In this issue of Blood, Koreth et al have reported that interleukin-2 (IL-2) is effective in chronic graft-versus-host disease (cGVHD) patients who had failed up to two prior lines of therapy.1
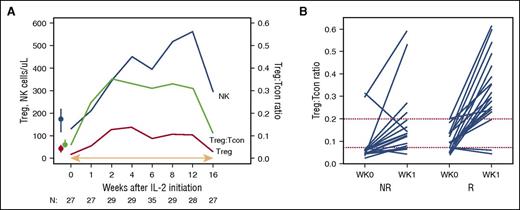
IL-2 induces both Treg expansion and an increase in the median Treg:Tcon ratio, which subsequently decreases when IL-2 therapy is withdrawn. NR, nonresponder; R, responder. See Figure 2A-B in the article by Koreth et al that begins on page 130.
IL-2 induces both Treg expansion and an increase in the median Treg:Tcon ratio, which subsequently decreases when IL-2 therapy is withdrawn. NR, nonresponder; R, responder. See Figure 2A-B in the article by Koreth et al that begins on page 130.
This study extends their previous report, which showed that IL-2 was safe and was associated with response in cGVHD.2 In the current study, 61% of patients responded as measured by the National Institutes of Health (NIH) cGVHD criteria, although no complete responses were achieved. A substantial proportion of patients were able to taper steroids when continuing on IL-2 after the 12-week initial study. Furthermore, for those continuing on treatment, responses were durable and IL-2 was given safely for up to 2 years.
This trial is important because effective therapy for cGVHD is a huge unmet medical need. Although the incidence of cGVHD has been increasing,3 reflecting the popularity of peripheral blood stem cells over bone marrow grafts, effective therapies are lacking. Initial treatment of cGVHD, usually consisting of steroids with or without a calcineurin inhibitor, is effective in only 50% of patients. There is no established second line therapy, with therapies being chosen based on availability, logistic concerns (eg, access to extracorporeal photopheresis), and side effect profiles.4,5 The majority of trials are phase 2 studies, with few randomized trials. Another challenge is a lack of clear understanding of the pathophysiology of cGVHD, the initiating events, immunologic changes as the disease progresses, and tolerance induction when cGVHD resolves.
The main conclusion of this paper is that by increasing the proportions of regulatory T cells (Tregs), as well as natural killer (NK) cells, in patients early in the course of cGVHD, IL-2 may reverse cGVHD manifestations and reduce progression (see figure). This is reflected in the study being successful as it reached the primary end point of an overall response rate >40% at 12 weeks. Responders also showed a trend toward better survival, which supports the validity of the response measure used here (NIH consensus guidelines).
This report is novel in that it reports on both the short- and long-term tolerability and efficacy of IL-2 in cGVHD, in a prospective, well-planned, and well-analyzed therapy trial. These findings both confirm and extend upon the previously reported mechanism of action of IL-2. This therapy induces both Treg expansion and an increase in the median Treg:Tcon (CD4 T cell) ratio, which decreases when IL-2 therapy is withdrawn. Additionally, a Treg:Tcon threshold ratio of ≥0.07 at baseline was a predictor of IL-2 clinical response, reported in the previous IL-2 phase 1 study and confirmed in this study. This baseline ratio thus identifies patients who are most likely to benefit from this therapy. Patients who are more recently diagnosed with cGVHD and younger in age are also more likely to respond; this suggests that increasing the Treg:Tcon ratios early on in cGVHD therapy provides a mechanism for improvement, but other immunologic pathways are involved later in the course of the disease. As for the age of the patients, it is known that Treg expansion is not as robust in older patients, most likely a reflection of decreased thymus function with age.6 This suggests that limiting this therapy to younger patients is appropriate.
Patients receiving IL-2 were allowed to be on additional immunosuppressive therapies, and those enrolled on continued treatment had steroids and other immunosuppression tapered as tolerated. Although it is a common strategy to layer treatments, having a control arm without IL-2 but the same immunologic monitoring of the Treg:Tcon ratio is important in future studies, so as to fully evaluate the benefit of this therapy. This is especially important given the observation of cGVHD “burnout,” in which cGVHD manifestations resolve with time, with eventual discontinuation of systemic immunosuppression.7,8
Patients taking concomitant sirolimus or calcineurin inhibitors were excluded from this study due to an increased risk of thrombotic microangiopathy. Because these two treatments are commonly used in initial therapy, this exclusion raises the question of broad application of IL-2 in cGVHD. In addition, the effect of IL-2 on ocular or oral cGVHD was not captured in this study, due to the use of topical therapy. Thus, the effect of IL-2 on these two organs is unknown.
Outstanding issues include the feasibility of daily, subcutaneous IL-2 injections for as long as 2 years. Quality of life and functional status after transplant and economic costs are all important concerns in this patient population. Patients who have already gone through a stem cell transplant may not be willing to be on long-term daily injections and the optimal duration of therapy has not been defined. Impact on quality of life would need to be the subject of additional study, ideally in a double-blinded randomized clinical trial.
Although this report compellingly establishes and describes low-dose IL-2 therapy as a therapeutic option for cGVHD patients who failed steroids, how IL-2 fits into the cGVHD treatment algorithm (especially with the B-cell–directed therapies) remains an unanswered question. The authors must be commended for executing this research because well-planned prospective therapy trials in cGVHD are scarce. Why more patients are not enrolled in cGVHD clinical trials is an important question for the transplant community.
The opinions expressed herein are those of the authors and do not represent the official position of the NIH or the US government.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal