Key Points
Recurrent mutations in chromatin modifiers and cohesin were observed in t(8;21) AML, but not inv(16) AML.
t(8;21) AML patients with mutations in kinase signaling plus chromatin modifiers or cohesin members had the highest risk of relapse.
Abstract
Acute myeloid leukemia (AML) with t(8;21) or inv(16) have been recognized as unique entities within AML and are usually reported together as core binding factor AML (CBF-AML). However, there is considerable clinical and biological heterogeneity within this group of diseases, and relapse incidence reaches up to 40%. Moreover, translocations involving CBFs are not sufficient to induce AML on its own and the full spectrum of mutations coexisting with CBF translocations has not been elucidated. To address these issues, we performed extensive mutational analysis by high-throughput sequencing in 215 patients with CBF-AML enrolled in the Phase 3 Trial of Systematic Versus Response-adapted Timed-Sequential Induction in Patients With Core Binding Factor Acute Myeloid Leukemia and Treating Patients with Childhood Acute Myeloid Leukemia with Interleukin-2 trials (age, 1-60 years). Mutations in genes activating tyrosine kinase signaling (including KIT, N/KRAS, and FLT3) were frequent in both subtypes of CBF-AML. In contrast, mutations in genes that regulate chromatin conformation or encode members of the cohesin complex were observed with high frequencies in t(8;21) AML (42% and 18%, respectively), whereas they were nearly absent in inv(16) AML. High KIT mutant allele ratios defined a group of t(8;21) AML patients with poor prognosis, whereas high N/KRAS mutant allele ratios were associated with the lack of KIT or FLT3 mutations and a favorable outcome. In addition, mutations in epigenetic modifying or cohesin genes were associated with a poor prognosis in patients with tyrosine kinase pathway mutations, suggesting synergic cooperation between these events. These data suggest that diverse cooperating mutations may influence CBF-AML pathophysiology as well as clinical behavior and point to potential unique pathogenesis of t(8;21) vs inv(16) AML.
Introduction
Core binding factor (CBF) acute myeloid leukemia (AML) includes AML with t(8;21)(q22;q22) and inv(16)(p13q22)/t(16;16)(p13;q22) chromosomal rearrangements (abbreviated as t(8;21) as inv(16), respectively), leading to the RUNX1-RUNX1T1 and CBFB-MYH11 fusion genes, respectively. CBF AML is among the most common cytogenetic subtypes of AML because t(8;21) and inv(16) together account for approximately 25% of pediatric and 15% of adult de novo AML patients.1 Their identification is critical in routine practice because the presence of these alterations significantly affects clinical management of AML.2 CBF AML is considered to have a good prognosis relative to other AML subtypes, and treatments using high-dose cytarabine-based chemotherapy have resulted in markedly improved outcome. Nonetheless, relapse occurs up to 40% in such patients, indicating clinical heterogeneity among CBF AML patients.3-6
Since the first description of t(8;21) and inv(16) AML in 19737 and 1983,8 respectively, a great deal has been learned about the molecular consequences of both rearrangements. Both alterations result in disruption of genes encoding subunits of the CBF complex (ie, RUNX1 and CBFB), a heterodimeric transcription factor complex that regulates the expression of genes required for normal hematopoiesis.9,10 Homozygous disruption of Runx1 or Cbfb in mice results in identical developmental defects, including failure to develop definitive hematopoiesis and embryonic death.11 At the same time, experience from murine models has demonstrated that the expression of the RUNX1-RUNX1T1 or CBFB-MYH11 fusion proteins alone induces aberrant self-renewal, but is insufficient to induce fulminant leukemia.11 Consistent with this, preleukemic cells harboring RUNX1-RUNX1T1 or CBFB-MYH11 fusion genes were identified >10 years before clinical development of AML as well as following long-term clinical remission of AML.12,13 CBF AML is therefore considered a model for the multistep pathogenesis of leukemia, in which AML development requires cooperation from disruption of a transcription factor (such as the CBF complex) that impairs differentiation, plus activating mutations that increase proliferation (the “2-hit model” of leukemogenesis).14 Further evidence supporting this model comes from the fact that frequent mutations activating tyrosine kinase (TK) signaling (including KIT, FLT3, and N/KRAS family genes) are frequently observed in both CBF AML subtypes.3
Given the similarities in prognostic features and involvement of CBF transcription factors in their pathogenesis, t(8;21) and inv(16) AML have been recognized as unique entities within AML and are usually grouped and reported together in clinical studies. However, patients with t(8;21) or inv (16) AML differ with respect to several biological and clinical features.15 Morphologically, patients with t(8;21) AML frequently present with the French-American-British morphological subtype M2 or AML with maturation, whereas patients with inv(16) more often are diagnosed with the French-American-British subtype M4Eo or acute myelomonocytic leukemia with abnormal marrow eosinophils.16 Moreover, gene expression profiling of CBF AML segregate t(8;21) and inv(16) patients into distinct subgroups,17 reflecting different pathways activated in each subtype of CBF AML.18 Although a genetic basis for morphologic and transcriptional differences between t(8;21) and inv(16) AML were previously unknown, frequent mutations in ASXL1 and ASXL2 were recently described specifically in t(8;21) AML patients. ASXL1/2 mutations have been described in ∼35% of t(8;21) AML but are absent in inv(16)AML.19,20 Interestingly, ASXL2 mutations are not recurrent in subsets of AML other than t(8;21) AML, suggesting an important potential functional intersection between ASXL2 mutations and the RUNX1-RUNX1T1 fusion specifically.21 Besides ASXL1/2 mutations, however, no other recurrent mutations specific to 1 or both CBF AML subsets are currently known.
Given that (1) up to 40% of patients with CBF AML relapse and that (2) CBF disruption is not sufficient to induce AML on its own, we hypothesized that additional recurrent genetic abnormalities may be enriched in 1 or more subsets of CBF AML patients. Through extensive mutational analysis of a large and well-annotated cohort of CBF AML patients, we identified a series of recurrent genetic alterations in genes encoding epigenetic modifiers and cohesin members with direct relevance specifically in t(8;21) AML. Moreover, we identified additional importance of allelic ratios of mutations affecting TK signaling across CBF AML. These data suggest that t(8;21) and inv(16) AML may have distinct pathophysiology and that comprehensive genetic analysis may be used to refine prognostication in CBF AML.
Methods
Patients and treatments
This study included 215 patients with CBF AML, including 106 with t(8;21) and 109 with inv(16) AML. The cohort included 142 adults treated in the Phase 3 Trial of Systematic Versus Response-adapted Timed-Sequential Induction in Patients With Core Binding Factor Acute Myeloid Leukemia (CBF2006) trial3 (EudraCT 2006 005163-26; ClinicalTrials.gov NCT00428558) as well as 73 children treated in the Treating Patients with Childhood Acute Myeloid Leukemia with Interleukin-2 (ELAM02) trial (ClinicalTrials.gov NCT00149162) (supplemental Figure 1, available on the Blood Web site). Studies were approved by the Ethics Committee of Nimes University Hospital and by the Institutional Review Board of the French Regulatory Agency and were conducted in accordance with the Declaration of Helsinki protocol.
Mutational analysis by high-throughput sequencing
Bone marrow samples from CBF AML patients at diagnosis were studied by high-throughput sequencing (HTS) of 40 genes recurrently mutated in myeloid malignancies. This included genes encoding proteins involved in signal transduction (CBL [exons 8-9], CSF3R [exons 3-18], FLT3 [exons 14-15 + 20], JAK2 [exons 12 + 14], KIT [exons 8-13 + 17], KRAS [exons 2-3], MPL [exon 10], NRAS [exons 2-3], PTPN11 [exons 3 + 13]), transcription (BCOR [exons 2-15], BCORL1 [exons 1-12], CEBPA [exon 1], ETV6 [exons 1-8], GATA1 [exon 2], GATA2 [exons 2-6], IKZF1 [exons 1-8] RUNX1 [exons 1-6]), chromatin modification (ASXL1 [exon 12], ASXL2 [exons 1-12], EZH2 [exons 2-20], KDM6A [exons 1-29], KMT2A [exons 5-8]), DNA methylation (DNMT3A [exons 2-23], IDH1 [exon 4], IDH2 [exon 4], TET2 [exons 3-11]), RNA splicing (SF3B1 [exons 13-18], SRSF2 [exon 1], U2AF1 [exons 2 + 6], ZRSR2 [exons 1-11]), cohesin complex (RAD21 [exons 2-14], SMC1A [exons 1-25], SMC3 [exons 1-29], STAG2 [exons 3-35]), tumor suppression (PHF6 [exons 2-10], TP53 [exons 2-11], WT1 [exons 7 + 9]] and other pathways [CALR [exon 9], NPM1 [exon 11], SETBP1 [exon 4]). For all but ASXL2, libraries were prepared using Haloplex Target Enrichment System (Agilent Technologies) according to the manufacturer’s instructions and run on MiSeq (Illumina). A high depth of coverage (>2000×) was obtained for all genes (supplemental Figure 2), allowing detection of mutations with a variant allele frequency (VAF) until 1%. Raw HTS data were analyzed with 2 distinct softwares: SureCall (Agilent Technologies) and SeqNext (JSI Medical System). Frameshift and nonsense variants were always considered as relevant mutations. Single nucleotide variants were retained in the absence of description into public databases of human polymorphisms, and effects on protein function were predicted with 6 established prediction tools: SIFT (Sorting Intolerant From Tolerant), PolyPhen-1, PolyPhen-2, Multivariate Analysis of Protein Polymorphism, Support Vector Machines based Predictor of human Deleterious Single Nucleotide Polymorphism, and SNAP (Screening for Non-Acceptable Polymorphisms).22 All variants were validated with another HTS technology with library preparation using the Ampliseq System and sequencing on a Personal Genome Machine (PGM, Life Technologies). Data from PGM sequencing were processed by Torrent Browser (Life Technologies) and SeqNext (JSI Medical System). ASXL2 sequencing was performed as previously described.19 Notably, because of technical limitations, the mutation c.1934dupG in ASXL1 cannot be detected with PGM sequencing, justifying its systematic validation by Sanger sequencing as previously described.19 In 1 case, this ASXL1 variant could not be verified by Sanger sequencing because of a low VAF (4%), but was retained by visual check of the reads (UPN 53).
Other cytogenetic and molecular analyses
The presence of the t(8;21) or the inv(16)/t(16;16) rearrangements were determined by karyotype (as well as additional cytogenetic abnormalities) and/or fluorescence in situ hybridization and/or evidence of RUNX1-RUNX1T1 or CBFB-MYH11 fusion transcripts, as previously described.3 The presence of the FLT3-internal tandem duplication (ITD) was not determined by HTS, but systematic screening was performed for all patients, as previously described.23 Minimal residual disease was evaluated with RUNX1-RUNX1T1 or CBFB-MYH11 real-time quantitative polymerase chain reaction analysis, as previously described.3
Statistical methods
Failure time data were analyzed and compared after censoring at transplant for patients who received allogeneic stem cell transplantation (SCT) in first complete remission (CR). Cumulative incidence of relapse (CIR) and overall survival (OS) were estimated by the Kaplan-Meier method. CIR was estimated taking into account death in first CR for competing risk. CIR and OS were compared by cause-specific hazard Cox models after stratification on the trial (CBF2006 vs ELAM02). Comparisons between patient subgroups were performed by the Mann-Whitney test for continuous variables and by Fisher’s exact test for categorical variables. Optimal cut-points for allelic ratios were determined by maximally selected log-rank statistics.24 Specific hazards of relapse (SHRs) and HRs are given with 95% confidence interval (CI). Univariate and multivariate analysis assessing the impact of categorical and continuous variables were performed with a Cox model. Proportional-hazards assumption was checked before conducting multivariate analyses.25 Covariates with a P value < .1 in univariate analysis were included in the multivariable models. All statistical tests were performed with the Stata/IC 12.1 software (StataCorp, College Station, TX).
Results
Patients’ characteristics at diagnosis
Patients’ characteristics as well as additional molecular and cytogenetic aberrations are shown in Table 1 according to CBF subtype. Median age was 32 years (patients were aged from 1 to 17 years in the ELAM02 trial and from 18 to 60 years in the CBF2006 trial). The median follow-up was 5.3 years. Adult and pediatric patients were grouped together for further investigations because CIR was similar regardless of the trial (supplemental Figure 3). Patients with inv(16) AML were younger (median age, 25 vs 37; P = .004) and had a higher white blood cell (WBC) count (median, 34.4 vs 11.5; P < .001) than those with t(8;21) AML.
Patient characteristics according to CBF subtype
| . | CBF-AML . | AML with inv(16) . | AML with t(8;21) . | P value . |
|---|---|---|---|---|
| Patients, n | 215 | 109 | 106 | |
| Median age, y (range) | 32 (1-60) | 37 (1-60) | 25 (2-59) | .004* |
| Median WBC, ×109/L (range) | 18.4 (1-232) | 34.4 (1-232) | 11.5 (1.3-163) | <.001* |
| Gender (male/female) | 119/96 | 61/48 | 58/48 | .891 |
| Trial (CBF2006/ELAM02) | 142/73 | 79/30 | 63/43 | .046* |
| Outcome | ||||
| Deaths, n (%) | 35 (16) | 15 (14) | 20 (18) | .358 |
| Relapses, n (%) | 70 (33) | 34 (31) | 36 (33) | .771 |
| Number of mutations per patient | 2 (0-6) | 1 (0-5) | 2 (0-6) | .277 |
| Gene mutations | ||||
| Tyrosine kinase pathway, n (%) | 156 (73) | 87 (80) | 69 (65) | .021* |
| KIT exon 8, n (%) | 37 (17) | 22 (20) | 15 (14) | .352 |
| KIT exon 17, n (%) | 42 (20) | 16 (15) | 26 (25) | .080 |
| Codon D816 (exon 17), n (%) | 30 (14) | 13 (12) | 17 (16) | .421 |
| Codon N822 (exon 17), n (%) | 12 (6) | 2 (2) | 10 (9) | .032* |
| KIT exon 11, nm(%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| KIT all, n (%) | 78 (36) | 36 (33) | 42 (40) | .325 |
| FLT3-TKD, n (%) | 28 (13) | 24 (22) | 4 (4) | <.001* |
| FLT3-ITD, n (%) | 14 (7) | 3 (3) | 11 (10) | .028* |
| FLT3 all, n (%) | 41 (19) | 26 (24) | 15 (14) | .083 |
| RTK (KIT and/or FLT3), n (%) | 105 (49) | 53 (49) | 52 (49) | 1.000 |
| NRAS, n (%) | 66 (31) | 42 (39) | 24 (23) | .012* |
| Codon G12, n (%) | 24 (11) | 14 (13) | 10 (9) | .518 |
| Codon G13, n (%) | 19 (9) | 9 (8) | 10 (9) | .813 |
| Codon Q61, n (%) | 34 (16) | 26 (24) | 8 (8) | .001* |
| KRAS, n (%) | 36 (17) | 29 (27) | 7 (7) | <.001* |
| Codon G12, n (%) | 15 (7) | 14 (13) | 1 (1) | <.001* |
| Codon G13, n (%) | 13 (6) | 8 (7) | 5 (5) | .569 |
| Codon Q61, n (%) | 6 (3) | 5 (5) | 1 (1) | .212 |
| RAS (NRAS and/or KRAS), n (%) | 87 (40) | 59 (54) | 28 (26) | <.001* |
| JAK2, n (%) | 3 (1) | 0 (0) | 3 (3) | .118 |
| PTPN11, n (%) | 8 (4) | 5 (5) | 3 (3) | .722 |
| CBL, n (%) | 2 (1) | 0 (0) | 2 (2) | .242 |
| Chromatin modifiers, n (%) | 51 (24) | 6 (6) | 45 (42) | <.001* |
| ASXL1, n (%) | 11 (5) | 0 (0) | 11 (10) | <.001* |
| ASXL2, n (%) | 23 (11) | 0 (0) | 23 (22) | <.001* |
| ASXL (ASXL1 or ASXL2), n (%) | 34 (16) | 0 (0) | 34 (32) | <.001* |
| KDM6A, n (%) | 9 (4) | 3 (3) | 6 (6) | .328 |
| EZH2, n (%) | 7 (3) | 0 (0) | 7 (7) | .006* |
| BCOR, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| BCORL1, n (%) | 6 (3) | 3 (3) | 3 (3) | 1.000 |
| DNA methylation, n (%) | 10 (5) | 2 (2) | 8 (8) | .057 |
| TET2, n (%) | 5 (2) | 0 (0) | 5 (5) | .028* |
| IDH1, n (%) | 3 (1) | 2 (2) | 1 (1) | 1.000 |
| IDH2, n (%) | 2 (1) | 0 (0) | 2 (2) | .242 |
| Cohesin, n (%) | 19 (9) | 0 (0) | 19 (18) | <.001* |
| RAD21, n (%) | 8 (4) | 0 (0) | 8 (8) | .003* |
| SMC1A, n (%) | 5 (2) | 0 (0) | 5 (5) | .028* |
| SMC3, n (%) | 5 (2) | 0 (0) | 5 (5) | .028* |
| STAG2, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| Transcription factors, n (%) | 6 (3) | 1 (1) | 5 (5) | .116 |
| ETV6, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| RUNX1, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| GATA2, n (%) | 2 (1) | 0 (0) | 2 (2) | .242 |
| IKZF1, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| Spliceosome, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| SRSF2, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| Tumor suppressors, n (%) | 11 (5) | 7 (6) | 4 (4) | .538 |
| PHF6, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| WT1, n (%) | 11 (5) | 7 (6) | 4 (4) | .538 |
| Additional cytogenetic abnormalities | ||||
| Loss X or Y, n (%) | 54 (25) | 0 (0) | 54 (51) | <.001* |
| Del(9q), n (%) | 16 (7) | 0 (0) | 16 (15) | <.001* |
| Del(7q), n (%) | 21 (10) | 11 (10) | 10 (9) | 1.000 |
| Trisomy 8, n (%) | 18 (8) | 12 (11) | 6 (6) | .218 |
| Trisomy 22, n (%) | 12 (6) | 12 (11) | 0 (0) | <.001* |
| . | CBF-AML . | AML with inv(16) . | AML with t(8;21) . | P value . |
|---|---|---|---|---|
| Patients, n | 215 | 109 | 106 | |
| Median age, y (range) | 32 (1-60) | 37 (1-60) | 25 (2-59) | .004* |
| Median WBC, ×109/L (range) | 18.4 (1-232) | 34.4 (1-232) | 11.5 (1.3-163) | <.001* |
| Gender (male/female) | 119/96 | 61/48 | 58/48 | .891 |
| Trial (CBF2006/ELAM02) | 142/73 | 79/30 | 63/43 | .046* |
| Outcome | ||||
| Deaths, n (%) | 35 (16) | 15 (14) | 20 (18) | .358 |
| Relapses, n (%) | 70 (33) | 34 (31) | 36 (33) | .771 |
| Number of mutations per patient | 2 (0-6) | 1 (0-5) | 2 (0-6) | .277 |
| Gene mutations | ||||
| Tyrosine kinase pathway, n (%) | 156 (73) | 87 (80) | 69 (65) | .021* |
| KIT exon 8, n (%) | 37 (17) | 22 (20) | 15 (14) | .352 |
| KIT exon 17, n (%) | 42 (20) | 16 (15) | 26 (25) | .080 |
| Codon D816 (exon 17), n (%) | 30 (14) | 13 (12) | 17 (16) | .421 |
| Codon N822 (exon 17), n (%) | 12 (6) | 2 (2) | 10 (9) | .032* |
| KIT exon 11, nm(%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| KIT all, n (%) | 78 (36) | 36 (33) | 42 (40) | .325 |
| FLT3-TKD, n (%) | 28 (13) | 24 (22) | 4 (4) | <.001* |
| FLT3-ITD, n (%) | 14 (7) | 3 (3) | 11 (10) | .028* |
| FLT3 all, n (%) | 41 (19) | 26 (24) | 15 (14) | .083 |
| RTK (KIT and/or FLT3), n (%) | 105 (49) | 53 (49) | 52 (49) | 1.000 |
| NRAS, n (%) | 66 (31) | 42 (39) | 24 (23) | .012* |
| Codon G12, n (%) | 24 (11) | 14 (13) | 10 (9) | .518 |
| Codon G13, n (%) | 19 (9) | 9 (8) | 10 (9) | .813 |
| Codon Q61, n (%) | 34 (16) | 26 (24) | 8 (8) | .001* |
| KRAS, n (%) | 36 (17) | 29 (27) | 7 (7) | <.001* |
| Codon G12, n (%) | 15 (7) | 14 (13) | 1 (1) | <.001* |
| Codon G13, n (%) | 13 (6) | 8 (7) | 5 (5) | .569 |
| Codon Q61, n (%) | 6 (3) | 5 (5) | 1 (1) | .212 |
| RAS (NRAS and/or KRAS), n (%) | 87 (40) | 59 (54) | 28 (26) | <.001* |
| JAK2, n (%) | 3 (1) | 0 (0) | 3 (3) | .118 |
| PTPN11, n (%) | 8 (4) | 5 (5) | 3 (3) | .722 |
| CBL, n (%) | 2 (1) | 0 (0) | 2 (2) | .242 |
| Chromatin modifiers, n (%) | 51 (24) | 6 (6) | 45 (42) | <.001* |
| ASXL1, n (%) | 11 (5) | 0 (0) | 11 (10) | <.001* |
| ASXL2, n (%) | 23 (11) | 0 (0) | 23 (22) | <.001* |
| ASXL (ASXL1 or ASXL2), n (%) | 34 (16) | 0 (0) | 34 (32) | <.001* |
| KDM6A, n (%) | 9 (4) | 3 (3) | 6 (6) | .328 |
| EZH2, n (%) | 7 (3) | 0 (0) | 7 (7) | .006* |
| BCOR, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| BCORL1, n (%) | 6 (3) | 3 (3) | 3 (3) | 1.000 |
| DNA methylation, n (%) | 10 (5) | 2 (2) | 8 (8) | .057 |
| TET2, n (%) | 5 (2) | 0 (0) | 5 (5) | .028* |
| IDH1, n (%) | 3 (1) | 2 (2) | 1 (1) | 1.000 |
| IDH2, n (%) | 2 (1) | 0 (0) | 2 (2) | .242 |
| Cohesin, n (%) | 19 (9) | 0 (0) | 19 (18) | <.001* |
| RAD21, n (%) | 8 (4) | 0 (0) | 8 (8) | .003* |
| SMC1A, n (%) | 5 (2) | 0 (0) | 5 (5) | .028* |
| SMC3, n (%) | 5 (2) | 0 (0) | 5 (5) | .028* |
| STAG2, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| Transcription factors, n (%) | 6 (3) | 1 (1) | 5 (5) | .116 |
| ETV6, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| RUNX1, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| GATA2, n (%) | 2 (1) | 0 (0) | 2 (2) | .242 |
| IKZF1, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| Spliceosome, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| SRSF2, n (%) | 2 (1) | 1 (1) | 1 (1) | 1.000 |
| Tumor suppressors, n (%) | 11 (5) | 7 (6) | 4 (4) | .538 |
| PHF6, n (%) | 1 (0) | 0 (0) | 1 (1) | .493 |
| WT1, n (%) | 11 (5) | 7 (6) | 4 (4) | .538 |
| Additional cytogenetic abnormalities | ||||
| Loss X or Y, n (%) | 54 (25) | 0 (0) | 54 (51) | <.001* |
| Del(9q), n (%) | 16 (7) | 0 (0) | 16 (15) | <.001* |
| Del(7q), n (%) | 21 (10) | 11 (10) | 10 (9) | 1.000 |
| Trisomy 8, n (%) | 18 (8) | 12 (11) | 6 (6) | .218 |
| Trisomy 22, n (%) | 12 (6) | 12 (11) | 0 (0) | <.001* |
Adult and pediatric patients are reported together.
P < .05.
Additional aberrations are found in >90% of CBF AML
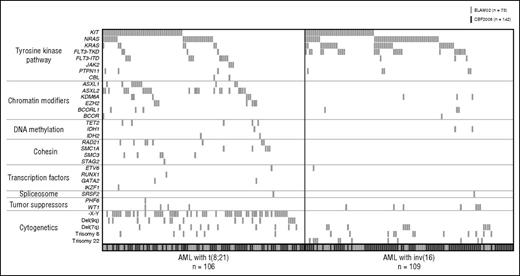
Mutation frequencies are reported in Table 1. All identified variants with their VAFs are reported in supplemental Table 1. Among the 215 patients analyzed, 182 (85%) had at least 1 mutation. Among them, 72 patients had 1 alteration, 51 had 2, 41 had 3, 13 had 4, 4 had 5, and 1 had 6. Considering the most common cytogenetic abnormalities in CBF AML together with mutations, additional aberrations were found in 93% of CBF AML (96% and 90% of t(8;21) AML and inv(16) AML, respectively). Figure 1 depicts the identified mutations as well as additional cytogenetic aberrations according to CBF AML subtype.26 Notably, mutational patterns appeared to be quite similar regardless of age group (supplemental Table 2).
Molecular alterations in CBF AML. Each column represents the mutation pattern in 1 patient, and each colored box represents a mutation of the gene. Genes have been grouped in 7 categories according to reference.26 Patients are grouped into AML with t(8;21) (left) and AML with inv(16) (right). Adult (CBF2006 trial, dark gray boxes) and pediatric patients (ELAM02, light gray boxes) are reported together.
Molecular alterations in CBF AML. Each column represents the mutation pattern in 1 patient, and each colored box represents a mutation of the gene. Genes have been grouped in 7 categories according to reference.26 Patients are grouped into AML with t(8;21) (left) and AML with inv(16) (right). Adult (CBF2006 trial, dark gray boxes) and pediatric patients (ELAM02, light gray boxes) are reported together.
Mutations disrupting tyrosine kinase signaling are the most frequent events in CBF AML
Among all genes sequenced, the most common mutations involved genes affecting TK signaling (especially KIT, FLT3, and N/KRAS mutations). A higher mutation incidence in these genes was identified here compared with prior studies using standard polymerase chain reaction and direct sequencing,3,23,27 likely because HTS and cross-validation allowed for detection of mutations with very low VAFs (supplemental Figure 4).
KIT mutations were found in 40% of AML with t(8;21) and 33% of AML with inv(16) (P = .345). Most KIT mutations were small deletions and or insertions in exon 8, leading to replacement of codon D419 or point mutations in exon 17. All but 1 exon 17 mutation involved codons D816 and N822 in the activation loop of the kinase domain. KIT exon 11 mutations were only detected in 2 patients. Two patients harbored rare KIT variants K509I in exon 9 (UPN206) and N655K in exon 13 (UPN3), previously reported in pediatric mastocytosis28 and gastrointestinal stromal tumor,29 respectively, and demonstrated to cause constitutive ligand-independent activation of KIT (supplemental Figure 5A).
FLT3-TKD mutations (especially at codon D835) were involved in 22% of inv(16) AML, but only 4% of t(8;21) AML (P < .001). On the other hand, FLT3-ITD was present in only 3% of inv(16) AML, whereas they occurred in 10% of t(8;21) AML (P = .028).
Notably, adult and pediatric patients with inv(16) AML differ by the pattern of mutations in genes coding for receptor tyrosine kinases (RTK) KIT and FLT3, a feature not shared by t(8;21) AML patients. Indeed, FLT3-TKD mutations have an incidence of 28% in adult inv(16) AML, whereas they account for 7% of pediatric cases (P = .019). On the other hand, KIT mutations are found in 50% of pediatric inv(16) AML cases, but were present in 27% of adult cases (P = .024) (supplemental Figure 6).
As previously reported,30 RAS (NRAS or KRAS) mutations were the most frequent mutations in inv(16) AML. RAS mutations were found in 54% of inv(16) AML and 26% of t(8;21) AML (P < .001). All but 4 RAS mutations involved the hotspots at codons G12, G13, and Q61 (supplemental Figure 5B).
Other events in TK pathway included rare mutations in JAK2 (V617F) CBL and PTPN11.
Cooccurring mutations in TK pathway gene members are frequent in CBF-AML
Seventy-five CBF AML patients (35%) had 2 or more mutations in genes coding for TK pathway effectors (involving the same gene or different genes, especially KIT, FLT3, and RAS genes). Among them, 6 patients had 2 or more KIT mutations, 25 patients had 2 or more RAS mutations, and 8 patients had 2 or more FLT3-ITD or TKD mutations. These findings highlight the multiclonality of CBF AML (supplemental Figure 7). In all but 6 CBF AML patients, the total of VAFs for TK pathway mutations was less than 50% (corresponding to 1 heterozygous mutation per cell). In the 6 remaining patients, 2 had mutations in the CBL gene, which is known to be involved in uniparental disomies,31 and 1 patient (UPN 71) had a KIT mutation with a VAF at 62%. In this patient, review of the karyotype showed duplication of the long arm of the chromosome 4 (probably containing the mutated KIT gene). Considering the redundant effects of these mutations, it is likely they occur in distinct clones although further investigations are needed to study clonal architecture and extend those findings.
Mutations of epigenetic regulators and cohesin complex are common in t(8;21) but rare in inv(16) CBF AML
Mutations in genes encoding epigenetic regulators that control chromatin conformation were found in 42% of t(8;21) AML cases, but only in 6% of inv(16) AML cases (P < .001). These mutations were largely mutually exclusive with 1 another (supplemental Figure 8). Among t(8;21) AML patients, ASXL1 or ASXL2 mutations occurred together in 32% of cases (all mutations were frameshift and nonsense mutations). EZH2 mutations were identified in 7% of t(8;21) AML. All but 1 were located within the post-SET domain, normal expression of which is essential for the conformation of the protein and its subsequent catalytic activity.32 Other alterations within chromatin modifiers included KDM6A, BCOR, and BCORL1 mutations in 6%, 1%, and 3% of patients with t(8;21) AML.
Likewise, mutations in genes encoding members of the cohesin complex were identified specifically in patients in t(8;21) AML, but not in inv(16) AML. Cohesin mutations were present in 18% of t(8;21) AML, but in none of the inv(16) AML patients (P < .001). All cohesin mutations were mutually exclusive among each other (supplemental Figure 9). All RAD21 and STAG2 mutations were nonsense or out-of-frame frameshift mutations. SMC1A and SMC3 mutations were missense mutations and involved functional domains (mostly hinge domain and adenosine triphosphatase heads). Two patients harbored the same SMC1A variant R96H, previously reported in AML.33 We found a hotspot in SMC3 because 3 of 5 mutations involved the codon R661 in the hinge domain of the protein. Mutations involving this codon have been previously described in cancer as well as in the leukemia-derived cell line MOLM-7.34
Finally, mutations in effectors that control DNA methylation (TET2, IDH1 R132H/L, or IDH2 R140) were identified in 8% of t(8;21) AML cases and in 2% of inv(16) AML cases with mutual exclusivity (P = .57).
Outcome
The CR rate in this cohort of 215 patients was 98.1% (211/215). The 5-year CIR was 33.2% (95% CI, 27.2-40.2) with no difference between the CBF2006 and ELAM02 (35.2% [95% CI, 27.9-43.9] vs 29.3% [95% CI, 19.8-42.1], P = .415, supplemental Figure 3). The 5-years OS was 83.6% [95% CI, 77.7-88.0]. A total of 14 patients received allogeneic SCT in first CR and were censored at SCT time for prognostic analyses. Five-year CIR was estimated at 31% (95% CI, 23-41) in patients with inv(16) AML and 35% (95% CI, 27-46) in patients with t(8;21) AML. All but 1 patient, who died during induction course, entered CR. Thirty-four patients died during follow-up: 9 died in first CR, 19 died after hematological relapse, and 6 adults died of allogeneic SCT complications. Seventy patients had hematological relapse.
Importance of VAFs for evaluating impact of TK pathway mutations on outcome
Univariate prognosis analyses for CIR are summarized in supplemental Table 3. Altogether, mutations in genes activating TK signaling were associated with a higher cumulative incidence of relapse (SHR, 2.81 [95% CI, 1.39-5.69]; P = .004) especially in t(8;21) AML patients (SHR, 5.22 [95% CI, 1.82-14.96]; P = .002). This was also evident across RTK mutations (KIT and/or FLT3 mutations) (SHR, 1.72 [95% CI, 1.05-2.81]; P = .031).
Given the range in VAFs identified in mutations activating TK signaling, we determined optimal cut-points for VAFs in the most frequent mutations (ie, KIT, FLT3-TKD, NRAS, and KRAS mutations) by maximally selected log-rank statistics.24 KIT mutations were associated with a significant higher CIR for t(8;21) AML patients with a mutant allelic ratio of 35% or greater (KIT≥35%). Patients in this particular subgroup had a particularly adverse outcome with a 5-year CIR of 69.4% vs 30.7% and 31.9%% for KIT<35% and KITwildtype, respectively (P = .008) (supplemental Figure 10). In patients with t(8;21) and KIT mutation, the only baseline characteristic that was associated with a higher incidence of relapse was logWBC (SHR, 3.72 [95% CI, 0.97-14.28]; P = .056). In bivariate analysis, both logWBC and KIT≥35% remained significantly associated with a poorer prognosis (logWBC: SHR, 8.45 [95% CI, 1.93-36.90], P = .005; KIT≥35%: SHR, 8.83 [95% CI, 2.45-31.76], P = .001).
Similarly, FLT3-TKD mutations were associated with a higher CIR in CBF AML patients with a mutant allelic ratio of 10% or greater (FLT3-TKD≥10%) when compared with lower ratio and nonmutated patients (SHR, 2.28 [95% CI, 1.16-4.50]; P = .018). The 5-year CIR was 58.8% (95% CI, 37.4-81.4) in patients with FLT3-TKD≥10%, 20.0% (95% CI, 5.4-59.1) in patients with FLT3-TKD<10%, and 31.5% (95% CI, 25.2-39.0) in patients without FLT3-TKD. Conversely, high NRAS and KRAS mutant allelic ratios (>35%) were both favorable factors for CIR in CBF AML (5-year CIR was 13% for NRAS≥35% vs 30.2% for NRASwt [P = .033] and 0% for KRAS≥35% vs 31.9% for KRASwt, respectively [P = .008]). Patients with high NRAS or KRAS mutant allelic ratios were characterized by the lack of RTK mutations, which could explain this favorable outcome (supplemental Figure 11).
Mutations in epigenetic modifying or cohesin genes are associated with a poor prognosis in t(8;21) AML patients with TK pathway mutations
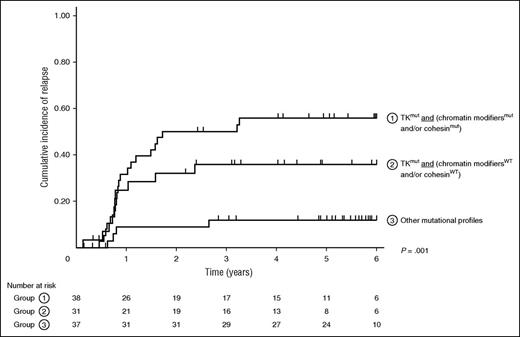
Besides mutations in genes affecting TK signaling, as previously reported, we observed a trend toward a higher SHR for ASXL1/2 mutations (ASXL1 or ASXL2) in patients with t(8;21) AML (SHR, 1.71 [95% CI, 0.88-3.33]; P = .113), which did not reach statistical significance. Interestingly, in this CBF AML subtype, patients who had mutations in both TK pathways and chromatin modifiers and/or cohesin genes had the worst prognosis with a 5-year CIR of 54.1% (95% CI, 39.7-69.9) when compared with patients with TK mutations without chromatin modifiers and/or cohesin gene mutations (5-year CIR, 33.9% [95% CI, 25.8-43.8]), or to patients without TK mutations (5-year CIR, 16.3% [95% CI, 8.9-29.1]; Figure 2). In multivariate analysis (Table 2), the association of mutations in TK pathway genes and chromatin modifiers or cohesin genes remained associated with the highest hazard of relapse in patients with t(8;21) AML (SHR, 5.44 [95% CI, 1.82-16.27]; P = .002).
Kaplan-Meier curves for CIR in patients stratified according to their mutational profile. Patients are separated in 3 groups: (1) TK pathway mutation associated with at least 1 mutation involving chromatin modifier or cohesin genes, (2) TK pathway mutation without mutations involving chromatin modifier or cohesin genes, and (3) other mutational profiles.
Kaplan-Meier curves for CIR in patients stratified according to their mutational profile. Patients are separated in 3 groups: (1) TK pathway mutation associated with at least 1 mutation involving chromatin modifier or cohesin genes, (2) TK pathway mutation without mutations involving chromatin modifier or cohesin genes, and (3) other mutational profiles.
Univariate and multivariate analyses for specific hazard of relapse in patients with t(8;21) AML
| . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|
| . | Patients . | SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . |
| Age* | 106 | 1.00 | 0.97-1.03 | .938 | — | — | — |
| LogWBC* | 106 | 2.47 | 1.18-5.19 | .017† | 1.94 | 0.89-4.27 | .097 |
| TKmut and (chromatin modifiersWT and cohesinWT) | 31/106 | 3.88 | 1.20-12.54 | .023† | 3.96 | 1.22-12.91 | .022† |
| TKmut and (chromatin modifiersmut and/or cohesinmut) | 38/106 | 6.21 | 2.11-18.26 | .001† | 5.44 | 1.82-16.27 | .002† |
| . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|
| . | Patients . | SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . |
| Age* | 106 | 1.00 | 0.97-1.03 | .938 | — | — | — |
| LogWBC* | 106 | 2.47 | 1.18-5.19 | .017† | 1.94 | 0.89-4.27 | .097 |
| TKmut and (chromatin modifiersWT and cohesinWT) | 31/106 | 3.88 | 1.20-12.54 | .023† | 3.96 | 1.22-12.91 | .022† |
| TKmut and (chromatin modifiersmut and/or cohesinmut) | 38/106 | 6.21 | 2.11-18.26 | .001† | 5.44 | 1.82-16.27 | .002† |
Tested as continuous variable.
P < .05.
Discussion
AML with t(8;21) and inv(16), collectively referred to as CBF AML, represent 2 of the most common genetic abnormalities in AML. Both entities disrupt the normal function of the heterodimeric transcription factor CBF complex and have similar clinical outcomes. However, the molecular genetic abnormalities potentially explaining differences between these 2 subtypes of AML have not been explored in detail. Although implication of TK pathways in CBF AML leukemogenesis has been widely studied,15 only a few reports have identified cooperating mutations in CBF AML outside of TK pathway alterations. Although the “2-hit model” of leukemogenesis mentioned earlier is biologically relevant, it is impossible to ignore the multitude of genetic and epigenetic aberrations that have recently been described in human leukemia.35 Within CBF AML, ASXL1 and ASXL2 mutations were recently reported to occur exclusively in AML with t(8;21), but not in AML with inv(16).19,20,36 The present study extends those findings. We performed extensive mutational analysis by HTS in 215 patients with CBF AML from 1 to 60 years. As expected, mutations in TK pathways were the most frequent aberrations in both subtypes (65% and 80% of t(8;21) AML and inv(16) AML respectively; P = .021). Mutations in TK pathway, especially RTK mutations (KIT and FLT3 mutations), were associated with a higher SHR and higher CIR. KIT mutations with high mutant allelic ratio appeared to significantly affect prognosis of t(8;21) AML patients in accordance with a previous report by Allen et al.37 Conversely, high NRAS and KRAS mutant allelic ratios were associated with the lack of RTK mutations and a favorable outcome. FLT3-TKD mutations were associated with a higher CIR in the present study, especially in inv(16) AML. These results are in accordance with previous studies showing inferior OS and PFS in inv(16) AML,30,38 although it remains controversial.39,40
However, TK pathways mutations appear to constitute only a portion of the genetic landscape of CBF AML. Interestingly, the 2 CBF AML subtypes showed greatly distinct mutational profiles. Mutations in genes that regulate chromatin conformation (ASXL1/2, EZH2, KDM6A, BCOR/BCORL1) or implicated in the cohesin complex (RAD21, SMC1A, SMC3, STAG2) were observed almost exclusively in t(8;21) AML. In line with those findings, using whole exome sequencing in 13 CBF AML patients, Sood et al recently identified cohesin and chromatin modifiers mutations in t(8;21) but not in inv(16) patients.41
Functionally, ASXL1, ASXL2, and EZH2 are Polycomb group-associated proteins that influence chromatin configuration and thus gene transcription by directing modifications at specific chromatin marks. EZH2 is the catalytic component of Polycomb Repressive Complex 2 (PRC2) and serves as a H3K27 methyltransferase activity. ASXL1 and ASXL2 are part of the Polycomb repressive deubiquitinase complex, which removes an ubiquitin from H2AK119 marks,42 but studies have shown that ASXL proteins may also function in recruitment and/or stabilization of the PRC2 complex to specific loci.43 KDM6A (also known as UTX) is an H3K27 demethylase that counters the enzymatic activity of PRC2. On the other hand, BCOR and BCORL1 are part of a complex similar to the PRC1.44
Cohesin is a multimeric protein complex that is conserved across species and is composed of 4 core subunits (SMC1A, SMC3, RAD21, and STAG proteins) together with a number of regulatory proteins such as NIPBL, PDS5, or ESCO proteins.34 The 4 subunits form a ring structure that regulates chromosome segregation during meiosis and mitosis, but recent data suggest additional functions such as double-stranded DNA repair and regulation of transcription.42,45 In a previous study by Thol et al, recurrent cohesin mutations have been reported in about 6% of AML patients.45 However, cohesin mutations concerned less than 2% of CBF AML in this study. Interestingly, it has been shown that the cohesin complex could functionally interact with Polycomb group proteins to control gene transcription.46 It is an interesting observation that ASXL gene mutations47 as well as cohesin gene mutations48 are enriched in patients with RUNX1-mutated AML. Recently, several experiments have linked cohesin and RUNX1 in hematopoiesis and leukemogenesis. Notably, runx genes expression has been showed to be dependent of rad21 expression in a zebrafish model.49 Kon et al showed that forced expression of wild-type RAD21 in the Kasumi-1 cell line (carrying both t(8;21) and RAD21 mutation p.K330PfsX6) induced significant growth suppression.34 Moreover, haploinsufficiency of cohesin proteins appears to be associated with myeloid transformation and aberrant self-renewal linked with broad changes in chromatin occupancy.50,51 Indeed, Mazumdar et al showed that cohesin mutations led to a state of elevated chromatin accessibility and higher binding at RUNX1 binding sites.50 These findings suggest links between alterations in chromatin structure, mediated by cohesin or chromatin modifiers mutations, and cooperativity with the RUNX1-RUNXT1 fusion oncoprotein.
In our cohort, 18% of t(8;21) AML patients have mutations involving the cohesin member and 42% of t(8;21) AML patients have mutations involving chromatin modifiers. Overall, 52% of t(8;21) AML patients have at least 1 mutation in 1 of these 2 groups of genes. These findings suggest an important pathway that is specific to t(8;21) AML leukemogenesis and may have biological and clinical significance. Accordingly, transcriptome profiling supports the notion that t(8;21) and inv(16) AML are characterized by different genetic programs.18 It is likely that future studies focused on the molecular basis of shared pathways as well as pathways specific to the 2 CBF AML subtypes may guide the development of new treatment approaches. Patients with t(8;21) AML who had at least 1 TK pathway mutation associated with at least 1 mutation in a chromatin modifier or cohesin gene had the worst prognosis, which could indicate synergic cooperation between these events. Finally, evaluation of drugs targeting these pathways and translational research integrating these molecular findings with clinical trials will likely improve the treatment of patients with CBF AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lamya Haddaoui (Tumour Bank for the Groupe Ouest Est d'Etude des Leucémies et Autres Maladies du Sang, N° BB-033-00073, Hôpital Cochin, Paris) and Christophe Roumier and Olivier Nibourel (Tumour Bank for the Acute Leukemia French Association certification NF 96900-2014/65453-1, Centre Hospitalier Regional Universitaire Lille) for handling, conditioning, and storing patient samples. The work of all clinical research assistants is also acknowledged.
This work was supported by the French National Cancer Institute (PRT-K 2010-285 and PRT-K 2012-043).
Authorship
Contribution: E.J. was the principal investigator of the Core Binding Factor 2006 study; G.L. was the principal investigator of the Treating Patients with Childhood Acute Myeloid Leukemia with Interleukin-2 study; H.D., H.L., and C.P. created the patient database; K.C.-L. and C.R. ensured data management; N.B. performed statistical analysis; E.J., N.B., J.-B.M., N.I., H.D., G.L., and A.P. enrolled patients in the studies; N.D., A.M.-R., M.B., S.G., A.R., M.F., C.P., C.L., P.C., and H.L. performed genetic analysis and analyzed mutational data; N.D. and C.P. performed the research and wrote the paper; and C.P., O.A.-W., and J.-B.M. revised the manuscript which was approved by all the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claude Preudhomme, Laboratory of Hematology, Biology and Pathology Center, Lille University Hospital, Professor J. Leclercq Blvd, 59037 Lille Cedex, France; e-mail: claude.preudhomme@chru-lille.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal