Abstract
Background: Acquired aplastic anemia (AA) is occasionally preceded or complicated by the other autoimmune diseases such as rheumatoid arthritis and ulcerative colitis (UC), but the pathogenetic links between these diseases remain unknown. A number of case reports attributed the development of AA in UC patients to mesalazine that had been administered before the onset of AA. However, some UC patients who were referred to our clinical because of "mesalazine-induced AA" had increased percentages of glycosylphosphatidylinositol-anchored protein-deficient (PNH-type) cells that serve as a marker for immune-mediated bone marrow (BM) failure. This suggested an immune pathophysiology of UC-associated AA similar to that of idiopathic AA.
Methods: To address this issue, we analyzed clinical and laboratory data including HLA-DRB1 alleles and PNH-type cells of 14 AA patients (six males and eight females) associated with UC who were referred to our clinic from 2001 through 2015.
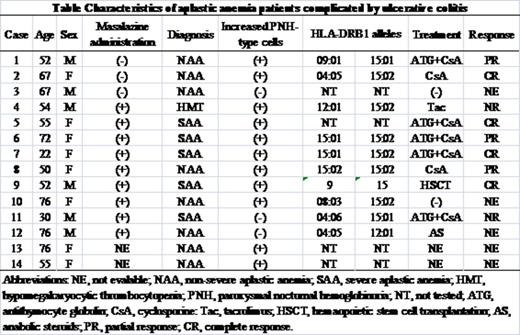
Results: The median age of the patients was 55 (range, 22-76). Diagnoses of the 14 patients were severe AA in eight, non-severe AA in five, and hypomegakaryocytic thrombocytopenia in one. UC preceded AA in 11 patients and nine of them received mesalazine at the time of AA diagnosis, while three AA patients developed UC 12, 72, and 36 months after diagnosis of AA. Increased percentages of PNH-type granulocytes (0.35% to 3.2%) were detected in 11 (79%) patients. Of 9 PNH(+) patients with AA preceded by UC, seven had received mesalazine while two had not been treated with mesalazine. The prevalence of increased PNH-type cells in AA patients who developed UC was 67% (2/3). Six patients were positive for DRB1*15:02, a well-known allele associated with susceptibility both to UC and AA in Japanese patients (Table). Another major DR15 allele, DRB1*15:01, was possessed by four patients. Seven patients received immunosuppressive therapy with either cyclosporine (CsA) alone (two patient) or CsA+antithymocyte globulin (five patients) and six of them achieved a remission of AA.
Conclusions: The high prevalence of increased PNH-type cells indicates that AA complicated by UC is a legitimate immune-mediated BM failure and that mesalazine may be irrelevant to the development of AA. The high frequency of DRB1*15:02 suggests the two diseases share an immune pathophysiology. Genome wide association studies on UC patients may help to identify genes associated with a susceptibility to AA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal