, 50) | |
| Median no. of prior treatments* (range) | 2 (1, 4) | |
| Median time from HSCT to start of IL-2, d (range) | 616 (270, 2145) | |
| Median time from cGVHD to start of IL-2, d (range) | 317 (28, 1880) | |
| Median time from HSCT to cGVHD, d (range) | 266 (46, 996) | |
| Response at week 12 | ||
| Partial response | 20 | 57.1 |
| Stable disease (including minor response) | 10 | 28.6 |
| Progressive disease | 3 | 8.6 |
| Nonevaluable† | 2 | 5.7 |
| No. of patients initiating extended IL-2 treatment | 23 | 65.7 |
| . | N . | % . |
|---|---|---|
| Total | 35 | 100 |
| Median age, y (range) | 51 (22, 72) | |
| Patient sex | ||
| F | 17 | 48.6 |
| M | 18 | 51.4 |
| Patient-donor sex match | ||
| FF | 9 | 25.7 |
| FM | 7 | 20 |
| MF | 8 | 22.9 |
| MM | 11 | 31.4 |
| HLA typing (A, B, C, DRB1) at HSCT | ||
| Matched unrelated | 18 | 51.4 |
| Matched related | 14 | 40 |
| Mismatched related | 3 | 8.6 |
| Conditioning intensity at HSCT | ||
| MAC | 18 | 51.4 |
| RIC | 17 | 48.6 |
| Primary disease | ||
| Acute lymphoid leukemia | 3 | 8.6 |
| Acute myeloid leukemia | 9 | 25.7 |
| CLL/SLL/PLL | 4 | 11.4 |
| Chronic myeloid leukemia | 1 | 2.9 |
| Hodgkin lymphoma | 1 | 2.9 |
| Myelodyplastic syndromes (MDS) | 7 | 20 |
| MM/PCD | 1 | 2.9 |
| Myeloproliferative disorder (MPD) | 1 | 2.9 |
| Mixed MDS/MPD | 1 | 2.9 |
| Non-Hodgkin lymphoma | 6 | 17.1 |
| Other acute leukemia | 1 | 2.9 |
| Disease Risk Index (DRI) | ||
| Low | 7 | 20 |
| Intermediate | 17 | 48.6 |
| High | 10 | 28.6 |
| Very high | 1 | 2.9 |
| Graft source at HSCT | ||
| Bone marrow (BM) | 1 | 2.9 |
| Peripheral blood stem cell (PBSC) | 32 | 94.1 |
| BM + PBSC | 1 | 2.9 |
| Median no. of cGVHD sites (range) | 4 (1, 7) | |
| Global cGVHD severity (per NIH criteria) | ||
| Mild | 6 | 17.1 |
| Moderate | 22 | 62.7 |
| Severe | 7 | 20 |
| Median prednisone dose at baseline (range) | 20 (2.5, 50) | |
| Median no. of prior treatments* (range) | 2 (1, 4) | |
| Median time from HSCT to start of IL-2, d (range) | 616 (270, 2145) | |
| Median time from cGVHD to start of IL-2, d (range) | 317 (28, 1880) | |
| Median time from HSCT to cGVHD, d (range) | 266 (46, 996) | |
| Response at week 12 | ||
| Partial response | 20 | 57.1 |
| Stable disease (including minor response) | 10 | 28.6 |
| Progressive disease | 3 | 8.6 |
| Nonevaluable† | 2 | 5.7 |
| No. of patients initiating extended IL-2 treatment | 23 | 65.7 |
Patient, transplantation, and cGVHD characteristics of the phase 2 trial.
CLL, chronic lymphocytic leukemia; F, female; M, male; MAC, myeloablative conditioning; MM, multiple myeloma; PCD, plasma cell dyscrasia; PLL, prolymphocytic leukemia; RIC, reduced-intensity conditioning; SLL, small lymphocytic leukemia.
Concurrent immune-suppression therapies beside corticosteroids included tacrolimus (n = 17), sirolimus (n = 5), mycophenolate mofetil (n = 5).
Two patients received the treatment <6 weeks, thus were not evaluable for response.
Results
Patient characteristics
Thirty-five patients were enrolled between July 2011 and January 2014 (Table 1). Median age was 51 years (range, 22-72 years). Median days since HSCT was 616 days (range, 270-2145 days) and days since cGVHD onset was 317 days (range, 28-1880 days). At enrollment, patients had a median of 4 (range, 1-7) sites of cGVHD involvement (supplemental Table A, available on the Blood Web site). The baseline median prednisone dose was 20 mg per day (range, 2.5-50). The median follow-up in survivors was 34 months (range, 21-50 months).
Safety and tolerability
All 35 patients who started IL-2 were evaluable for safety (supplemental Table B). Two patients withdrew early and 5 required IL-2 dose reduction for constitutional adverse events (AEs; n = 6) and thrombocytopenia (n = 1). IL-2–related constitutional AEs of flu-like symptoms, fatigue, malaise, arthralgia/myalgia were grade-2 (n = 3) and grade-3 (n = 3). One patient had grade 2 injection-site pain (n = 1). Two patients developed grade 3 infections (respiratory syncytial virus, Streptococcus viridians). Two patients had grade 3 PE/deep vein thrombosis. None developed TMA. Transient skin GVHD flares did not occur at IL-2 initiation.
Clinical response and survival outcomes
Twenty of 33 evaluable patients (61%) had objective PR by week 12, but there were no CR. Ten patients (30%) had SD including MR not meeting PR criteria. Three patients (9%) progressed (supplemental Table B). Response sites included liver (n = 6 of 13, 46%), skin (n = 9 of 27, 33%), gastrointestinal (GI) tract (n = 3 of 10, 30%), lung (n = 3 of 15, 20%; with stable forced expiratory volume in 1 second in 5 patients, 33%), and joint/muscle/fascia (JMF) (n = 4 of 23, 17%).
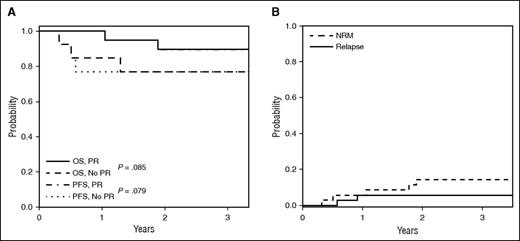
Twenty-three patients with PR or SD with minor response elected to continue daily IL-2 after a mandatory 4-week treatment hiatus. Three-year OS and PFS were both 83%. The 3-year OS and PFS of 90% in responders compares with 77% in nonresponders (P = .085 and P = .079, respectively) (Figure 1A). On extended follow-up of the phase 2 cohort, 7 patients died: 1 nonevaluable patient (pulmonary GVHD/respiratory failure), 2 clinical responders (1-second malignancy; 1 cGVHD progression off IL-2), and 4 nonresponders (3 pulmonary GVHD/respiratory failure; 1 unknown). Two patients relapsed (Figure 1B).
Survival outcomes. (A) OS and PFS for the phase 2 cohort for responders (PR) and nonresponders (PD, SD, MR). Landmark analysis by week 12 response is indicated, and results did not differ when assessed from study entry. (B) Cumulative incidence of relapse and NRM for the phase 2 cohort.
Survival outcomes. (A) OS and PFS for the phase 2 cohort for responders (PR) and nonresponders (PD, SD, MR). Landmark analysis by week 12 response is indicated, and results did not differ when assessed from study entry. (B) Cumulative incidence of relapse and NRM for the phase 2 cohort.
Responders improved in skin manifestations (keratosis pilaris, poikiloderma, lichen planus-like features, maculopapular rash, nail involvement, and superficial and deep sclerosis), diarrhea, fasciitis and joint mobility, cough/dyspnea on exertion, and liver and/or pulmonary function tests. PRO assessments were limited by study size and the exclusion of uninformative cGVHD sites. Sites with no involvement at baseline but symptomatic at week 12 were included. Pre-to-posttreatment paired analysis was performed for overall score as well as each subscale of PRO. For the overall cohort, PRO of skin symptoms improved from baseline median score of 3 (range, 0-19) to a week 12 median score of 2 (range, 0-14; P = .035). There was no difference in pre-to-posttreatment change in overall or other subscale scores or between responders and nonresponders.
Clinical laboratory parameters
Twelve-week daily IL-2 treatment did not induce systematic cytopenias, TMA/renal failure, or hepatic dysfunction in the phase 2 cohort. Asymptomatic peripheral eosinophilia was anticipated,27 rising from a median of 1% (range, 0-9) at baseline to a peak of 8.5% (interquartile range [IQR], 0-51) at 4 weeks (P < .001), declining thereafter to 4% (IQR, 1-46) by 12 weeks, but did not vary with clinical response. Thyroid function was unimpaired.
Immunologic response
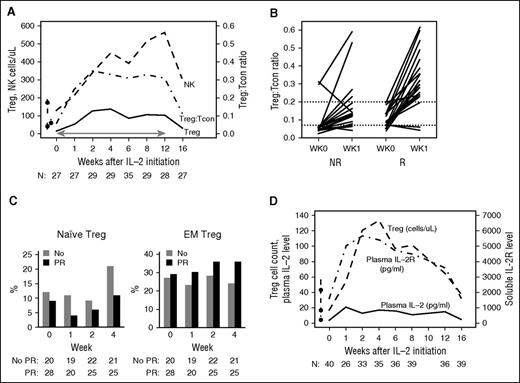
Prior to starting low-dose IL-2, the baseline median Treg count was 17 cells per μL (IQR, 9-41). This was low compared with healthy donor value of 43 cells per μL (IQR, 27-57) (P = .0001) (Figure 2A). Tregs increased rapidly after IL-2 therapy, peaking at 4 weeks at a median of 138 cells per μL (IQR, 52-188). This peak level was greater than eightfold higher than baseline (P < .001). Levels of circulating Tregs plateaued then slowly decreased. After 12 weeks of IL-2 therapy, median Treg count was 104 cells per μL (IQR, 54-167). Tregs declined but remained above baseline 4 weeks after IL-2 therapy was stopped, with a median Treg count of 31 cells per μL (IQR, 23-64).
Immunologic outcomes. (A) Immunologic impact of 12 weeks of low-dose IL-2: median Treg, Tcon count, and Treg:Tcon ratio in the study cohort during 12-week IL-2 and 4 weeks off IL-2. Line with arrows indicates IL-2 therapy. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom. (B) Treg:Tcon ratios and IL-2 clinical response: week 0 and week 1 Treg:Tcon ratios in clinical responders (R: PR) and nonresponders (NR: PD/SD/MR) are displayed separately. Each line indicates an individual patient’s data at baseline and week 1. Threshold ratios of 0.07 (baseline) and 0.20 (week 1) are indicated by the dashed lines. (C) Naive and EM Treg percentage by IL-2 clinical response: week 0 and week 1, 2, and 4 naive and EM Treg percentage for clinical responders (R: PR) and nonresponders (NR: PD/SD/MR) are displayed separately. P values at relevant time points are indicated. Number of patients evaluated at each time point is indicated at the bottom. (D) Plasma IL-2, sIL-2R, and Treg cell counts during IL-2 therapy. Median plasma IL-2, plasma sIL-2R, and Treg cell counts during 12-week IL-2 and 4 weeks off IL-2. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom of the image.
Immunologic outcomes. (A) Immunologic impact of 12 weeks of low-dose IL-2: median Treg, Tcon count, and Treg:Tcon ratio in the study cohort during 12-week IL-2 and 4 weeks off IL-2. Line with arrows indicates IL-2 therapy. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom. (B) Treg:Tcon ratios and IL-2 clinical response: week 0 and week 1 Treg:Tcon ratios in clinical responders (R: PR) and nonresponders (NR: PD/SD/MR) are displayed separately. Each line indicates an individual patient’s data at baseline and week 1. Threshold ratios of 0.07 (baseline) and 0.20 (week 1) are indicated by the dashed lines. (C) Naive and EM Treg percentage by IL-2 clinical response: week 0 and week 1, 2, and 4 naive and EM Treg percentage for clinical responders (R: PR) and nonresponders (NR: PD/SD/MR) are displayed separately. P values at relevant time points are indicated. Number of patients evaluated at each time point is indicated at the bottom. (D) Plasma IL-2, sIL-2R, and Treg cell counts during IL-2 therapy. Median plasma IL-2, plasma sIL-2R, and Treg cell counts during 12-week IL-2 and 4 weeks off IL-2. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom of the image.
The baseline median Tcon count was 274 cells per μL (IQR, 96-605) compared with 686 cells per μL (IQR, 567-873) in healthy donors (P < .0001). Tcons did not change significantly during IL-2 therapy. The median Tcon count was 310 cells per μL (IQR, 170-465) after 12 weeks, and 398 cells per μL (IQR, 180-757) 4 weeks after stopping IL-2. The median Treg:Tcon ratio therefore increased more than fivefold (P < .001) from 0.06 (IQR, 0.05-0.13) at baseline to 0.35 (IQR, 0.26-0.48) after 2 weeks. The Treg:Tcon ratio remained elevated at 0.31 (IQR, 0.27-0.39) through week 12, declined off IL-2 to 0.11 (IQR, 0.06-0.16), but remained significantly higher than baseline (P = .005) 4 weeks after stopping IL-2 (Figure 2A).
During IL-2 therapy, the median NK-cell count increased from 130 cells per μL (IQR, 91-219) at baseline to 563 cells per μL (IQR, 270-675) after 12 weeks (P < .001), declining to 296 (IQR, 167-423) at 4 weeks after discontinuation of therapy (Figure 2A). Conversely, median CD19+ B-cell count declined from a baseline of 297 (IQR 38-516) to 80 cells per μL (IQR, 14, 276) at 12 weeks (P = .01). There were no significant changes in CD3+CD8+ T-cell or CD3+CD56+ NKT cell counts during the 12-week treatment period.
Clinical response correlates
Patient sex, prior diagnosis, conditioning intensity, and donor type were not associated with clinical responses in the phase 2 study (Table 2). Responders were younger (50 vs 61 years, P = .044) and initiated IL-2 earlier (499 vs 903 days after HSCT, P = .005; 249 vs 461 days after cGVHD onset, P = .03). However, age was not significantly associated with response in multivariable logistic regression analysis when time from HSCT or from cGVHD onset to start of IL-2 treatment (both highly correlated [r = 0.89]) was included.
Clinical predictors of IL-2 response
| . | No response (MR/SD/PD) . | Response (PR) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N . | Median . | Minimum . | Maximum . | N . | Median . | Minimum . | Maximum . | ||
| Age, y | 13 | 61 | 29 | 70 | 20 | 50 | 22 | 72 | .044 |
| No. of prior therapies | 13 | 3 | 1 | 4 | 20 | 2 | 1 | 4 | .6 |
| Prednisone start dose, mg | 13 | 25 | 3 | 40 | 20 | 20 | 5 | 50 | .9 |
| No. of cGVHD sites | 13 | 4 | 1 | 6 | 20 | 5 | 2 | 7 | .27 |
| Time from HSCT to start of IL-2, d | 13 | 917 | 348 | 2145 | 20 | 508 | 270 | 1065 | .005 |
| Time from cGVHD to start of IL-2, d | 13 | 461 | 119 | 1880 | 20 | 249 | 28 | 847 | .03 |
| Time from HSCT to cGVHD, d | 13 | 265 | 153 | 996 | 20 | 266 | 46 | 433 | .35 |
| Prednisone dose reduction, week 12, % | 13 | 0 | 0 | 50 | 20 | 25 | 0 | 75 | .15 |
| . | No response (MR/SD/PD) . | Response (PR) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N . | Median . | Minimum . | Maximum . | N . | Median . | Minimum . | Maximum . | ||
| Age, y | 13 | 61 | 29 | 70 | 20 | 50 | 22 | 72 | .044 |
| No. of prior therapies | 13 | 3 | 1 | 4 | 20 | 2 | 1 | 4 | .6 |
| Prednisone start dose, mg | 13 | 25 | 3 | 40 | 20 | 20 | 5 | 50 | .9 |
| No. of cGVHD sites | 13 | 4 | 1 | 6 | 20 | 5 | 2 | 7 | .27 |
| Time from HSCT to start of IL-2, d | 13 | 917 | 348 | 2145 | 20 | 508 | 270 | 1065 | .005 |
| Time from cGVHD to start of IL-2, d | 13 | 461 | 119 | 1880 | 20 | 249 | 28 | 847 | .03 |
| Time from HSCT to cGVHD, d | 13 | 265 | 153 | 996 | 20 | 266 | 46 | 433 | .35 |
| Prednisone dose reduction, week 12, % | 13 | 0 | 0 | 50 | 20 | 25 | 0 | 75 | .15 |
Additional nonsignificant variables: patient sex, conditioning regimen intensity (MAC, RIC), HLA match (HLA-matched related donor, HLA-matched unrelated donor, mismatched), prior hematologic malignancy (lymphoid, myeloid), specific cGVHD agents (eg, sirolimus, mycophenolate mofetil). Note: Other baseline patient and transplant characteristics listed in Table 1 are not included in this table and none of them was different between PR and no PR at the 0.05 level.
In the phase 1 study, we identified a baseline Treg:Tcon threshold ratio of ≥0.07 as a predictor of IL-2 clinical response.20 Analysis of patients in the phase 2 trial validated this association (Table 3). Additionally, a 1-week Treg:Tcon ratio of ≥0.2 also strongly predicted clinical response in the phase 2 cohort. Combined data for 53 evaluable patients from both studies confirmed that responders had a higher median Treg:Tcon ratio both at baseline (0.09 vs 0.06, P = .02) and 1 week after treatment onset (0.31 vs 0.14, P = .004). Treg:Tcon threshold ratios of ≥0.07 at baseline and ≥0.2 after 1 week of IL-2 therapy were also highly predictive of clinical response (P = .003; P = .0003, respectively) (Table 3, Figure 2B). The receiver-operating-characteristic curve of the 1-week Treg:Tcon ratio cutoff yielded an area under the curve of 0.77 for prediction of IL-2 clinical response.
Immunologic predictors of IL-2 response: Treg:Tcon ratios
| Week . | Trials . | Treg:Tcon . | Nonresponse, % . | Response (PR), % . | P . |
|---|---|---|---|---|---|
| 0 | Phase 2 | ≥0.07 | 20 | 64.7 | .046 |
| Phase 1 and 2 combined | ≥0.07 | 25 | 71.4 | .003 | |
| 1 | Phase 2 | ≥0.2 | 27.3 | 100 | .00015 |
| Phase 1 and 2 combined | ≥0.2 | 26.3 | 85 | .0003 |
| Week . | Trials . | Treg:Tcon . | Nonresponse, % . | Response (PR), % . | P . |
|---|---|---|---|---|---|
| 0 | Phase 2 | ≥0.07 | 20 | 64.7 | .046 |
| Phase 1 and 2 combined | ≥0.07 | 25 | 71.4 | .003 | |
| 1 | Phase 2 | ≥0.2 | 27.3 | 100 | .00015 |
| Phase 1 and 2 combined | ≥0.2 | 26.3 | 85 | .0003 |
Treg:Tcon ratios at baseline (week 0) and 1 week after start of IL-2 therapy (week 1). Data for phase 2, and combined phase 1 and 2 trials are presented separately.
Early differences between clinical responders and nonresponders were also noted in the relative proportions of CD25+CD127−CD45RA+CD62L+ naive Treg and CD25+CD127−CD45RA−CD62L− effector memory (EM) Tregs. Naive Treg proportions fell more in responders from a baseline median of 0.09 (Q1-Q3, 0.04-0.17) to a median of 0.04 (Q1-Q3, 0.02-0.06) at week 1, thereafter recovering to a week 4 median of 0.11 (Q1-Q3, 0.07-0.18). In nonresponders, the naive Treg median percentage at baseline was similar at 0.12 (Q1-Q3, 0.07-0.24), but week 1 and week 4 medians were comparatively higher than responders at 0.11 (Q1-Q3, 0.05-0.17; P = .0056) and 0.21 (Q1-Q3, 0.14-0.31; P = .0046), respectively (Figure 2C). Conversely, EM Tregs, arising from differentiation of naive Tregs, rose more in clinical responders from a baseline median of 0.29 (Q1-Q3, 0.15-0.36) to a week 4 median of 0.36 (Q1-Q3, 0.26-0.47; P = .0039) compared with nonresponders, where the EM Treg median at baseline was similar at 0.27 (Q1-Q3, 0.15-0.4) but failed to rise with IL-2 therapy with a week 4 median of 0.24 (Q1-Q3, 0.16-0.28).
Plasma IL-2 and sIL-2R levels
Plasma IL-2 and sIL-2R levels were assessed in the combined phase 1 and 2 cohorts. Median IL-2 level rose from a baseline of 4 pg/mL (IQR, 2-8) to a peak of 21 pg/mL (IQR, 16-36) at week 1, plateaued in the 13-17 pg/mL range between weeks 2 and 12, and fell to 5 pg/mL (IQR, 3-8) 4 weeks after stopping IL-2 (Figure 2D). Median sIL-2R level also rose from a baseline of 1605 pg/mL (IQR, 122-2159) to a peak of 5656 pg/mL (IQR, 4465-9358) at week 2, then declined gradually to 3605 pg/mL (IQR, 2148-5503) by week 12, and fell to 1612 pg/mL (IQR, 1124-2172) 4 weeks after stopping IL-2. Changes in sIL-2R closely tracked changes in Treg count during therapy (Figure 2D). IL-2 or sIL-2R levels did not vary significantly between responders and nonresponders (data not shown).
Extended-duration IL-2 therapy
Thirty-five patients in the phase 1 and 2 studies elected to restart low-dose IL-2 after the mandatory 4-week hiatus (12 patients from phase 1 and 23 from phase 2). IL-2 was administered to 23 patients for over 1 year and to 15 patients for >2 years. The median duration of follow-up on extended IL-2 was 22 months (range, 4-80 months). Clinical characteristics of individual patients who received extended IL-2 therapy are summarized in supplemental Tables B-C.
Extended low-dose IL-2 therapy was well tolerated. Grade 2 IL-2–related AEs during extended therapy included myalgia (n = 1) and thrombocytopenia (n = 1). Grade 3 IL-2–related AEs included lung infection (n = 1), arthralgia (n = 1), and injection site induration (n = 2). The pulmonary infection (Aspergillus/Pseudomonas) was initially deemed possibly IL-2 related, but the patient remains on IL-2 without recurrent infections. One patient with prior coronary artery disease had angina and myocardial ischemia requiring restenting during extended-duration IL-2. He remains on IL-2 with preserved cardiac function. Five patients (14%) required IL-2 dose reduction, 2 patients (6%) had hematologic relapse, and 1 patient (3%) developed an oropharyngeal squamous cell cancer. Clinical benefit for responders on extended IL-2 therapy was sustained during taper of concomitant immunosuppression. The mean steroid dose taper during extended IL-2 therapy was 50% (range, −20 to 100). Seven patients discontinued systemic steroids, and another 4 patients discontinued all other immunosuppressive medications. Three patients initiated additional cGVHD therapies (2 extracorporeal photopheresis; 1 sirolimus) to deepen clinical response.
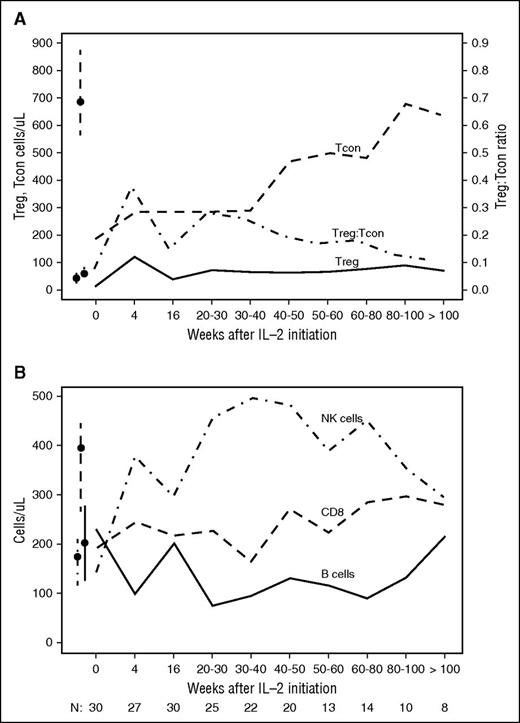
Peripheral blood Treg counts remained elevated the entire 2 years of IL-2 therapy (Figure 3A). The median Treg count was 65 cells per μL (IQR, 41-101) and 70 cells per μL (IQR, 44-113) at 50 to 60 weeks and >100 weeks, respectively. During this period, CD4 Tcon counts gradually increased and normalized 2 years after starting low-dose IL-2. The median Tcon count was 498 cells per μL (IQR, 393-516) and 635 cells per μL (IQR, 545-933) at 50 to 60 weeks and >100 weeks, respectively. This increase in CD4 Tcons resulted in the gradual normalization of the Treg:Tcon ratio during this period (Figure 3A). IL-2–induced NK-cell elevation was also sustained and remained significantly above normal levels during extended IL-2 therapy (Figure 3B) (389 cells per μL [IQR, 316-453] and 294 cells per μL [IQR, 219-556] at 50-60 weeks and >100 weeks, respectively). CD8 T cells also gradually rose during extended therapy but this increase was relatively small. After 2 years of IL-2 therapy, CD8 T-cell counts remained below normal levels. B-cell counts decreased during the initial period of IL-2 therapy but gradually recovered to normal levels as extended therapy continued (Figure 3B).
Immunologic impact of extended-duration IL-2 therapy. (A) Median Treg, Tcon count, and Treg:Tcon ratio during extended IL-2 is indicated. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom of the image. (B) Median NK, CD8 T, and CD19 B-cell counts during extended-duration IL-2 are indicated. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom.
Immunologic impact of extended-duration IL-2 therapy. (A) Median Treg, Tcon count, and Treg:Tcon ratio during extended IL-2 is indicated. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom of the image. (B) Median NK, CD8 T, and CD19 B-cell counts during extended-duration IL-2 are indicated. Box-and-whisker plots indicate median and IQR for healthy donors. Number of patients evaluated at each time point is indicated at the bottom.
Discussion
CD4+ Tregs have a central role in immune tolerance and controlling immune responses, and Treg dysfunction is well described in autoimmune disorders and cGVHD.12-14 Clinical adoptive transfer of Tregs purified and expanded ex vivo remains challenging, despite some evidence of benefit.28-30 Alternative approaches to expand Tregs in vivo utilizing systemic low-dose IL-2 have been reported in healthy donors,31 for GVHD prophylaxis,32 as well as hepatitis C virus–induced vasculitis33 and other autoimmune disorders.34-36 Early-phase studies indicated that this approach is well tolerated but efficacy in controlling allo- and autoimmune inflammation has not been established.
Our major goal was to evaluate the clinical efficacy of low-dose IL-2 in cGVHD patients with inadequate response to corticosteroids. Our previous phase 1 study in patients with advanced cGVHD identified a daily IL-2 dose that was well tolerated for 8 weeks with significant immunologic and clinical activity.20,21 In the phase 2 study, patients received the same daily IL-2 dose dispensed as a 2-week supply for home refrigeration, aliquoted as single-use syringes for daily SC self-administration, and treatment was extended for a 12-week period. Treatment was well tolerated even for those with extensive sclerosis. Only 2 patients withdrew early and none developed TMA noted in the phase 1 study. Only 3 patients had progressive cGVHD, and no hematologic malignancy relapses occurred by week 12. Notably, no cGVHD flares occurred upon initiation of IL-2 therapy. Objective partial response was noted in 20 of 33 evaluable patients (61%), involving multiple cGVHD sites (liver, skin, GI, JMF, lung). One limitation of our study was the exclusion of oral and ocular cGVHD response assessments a priori because concurrent use of topical therapies rendered us unable to assess IL-2 efficacy at these sites. Overall, however, the study was successful per its statistical design, which hypothesized a response rate >40% at 12 weeks. Interestingly, some responders noted recrudescent cGVHD during their mandatory 4-week treatment hiatus, indicating a need for continued IL-2 therapy.
PRO assessment supported the tolerability of low-dose IL-2 therapy, with improved skin symptoms by 12 weeks (P = .035). Improved Lee skin symptom scores have been associated with improved survival.37 However, a larger study is needed for confirmation and to evaluate alternative quality-of-life measures (eg, Functional Assessment of Cancer Therapy–Bone Marrow Transplant). Additionally, there was no difference in pre-to-posttreatment change in overall or subscale PRO scores between clinical responders and nonresponders. This is likely because the majority of patients were responders and some nonresponders showed symptomatic improvement despite not meeting NIH response criteria.
Low-dose IL-2 therapy induced rapid selective expansion of Treg and NK cells, with rapid increase in Treg:Tcon ratio in all treated patients. Although patients continued to receive daily IL-2 for 12 weeks, the expansion of peripheral Tregs peaked at 4 weeks and continued therapy did not lead to further Treg expansion. Measurement of IL-2 levels in plasma demonstrated that IL-2 levels peaked at 1 week and remained relatively low and stable despite continued daily therapy. These patterns likely reflect the short elimination half-life of IL-2, which has been reported to be only 1 to 2 hours after IV injection (Aldesleukin IB). In addition, both expansion of Tregs that express CD25 and increased levels of soluble CD25 may sequester IL-2 and limit the amount of IL-2 available to promote further Treg proliferation in vivo. These findings suggest that IL-2 dose escalation once Treg levels have plateaued may result in greater levels of Treg expansion in vivo. Importantly, all immunologic effects reverted to baseline when IL-2 treatment was stopped.
By combining results of both studies, we identified early predictors of IL-2 clinical response. Patients beginning IL-2 earlier after HSCT were more likely to benefit. Baseline and week 1 Treg:Tcon ratios of ≥0.07 and ≥0.2, respectively, were found to be strong predictors of clinical response. Additionally, clinical responders had a greater rise in effector memory Tregs and a corresponding fall in naive Tregs after initiating IL-2 therapy. This was most evident when Treg levels peaked 4 weeks after starting IL-2. Taken together, these findings suggest that the functional state and number of Tregs available for in vivo response to exogenous IL-2 may be an important clinical variable. In this setting, it may be possible to augment endogenous Tregs by adoptive transfer of donor-derived Treg in cGVHD patients. Subsequent administration of low-dose IL-2 could result in greater levels of functionally active Treg in vivo by promoting expansion of both adoptively transferred and endogenous Tregs.
In patients who benefited during the initial 12-week course of therapy and continued on extended low-dose IL-2, long-term therapy was safe with a trend to improved OS and PFS. This is an important consideration because cGVHD clinical responses per NIH criteria have not always correlated with a survival benefit. In part, this may reflect the long-term toxicities of therapies administered to control cGVHD.38-40 Notably, immunosuppression was not apparent during extended IL-2 therapy. Clinical responders receiving IL-2 did not develop opportunistic infections that are frequent in immunocompromised hosts. The relatively low incidence of opportunistic infections may reflect the taper of concurrent immunosuppressive agents as well as normalization of CD4 Tcon counts during 2 years of extended-duration IL-2 therapy.
Only 2 patients (6%) of the extended-duration cohort had relapses of the underlying hematologic malignancy. This is well within the 10% to 13% relapse rate reported in a prospective cGVHD cohort followed for 2 to 4 years,41 suggesting that antileukemic responses were not abrogated. IL-2–mediated NK-cell augmentation may be helpful in this regard. One oropharyngeal squamous cell cancer in a patient with oral cGVHD occurred after several years of extended IL-2 therapy. These low relapse and second malignancy rates are reassuring but remain an area of scrutiny. The long-term safety and efficacy of low-dose IL-2 therapy is also relevant for autoimmune diseases and solid organ transplantation, where chronic therapy will be needed to maintain Treg expansion.
In summary, 12 weeks of daily SC low-dose IL-2 therapy induced profound in vivo Treg enhancement, with objective clinical responses in over 60% of patients with steroid-refractory cGVHD. These results provide additional impetus to consider low-dose IL-2 therapy early after cGVHD onset and before development of “fixed” dysfunction and severe tissue damage. Corticosteroids are standard therapy for patients with new-onset cGVHD, but many do not respond adequately. The clinical effectiveness of low-dose IL-2 in steroid-refractory cGVHD suggests that this regimen could be effective primary therapy, and further studies in patients with newly diagnosed cGVHD are warranted. Long-term tolerance induction with IL-2 is a promising therapeutic strategy in patients with active cGVHD and potentially other disorders of impaired immune tolerance, but randomized trials will be necessary to confirm its efficacy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health National Cancer Institute grants RO1CA183559, RO1CA183560, and PO1CA142106; Leukemia & Lymphoma Society grant 6005-12; Prometheus Laboratories Inc; and the Ted and Eileen Pasquarello Research Fund.
Authorship
Contribution: J.K., H.T.K., B.R.B., J.H.A., J.R., and R.J.S. conceived and designed the study; J.K. wrote the protocol and is study principal investigator; J.K., E.P.A., P.A., C.S.C., V.T.H., Y.-B.C., D.A., J.H.A., and R.J.S. provided patients; J.K., C.G.R., M.J.C., K.D., J.W., S.N., J.R., K.T.J., and P.B.L. collected and assembled data; H.T.K. analyzed and interpreted data and performed statistical analysis; and all authors wrote the manuscript.
Conflict-of-interest disclosure: J.K. reports research funding from Prometheus Laboratories Inc, Otsuka Pharmaceuticals Inc, and Millennium Pharmaceuticals Inc; honoraria from Miltenyi Biotec GmbH; and advisory board fees from Takeda Pharmaceuticals Inc and Kadmon Corp. The remaining authors declare no competing financial interests.
Correspondence: John Koreth, Dana-Farber Cancer Institute, D2029, 450 Brookline Ave, Boston, MA 02215; e-mail: john_koreth@dfci.harvard.edu.