Abstract
The dramatic success of tyrosine kinase inhibitors (TKIs) has led to the widespread perception that chronic myeloid leukemia (CML) has become another chronic disease, where lifelong commitment to pharmacologic control is the paradigm. Recent trials demonstrate that some CML patients who have achieved stable deep molecular response can safely cease their therapy without relapsing (treatment free remission [TFR]). Furthermore, those who are unsuccessful in their cessation attempt can safely re-establish remission after restarting their TKI therapy. Based on the accumulated data on TFR, we propose that it is now time to change our approach for the many CML patients who have achieved a stable deep molecular response on long-term TKI therapy. Perhaps half of these patients could successfully achieve TFR if offered the opportunity. For many of these patients ongoing therapy is impairing quality of life and imposing a heavy financial burden while arguably achieving nothing. This recommendation is based on the evident safety of cessation attempts and TFR in the clinical trial setting. We acknowledge that there are potential risks associated with cessation attempts in wider clinical practice, but this should not deter us. Instead we need to establish criteria for safe and appropriate TKI cessation. Clinical trials will enable us to define the best strategies to achieve TFR, but clinicians need guidance today about how to approach this issue with their patients. We outline circumstances in which it would be in the patient's best interest to continue TKI, as well as criteria for a safe TFR attempt.
Introduction
As the era of up-front stem cell transplantation for chronic phase chronic myeloid leukemia (CML) came to an end, it was proposed that lifelong imatinib treatment might offer an “operational cure,” durable freedom from disease progression and disease-related symptoms and signs. A decade later, the field has moved on to the more ambitious goal of operational cure without the need for continuing tyrosine kinase inhibitor (TKI) treatment. In doing so, we must always bear in mind that patients with a good response to TKI treatment should not be exposed to unnecessary risk. Stopping effective treatment has until now been largely confined to the clinical trial setting. In this article, we acknowledge that many patients with a sustained deep molecular response would stop their TKI if they could do so safely, and we discuss patient selection criteria and institutional requirements for the provision of safe monitoring and reinstitution of TKI treatment outside a clinical trial.
Success of TKI therapy
The introduction of imatinib, and subsequent TKIs, for the treatment of CML in the early chronic phase has been one of the great success stories of modern medicine. The natural history of CML has been dramatically altered, so that patients who might once have died of progressive disease within 5 to 7 years of diagnosis are now more likely to die of other causes than from CML.1 Results from the United States Surveillance, Epidemiology, and End Results (SEER) database from the period 2000 to 2005 showed that the proportion of deaths in CML patients from cancers other than CML slightly exceeded those attributed to CML (36.1% vs 34.9%, respectively), with a further 29% of deaths from nonmalignant causes.1
There has been a corresponding increase in the overall survival of CML patients. Chronic phase CML patients treated with TKIs in clinical trials in the MD Anderson Cancer Center since 2000 had a 5-year overall survival estimated at 94.7% relative to the general population of the United States.2 Results from clinical trials in centers of excellence are frequently better than “real world” results, but population-based registries also confirm the improvement in survival since the introduction of TKIs. Based on data primarily from the first half of the decade in which TKIs were introduced, the 5-year relative survival was ∼70%, in comparison with relative survival as low as 10% to 20% a decade earlier when interferon (IFN)-α or allogeneic stem cell transplantation was the standard of care.3 More recent data are likely to be better still: in the same population-based study, the 5-year relative survival for CML patients diagnosed in 2002 was 63% and increased to 80% for patients diagnosed in 2006.
Assuming that the incidence of CML is relatively constant, the prevalence of the disease is dependent on patient survival. In fact, the incidence is likely increasing in most developed countries where the median age of the population is increasing. Considering the near-normal relative survival of CML patients, the number of patients living with CML is rising dramatically. One study projected that the peak prevalence of CML will be reached around the year 2050, with ∼180 000 CML patients living in the United States at that time (an approximate ninefold increase since the year 2000).4 In this context, the provision of lifelong TKI treatment is a substantial and growing financial burden for CML patients and health funding agencies. Generic imatinib is now available in some countries, but TKIs remain unaffordable for many patients, especially those for whom imatinib is not the preferred choice.
Deep molecular response
The phase 3 International Randomized Study of Interferon and STI571 (IRIS) trial compared imatinib with IFN plus cytarabine and showed a significant improvement in progression-free survival for patients randomized to the TKI, even though the majority of patients in the IFN arm crossed over to imatinib.5 This was accompanied by unprecedented rates of cytogenetic response: a complete cytogenetic response was observed in 8.5% of patients treated with IFN vs 73.8% of patients treated with imatinib. The level of minimal residual disease (MRD) achieved in chronic phase CML patients is so much better with TKI treatment that, in the great majority of patients, cytogenetic analysis is no longer informative after the first 6 months of TKI treatment.6 This therapeutic success ultimately led to the widespread adoption of molecular monitoring by sensitive real-time quantitative reverse transcriptase-polymerase chain reaction for Breakpoint Cluster Region-Abelson murine leukemia viral oncogene homolog 1 (BCR-ABL) transcripts (RQ-PCR). In turn, the goals of treatment are increasingly based on molecular definitions, as outlined in the respective guidelines of the European Leukemia Net7 and the National Comprehensive Cancer Network (available at www.nccn.org).
In the first 2 years of TKI treatment, the achievement of a major molecular response (MMR; BCR-ABL ≤0.1%) is a primary goal of treatment, because it is associated with very high rates of progression-free survival.8 Many patients achieve MMR in the first 6 to 12 months of treatment, but a large fraction of these patients will have a further reduction in the level of MRD with continuing TKI treatment, leading to progressively deeper molecular responses and even undetectable MRD (UMRD).9 The level of MRD that is undetectable is determined by the lower limit of detection of the assay in use. PCR-based methods vary in efficiency from run to run, as can RNA extraction. Sample quality may also vary, especially if there is a delay from collection to RNA stabilization. This inherent variability makes it difficult to standardize UMRD. Consequently, there has been a move away from reporting UMRD to reporting a fixed level of molecular response.10 The lower limit of detection of RQ-PCR currently averages around MR4.5 (BCR-ABL ≤0.0032%), so that UMRD and MR4.5 are approximately equivalent in many laboratories today. MR4.5 includes both patients with detectable BCR-ABL ≤0.0032% and patients with undetectable BCR-ABL in a sample with a calculated detection limit of ≥4.5 logs below standardized baseline.
MR4.5 occurs in ∼20% of imatinib-treated patients in the first 2 to 3 years, but this proportion rises to ∼40% after 5 to 7 years.9 This progressive slow recruitment of patients to MR4.5 raises the possibility that with very prolonged treatment, the level of MRD may continue to fall, as long as effective treatment is continued. Using more potent TKIs, such as nilotinib and dasatinib, the rates of MR4.5 in the first 2 years are higher,11,12 and it is hoped that this will ultimately lead to even higher proportions of patients with MR4.5. In the phase 3 Evaluating Nilotinib Efficacy and Safety in Clinical Trials newly diagnosed (ENESTnd) study, the rate of MR4.5 by 4 years was 40% with nilotinib 300 mg twice daily vs 23% with imatinib 400 mg daily.11 In the Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients (DASISION) study comparing dasatinib 100 mg with imatinib 400 mg daily, MR4.5 was achieved by 3 years in 22% vs 12% of patients, respectively.12
Problems with ongoing TKI therapy
A truly successful chemotherapy is one that is given for a finite period of time and is then no longer needed. This is a paradigm that most people are familiar with, following the example of cytotoxic chemotherapy for acute leukemia or Hodgkin lymphoma. For many CML patients, the chronic illness that they are aware of is not CML, but a syndrome of TKI side effects. In our experience, most patients who have achieved a deep molecular response eventually ask whether they can stop their TKI.
A study of patients treated with several TKIs found that approximately one-third of patients experienced moderate to severe TKI-related side effects.13 Importantly, the severity of TKI side effects tended to remain relatively constant over time. A study undertaken in imatinib-treated patients showed impaired physical and mental health status in patients <60 years of age, whereas older CML patients on average had a health status comparable to age-matched controls.14
In addition to these chronic TKI toxicities, there is growing awareness of other uncommon, but potentially serious, risks of long-term TKI treatment that may emerge after months or years. These risks include pleural effusion and pulmonary hypertension with dasatinib15 and vascular events with nilotinib.16 Imatinib appears to be relatively free of such late toxicities, but an accelerated decline in glomerular filtration rate has been reported.17 None of the TKIs are recommended for patients trying to conceive or during pregnancy or lactation.
Treatment-free remission: current status
More than 20 years ago, it was reported that some CML patients treated with IFN could remain in stable cytogenetic remission after their IFN was stopped, despite having detectable BCR-ABL by reverse transcriptase-PCR.18 In 2002, Mahon and colleagues reported on a small number of patients with UMRD during IFN treatment, who stopped IFN and remained in molecular remission.19 Patients in that study who had achieved MMR, but not UMRD, all showed molecular relapse. The maintenance of UMRD, or MRD detectable at a stable low level, without the need for ongoing treatment is commonly termed a treatment-free remission (TFR). The French CML Intergroup went on to report TFR in the Stop Imatinib (STIM) study in ∼40% of 100 imatinib-treated patients who had maintained UMRD for ≥2 years.20 In this study, half of the patients had received prior therapy with IFN, and the remainder were treated de novo with imatinib. The Australasian Leukaemia & Lymphoma Group Trial of Withdrawing Imatinib in Stable Remission (TWISTER) study reported almost identical results in 40 patients with similar clinical characteristics.21 Both studies reported a higher TFR rate in patients with prior IFN exposure, although this observation is potentially confounded by a selection bias, because high-risk patients may have been unlikely to remain on IFN for long enough to benefit from the switch to imatinib. Both studies reported higher TFR rates in Sokal low-risk patients, although this difference was not statistically significant in the smaller Australian study. Both studies also reported that most patients regained UMRD within 3 to 6 months of restarting imatinib treatment in response to a conservative RQ-PCR–defined trigger. In TWISTER, this trigger was loss of UMRD with any 2 consecutive positive RQ-PCR results. In STIM, the trigger also included a requirement for a significant (10-fold) rise in BCR-ABL between the first and subsequent RQ-PCR measurements.
A follow-up study (A-STIM; According to STIM) from the French group enrolled 80 patients, again with a similar mix of de novo patients and prior IFN treatment.22 A-STIM tested the use of a less stringent definition of molecular relapse as the trigger to restart therapy. Patients who lost UMRD remained under close observation, and imatinib was restarted only at loss of MMR. This enabled the investigators to determine the TFR rate using both the original STIM definition and the newer MMR-based definition. The TFR rate using the STIM definition was close to that of the 2 earlier studies, but a higher proportion of patients (64% at 2 years) maintained MMR without treatment. In contrast to the original STIM study, no effect of Sokal score was seen. Whether this difference is related to the relatively small numbers of high-risk patients or whether the effect of Sokal score differs between “grades” of TFR (UMRD vs MMR) is unclear. Remarkably, some patients in MMR with detectable BCR-ABL mRNA have maintained a stable level of response for periods in excess of 2 years, whereas the doubling time of BCR-ABL without treatment (<14 days) is normally such that cytogenetic or hematologic relapse would have been expected.23 The biology underlying this observation is an area of active study.
The large, multinational European Stop Kinase Inhibitor study (EuroSKI) study is enrolling patients treated with multiple TKIs, and uses both a less stringent entry criterion of MR4.0 for ≥12 months and the less stringent retreatment trigger: loss of MMR. At the time of the latest abstract, ∼480 patients had been enrolled, but outcome data were reported for only the first 100 patients with ≥6 months of follow-up.24 At this early time point, the TFR (MMR) rate was similar to A-STIM, but these results require confirmation with longer follow-up.
Updated results of the Korean Imatinib Discontinuation Study (KIDS) were recently published on a cohort of 82 patients who had received up-front imatinib (and 8 patients with prior IFN).25 Fifty-nine percent of the 90 patients remained in MMR at 2 years. The French group has reported interim results of the STIM2 study in abstract.26 This study is enrolling only patients treated de novo with imatinib. The preliminary analysis showed a TFR (MMR) rate that was similar to KIDS and A-STIM at ∼60%.26 First-line TKI treatment represents the bulk of contemporary patients, and studies in first-line patients will give bring the opportunity to study the effect of Sokal score and imatinib response kinetics without the confounding effects of prior IFN treatment. The key inclusion criteria used in TFR studies to date and the respective triggers for restarting TKI therapy are summarized in Table 1.
Summary of key inclusion criteria and TKI retreatment triggers used in clinical trials
| Study . | TKI . | Number of patients reported . | Required depth of MR . | Minimum duration of MR (years) . | Trigger to resume TKI . |
|---|---|---|---|---|---|
| STIM20 | Imatinib (±prior IFN) | 100 | UMRD (MR5.0) | 2 | Loss of UMRD* |
| TWISTER21 | Imatinib (±prior IFN) | 40 | UMRD (MR4.5) | 2 | Loss of UMRD |
| A-STIM22 | Imatinib (±prior IFN) | 80 | UMRD† | 2 | Loss of MMR |
| EuroSKI24 | Imatinib/nilotinib/dasatinib | 200 | MR4.0 | 1 | Loss of MMR |
| Stop 2GTKI28 | Nilotinib/dasatinib first and second line | 52 | UMRD (MR4.5) | 2 | Loss of MMR |
| KIDS25 | Imatinib (±prior IFN) | 90 | UMRD (MR4.5) | 2 | Loss of MMR |
| HOVON32 | Imatinib | 18 | UMRD (MR4.5) | 2 | Loss of UMRD |
| DADI27 | Second-line dasatinib | 63 | MR4.0 | 1 | Loss of MR4.0 |
| STIM226 | Imatinib | 124 | UMRD (MR4.5) | 2 | Loss of UMRD |
| Study . | TKI . | Number of patients reported . | Required depth of MR . | Minimum duration of MR (years) . | Trigger to resume TKI . |
|---|---|---|---|---|---|
| STIM20 | Imatinib (±prior IFN) | 100 | UMRD (MR5.0) | 2 | Loss of UMRD* |
| TWISTER21 | Imatinib (±prior IFN) | 40 | UMRD (MR4.5) | 2 | Loss of UMRD |
| A-STIM22 | Imatinib (±prior IFN) | 80 | UMRD† | 2 | Loss of MMR |
| EuroSKI24 | Imatinib/nilotinib/dasatinib | 200 | MR4.0 | 1 | Loss of MMR |
| Stop 2GTKI28 | Nilotinib/dasatinib first and second line | 52 | UMRD (MR4.5) | 2 | Loss of MMR |
| KIDS25 | Imatinib (±prior IFN) | 90 | UMRD (MR4.5) | 2 | Loss of MMR |
| HOVON32 | Imatinib | 18 | UMRD (MR4.5) | 2 | Loss of UMRD |
| DADI27 | Second-line dasatinib | 63 | MR4.0 | 1 | Loss of MR4.0 |
| STIM226 | Imatinib | 124 | UMRD (MR4.5) | 2 | Loss of UMRD |
DADI, Dasatinib Discontinuation study; HOVON, Stichting Hemato-Oncologie voor Volwassenen Nederland.
A-STIM allowed occasional low level positive RQ-PCR results during the 2 years of UMRD.
Loss of UMRD in STIM and STIM2 was defined as ≥2 consecutive samples with detectable BCR-ABL and a 1-log increase; in TWISTER and the HOVON study, any 2 consecutive samples at any level were considered loss of UMRD.
Data on TFR after more potent TKIs are currently very limited, but major studies are underway with both nilotinib and dasatinib. The first prospective TFR study with dasatinib was recently reported from Japan. The DADI study enrolled 63 patients treated with dasatinib second line after imatinib.27 Most of the patients switched due to intolerance or “patient preference,” with only 21% having European Leukemia Net suboptimal response to imatinib or treatment failure. Using loss of MR4.0 as the trigger to restart dasatinib, Imagawa and colleagues reported a TFR rate of 58% among those patients switching to dasatinib due to intolerance or patient preference. The TFR rate was only 8% in those with suboptimal response to imatinib. A lower rate of TFR for patients on a second-line TKI after suboptimal imatinib response was also seen in the preliminary results of the Stop 2GTKI study.28 The negative effect of prior suboptimal response was less striking in that study with TFR (MMR) sustained at 12 months in 42% of patients switching for inadequate response vs 67% of the remaining patients.
The minimum duration of TKI therapy required for inclusion in several TFR clinical trials has been as short as 3 years, but it is important to be aware that very few patients have successfully discontinued therapy after such a short time. The median duration of total TKI therapy was typically ∼5 to 8 years in the studies mentioned above. In the large EuroSKI study, only 16% of patients had <5 years of prior TKI treatment.24 A longer duration of TKI treatment has been associated with a higher probability of maintaining TFR: this difference was statistically significant in some studies20,24,29 but not all.27,30 Yet, other studies have shown no effect of total TKI duration.21,22,31,32 These were all retrospective analyses, differing in treatment history or molecular response/relapse definitions and often with relatively small numbers of patients. It is not possible to draw firm conclusions from these data, but there is a suggestion that attempting TFR after a shorter duration of TKI treatment may reduce the likelihood of a successful outcome. This observation leads to several clinically important questions. Is there an optimum duration of TKI treatment prior to attempting TFR, beyond which there is little or no further improvement in the probability of maintaining TFR? If so, it is likely that this duration will vary between patients, and there is a need for clinical or laboratory biomarkers that may improve prediction of TFR. Second, if a patient stops therapy “prematurely” (when he or she might have maintained TFR after several years of additional TKI therapy), has that person’s longer-term chance of TFR been compromised by the initial failed attempt at TFR?
Risks associated with a TFR attempt
For a patient in deep molecular remission continuing on TKI therapy, the risk of experiencing disease progression and dying from CML is negligible. In this context, any excess risk of CML progression is unacceptable in a patient without significant TKI toxicity. From all of the published reports of TFR to date, involving >1000 patients, we are aware of only 1 such case. This patient was not typical of the contemporary CML population and had started imatinib 10 years after her original diagnosis of CML. She lost MMR after stopping imatinib and was responsive to retreatment, but 8.5 months later developed blast crisis.22
An imatinib withdrawal syndrome consisting of diffuse myalgia/arthralgia may be encountered. In most cases, this syndrome can be managed with simple analgesia or nonsteroidal anti-inflammatory drugs. It can persist for months but is usually self-limiting.33 Occasional cases are severe enough to warrant prednisolone treatment. There are numerous reports of improvement in inflammatory arthritis during TKI therapy, and we encountered 1 patient in whom a TKI “withdrawal syndrome” turned out to be a flare of undiagnosed rheumatoid arthritis that was presumably masked by TKI treatment. The KIDS investigators reported that patients with an imatinib withdrawal syndrome had a higher probability of maintaining TFR.25
An issue that has not been formally explored is the psychological impact of being off TKI therapy. Some patients might experience significant anxiety relating to concerns of relapse, and almost 50% of patients in an Italian survey said that they would be afraid of losing their disease response.34 It is also possible that patients who are offered a TFR attempt might misinterpret this as permission to reduce or omit doses.
Which patients should not be considered for a TFR attempt?
There are rare CML patients, ∼2% to 3% of all newly diagnosed cases, with atypical BCR-ABL transcripts that cannot be monitored on the BCR-ABL International Scale. For these patients, clinically important RQ-PCR end points, such as MMR or MR4.5, cannot be measured with certainty. Therefore, these patients are not suitable candidates for a TFR attempt. Similarly, there are occasional patients whose original BCR-ABL transcript type at diagnosis is not known (eg, Ph-positive patients who did not have RQ-PCR early in treatment). There is a small risk that such a patient has atypical transcripts, where an apparent low level of MRD will be misleading. These patients may not be suitable to attempt TFR.
In patients with a prior history of TKI resistance, it is clear that the probability of maintaining TFR is lower, and patients should be made aware of this when discussing the option of TFR. A TFR attempt may still be appropriate in such patients if their quality of life is impaired by significant TKI toxicity. Almost all of the statements made about TFR are referring to adult chronic phase CML patients with no prior history of accelerated phase or blast crisis. In patients who have achieved a second chronic phase, we would be very reluctant ever to stop TKI outside of a clinical trial.
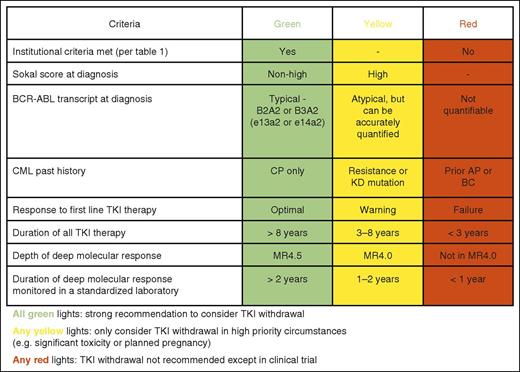
Given the rarity of CML in childhood, there are only very limited reports of TFR in children.35,36 The available information is concordant with the adult experience, and we would not preclude children from attempting TFR. However, this would be an area where clinical trials would be especially important and should always be considered the first priority when considering a TKI withdrawal attempt. Children and adolescents face special issues related to growth, self-image, and fertility, and because their lifelong duration of treatment will be so much greater, their potential for TKI toxicity must be greater. Advanced age is not a contraindication to TFR, and age does not appear to be a risk factor for molecular relapse. It is reported that the impairment of quality of life in older patients on TKIs is less than in younger patients,14 and therefore the incentive to attempt TFR may be less in older patients. Conversely, older patients may be at higher risk of specific TKI toxicities, such as pleural effusion and arterial thrombosis, and these risks need to be taken into account. Some of the key factors that determine a patient’s suitability for a TFR attempt are summarized in Figure 1.
Criteria to guide selection of patients suitable for a TFR attempt. KD, kinase domain; AP, accelerated phase; BC, blast crisis.
Criteria to guide selection of patients suitable for a TFR attempt. KD, kinase domain; AP, accelerated phase; BC, blast crisis.
How should TKI withdrawal attempts be managed?
The provision of timely RQ-PCR results is crucial to the safety of patients undergoing a TFR attempt. Most patients failing a TFR attempt do so with an exponential increase in BCR-ABL, rising ∼1 log per month. Consequently, there is a need for results to be available rapidly so that they can be acted on. The sensitivity of RQ-PCR testing is also crucial, both for assessing eligibility (confirming deep molecular response) and to detect impending relapse as soon as possible. Because most relapses occur within 6 months of stopping TKI, we would recommend close monitoring during that time. If high-quality RQ-PCR is not available or cannot be provided frequently and promptly, then a TFR attempt cannot be undertaken safely. It is also important that the RQ-PCR assay has been standardized and expressed on the International Scale so that the values of MR4.5 and MMR can be reliably determined.37 Institutional requirements for TFR are summarized in Table 2. Patients who are unwilling or unable to comply with frequent monitoring should not be offered a TFR attempt.
Institutional requirements for safe supervision of TFR
| 1. Availability of high quality internationally standardized, accurate, sensitive RQ-PCR laboratory |
| 2. Rapid turn-around of RQ-PCR test results, within 4 weeks |
| 3. Capacity to provide RQ-PCR tests every 4-6 weeks, when required |
| 4. Structured follow-up established to enable rapid intervention if BCR-ABL is rising |
| 1. Availability of high quality internationally standardized, accurate, sensitive RQ-PCR laboratory |
| 2. Rapid turn-around of RQ-PCR test results, within 4 weeks |
| 3. Capacity to provide RQ-PCR tests every 4-6 weeks, when required |
| 4. Structured follow-up established to enable rapid intervention if BCR-ABL is rising |
For patients remaining in TFR beyond the first 6 months, the risk of relapse is lower, and less frequent testing, eg, every 2 to 3 months, may be sufficient. The question of how long to continue monitoring is more difficult to answer. The latest reported loss of TFR was at 42 months, so there is clearly a need for monitoring beyond the first few years. In the CML allograft setting late relapses (beyond 5 years) do occur, averaging ∼0.5% to 1% per year.38 At present, the number of TFR patients with reported follow-up in excess of 5 years is so small that we cannot reliably estimate the risk of late relapse. Until such information is available, we would recommend indefinite RQ-PCR monitoring every 3 months.
Where the criteria for a safe TFR attempt are met (see Figure 1), the first step toward TKI withdrawal is a detailed conversation with the patient to ensure that he or she is fully informed of the potential risks and benefits and aware of the need for close follow-up. To minimize unnecessary anxiety, it is important to explain that molecular relapse is completely different from hematologic relapse and that clinically relevant consequences of molecular relapse are rare. Some vignettes to illustrate issues in the decision-making process are presented in Table 3.
Informed decision-making for TFR candidates: practical examples
| Case summary . | Recommendation . |
|---|---|
| Case 1: A 58-year-old woman has been taking imatinib 400 mg daily for 10 years. She has ongoing mild myalgia and arthralgia, but overall she reports that her quality of life is as good as that of many of her friends. She has maintained UMRD for 8 years. | In favor of a cessation attempt. Informed that there is a >50% likelihood that TFR could be achieved, but that the risks of stopping need to be weighed carefully against the risks of continuing, which in this case were very low. She decided not to stop TKI at this stage, saying that imatinib had been a normal part of her life for many years. Stopping imatinib would mean having more frequent blood tests, which she saw as an inconvenience with no meaningful gain. She was worried about the hypothetical risk that a period off therapy might lead to a loss of sensitivity to imatinib, arguing that even if this risk is small it is a risk that she need not take. |
| Case 2: A 78-year-old man treated with CML on imatinib 400 mg daily for 6 years. He has never achieved UMRD, but has maintained MR4.0 for the last 2 years. He is considering TKI cessation because of side effects (diarrhea and anemia). | In favor of a cessation attempt. Chances of TFR may be <50%, because he has received <8 years of TKI therapy and has only achieved MR4.0. However, he has substantial side effects, which in this case would be sufficient justification for a cessation attempt. Counseling here is important so that he understands the high probability that TKI withdrawal will not be successful. |
| Case 3: A 31-year-old woman with CML diagnosed 4.5 years ago. She received imatinib 600 mg daily for 2 years and then switched to nilotinib due to failure to achieve MMR. She rapidly achieved MR4.5 on nilotinib, which she has now maintained for 2 years. | Against proceeding with a cessation attempt at this stage. Recommended persisting with nilotinib for another 2 years and then consider a cessation attempt. The probability of TFR at this stage given the poor response to imatinib and the relatively short total duration of TKI is likely to be <50%. A premature attempt may significantly delay the time when she can successfully achieve TFR. Recognizing that fertility declines rapidly past the age of 30, there is a significant risk that the patient will go ahead without physician support and adequate monitoring. Some clinicians would stop TKI and use IFN as maintenance therapy in this situation. |
| Case summary . | Recommendation . |
|---|---|
| Case 1: A 58-year-old woman has been taking imatinib 400 mg daily for 10 years. She has ongoing mild myalgia and arthralgia, but overall she reports that her quality of life is as good as that of many of her friends. She has maintained UMRD for 8 years. | In favor of a cessation attempt. Informed that there is a >50% likelihood that TFR could be achieved, but that the risks of stopping need to be weighed carefully against the risks of continuing, which in this case were very low. She decided not to stop TKI at this stage, saying that imatinib had been a normal part of her life for many years. Stopping imatinib would mean having more frequent blood tests, which she saw as an inconvenience with no meaningful gain. She was worried about the hypothetical risk that a period off therapy might lead to a loss of sensitivity to imatinib, arguing that even if this risk is small it is a risk that she need not take. |
| Case 2: A 78-year-old man treated with CML on imatinib 400 mg daily for 6 years. He has never achieved UMRD, but has maintained MR4.0 for the last 2 years. He is considering TKI cessation because of side effects (diarrhea and anemia). | In favor of a cessation attempt. Chances of TFR may be <50%, because he has received <8 years of TKI therapy and has only achieved MR4.0. However, he has substantial side effects, which in this case would be sufficient justification for a cessation attempt. Counseling here is important so that he understands the high probability that TKI withdrawal will not be successful. |
| Case 3: A 31-year-old woman with CML diagnosed 4.5 years ago. She received imatinib 600 mg daily for 2 years and then switched to nilotinib due to failure to achieve MMR. She rapidly achieved MR4.5 on nilotinib, which she has now maintained for 2 years. | Against proceeding with a cessation attempt at this stage. Recommended persisting with nilotinib for another 2 years and then consider a cessation attempt. The probability of TFR at this stage given the poor response to imatinib and the relatively short total duration of TKI is likely to be <50%. A premature attempt may significantly delay the time when she can successfully achieve TFR. Recognizing that fertility declines rapidly past the age of 30, there is a significant risk that the patient will go ahead without physician support and adequate monitoring. Some clinicians would stop TKI and use IFN as maintenance therapy in this situation. |
Areas of future investigation
Several studies of IFN in combination with TKI have been completed in recent years or are in progress. Rates of deep molecular response in 2 independent studies in France and Scandinavia were higher in the pegylated IFN-imatinib combination arm than in the standard imatinib arm,39,40 but no such difference was seen in the equivalent arms of the German CML IV study.41 TFR rates for patients achieving deep molecular response with combination therapy have not yet been reported. These data may help to clarify whether a finite period of IFN exposure does have a durable effect on the probability of TFR.
Approximately 40% to 60% of patients with a deep molecular response stopping TKI will require retreatment and regain a deep molecular response. What hope is there for a second attempt at TFR? There is a report on the outcome of a small number of patients from the French TFR studies attempting TFR for a second time.42 The duration of UMRD prior to the second attempt was no longer than the period of UMRD prior to the first attempt, yet ∼20% of these patients remained in TFR at the time of the report. This is another surprising observation: the TKI and the depth of molecular response were unchanged, yet the outcome was different. Enabling patients to have a second attempt at TFR is likely to become an active area of investigation. The rational selection of alternative (or additional) therapies will depend on an improved understanding of the biological factors that determine TFR.
The divergent outcomes of patients in TFR studies with similar levels of minimal residual disease argue that additional biological factors have a major impact on outcome. Some of the most promising research into this question has been in the field of immunology. In several studies, natural killer (NK) cell numbers (or functional subsets of NK cells) have been shown to be higher in patients who sustain TFR than in those who experience molecular response.27,43,44 Immunologic anergy may be a feature of CML, and diverse immunologic effector responses including T-cell cytotoxicity and NK-cell effects may be involved.45,46 Which of these markers of immune function is most relevant is not yet clear, and none of these studies have yet demonstrated that an immunologic end point has positive predictive value for subsequent TFR, independent of clinical parameters. The availability of a biomarker that accurately predicts a very high or very low probability of TFR would be a significant step forward.
Enormous progress has been made in the quantification of BCR-ABL transcripts by RQ-PCR, but the measurement error of any PCR assay is increased by stochastic effects at low levels of the target, and a more sensitive assay could mitigate this effect. In addition, we and others have compared the quantification of BCR-ABL in genomic DNA and RNA in serial samples and have shown that individual patients may have consistently higher or lower expression of BCR-ABL mRNA for a given number of CML cells.47,48 This interindividual difference results in a measurement bias whereby RQ-PCR may underestimate the level of residual disease in some patients. Although genomic Q-PCR for BCR-ABL is not widely available, it is a promising research tool to elucidate the relationship between MRD and TFR outcome.
Not if, but when and how
In our opinion, the safety data from the TFR studies reported to date are sufficiently reassuring that we feel comfortable in offering all eligible patients a supervised test of TKI withdrawal. The optimum eligibility criteria are open to debate, but the available data suggest that patients should have a minimum of 12 months of deep molecular response with MR4.0 or better. A history of prior TKI resistance should be viewed as a relative contraindication to attempting TFR, and such patients should be aware of their higher risk of relapse before making a decision. There is some evidence that a longer duration of treatment/deep molecular response is associated with a higher probability of TFR, but there must be a practical balance between the additional burden of prolonged treatment and the potential to improve the TFR rate. There are still many unanswered questions concerning TFR, so eligible patients should be strongly encouraged to participate in clinical trials where possible. The absence of a suitable trial should not preclude a patient from stopping TKI treatment, but outside the structure of a clinical trial, it would be useful to have consensus recommendations to guide those clinicians for whom TFR represents a new area of practice.
We proposed some criteria for patient selection and some criteria for institutional capability to supervise a TFR attempt, primarily access to timely and accurate standardized RQ-PCR results. If all of these criteria can be met, then we believe that TFR should become a routine part of clinical practice. Unfortunately, in many regions of the world, the availability of quality RQ-PCR is not sufficient, which means that TFR cannot be attempted safely. This poses a dilemma for many clinicians who recognize that some of their patients could otherwise be appropriately offered an attempt at TFR. This represents a new challenge for the global CML community.
Authorship
Contribution: T.P.H. and D.M.R. wrote the paper.
Conflict-of-interest disclosure: T.P.H. has received research funding and honoraria from Novartis, BMS, and Ariad. D.M.R. has received research funding from Novartis and honoraria from Novartis and BMS.
Correspondence: Timothy Hughes, South Australian Health & Medical Research Institute, PO Box 11060, Adelaide, SA 5001, Australia; e-mail: tim.hughes@sahmri.com.