Key Points
Donor-derived Tc17 cells differentiate early after allogeneic transplant in response to IL-6 and alloantigen presentation by host DCs.
Tc17 are highly proinflammatory and pathogenic posttransplant, but exert limited or no GVL activity.
Abstract
IL-17–producing cells are important mediators of graft-versus-host disease (GVHD) after allogeneic stem cell transplantation (SCT). Here we demonstrate that a distinct CD8+ Tc17 population develops rapidly after SCT but fails to maintain lineage fidelity such that they are unrecognizable in the absence of a fate reporter. Tc17 differentiation is dependent on alloantigen presentation by host dendritic cells (DCs) together with IL-6. Tc17 cells express high levels of multiple prototypic lineage-defining transcription factors (eg, RORγt, T-bet) and cytokines (eg, IL-17A, IL-22, interferon-γ, granulocyte macrophage colony-stimulating factor, IL-13). Targeted depletion of Tc17 early after transplant protects from lethal acute GVHD; however, Tc17 cells are noncytolytic and fail to mediate graft-versus-leukemia (GVL) effects. Thus, the Tc17 differentiation program during GVHD culminates in a highly plastic, hyperinflammatory, poorly cytolytic effector population, which we term “inflammatory iTc17” (iTc17). Because iTc17 cells mediate GVHD without contributing to GVL, therapeutic inhibition of iTc17 development in a clinical setting represents an attractive approach for separating GVHD and GVL.
Introduction
Bone marrow/stem cell transplantation (BMT/SCT) remains an important curative therapy for hematologic malignancies, and its success is primarily a result of the generation of donor T cell–mediated graft-versus-leukemia (GVL) effects. The complicating factor for BMT/SCT recipients is the risk of developing graft-versus-host disease (GVHD) within the gastrointestinal tract, liver, and skin.1 GVHD requires the differentiation of donor T cells and is mediated by both the cytolytic pathways of donor T cells and proinflammatory cytokines. GVHD can be either acute (aGVHD) or chronic (cGVHD), both of which have a significant impact on patient morbidity and mortality, and, despite advances in immunosuppressive therapies, their incidence remains high.2,3 Therefore a major goal in BMT/SCT remains to prevent GVHD while maintaining GVL effects.
GVHD pathology is initiated by conditioning-induced damage/pathogen-associated molecular patterns and inflammatory cytokine release, which promotes host antigen-presenting cell activation and alloantigen-specific donor T-cell differentiation. Although it has been widely recognized that CD4+ T cell–derived interferon-γ (IFNγ) is central to this process,4 the role of IL-17 has been more recently established.5 IL-17 is expressed by both innate and adaptive immune cells including conventional T cells, mucosal-associated invariant T cells (MAIT), natural killer (NK), invariant NK T cells (iNKT), and γδ T cells,6-11 and is known to contribute to the pathogenesis of many autoimmune diseases as well as play a role in host defense from infection and malignancy.9,12-14 IL-17–producing CD4+ T cells (Th17) have been extensively characterized in a variety of disease settings; however, IL-17–producing CD8+ T cells (Tc17) have received comparatively little attention and as a result are relatively poorly understood.
We have previously reported that the mobilization of stem cells in donors with granulocyte colony-stimulating factor (G-CSF) promotes Th17 and Tc17 differentiation and that donor IL-17A, which is predominantly derived from Tc17, drives sclerodermatous GVHD after allogeneic SCT (allo-SCT).15 However, little is known about the nature of Tc17 that develop posttransplant, or their developmental requirements or functional capabilities. We used an IL-17 “fate mapping” reporter model to characterize Tc17 development, plasticity, and function in the context of GVHD and to investigate potential routes of therapeutic intervention.
Methods
Mice
Female C57Bl/6 (WT.B6 [H-2Db, CD45.2+]), B6D2F1 (H-2Db/d, CD45.2+), and Balb/c (H-2Dd, CD45.2+) mice were purchased from the Animal Resources Center (WA, Australia). Bm1 (B6.CBy H-2Kbm1), IL-17Cre (B6, H-2Db, CD45.2+), Rosa26eYFP (B6, H-2Db, CD45.2+), Rosa26iDTR (B6, H-2Db, CD45.2+), CD11c.DOG.F1 (B6.CD11c- DTR–OVA–eGFP x DBA/2 F1, H-2Db/d, CD45.2+), and IL-6−/− (B6, H-2Db, CD45.2+) mice were bred and housed at QIMR Berghofer. Rosa26iDTR mice were purchased from JAX (stock #007900), IL-17Cre and Rosa26eYFP mice were provided by Dr. B. Stockinger (Medical Research Council NIMR, Mill Hill, UK), and were crossed to generate IL-17creRosa26eYFP and IL-17creRosa26eYFP/iDTR heterozygous mice.16 Mice were housed in sterilized micro-isolator cages and received acidified autoclaved water (pH 2.5) after transplantation. All animal experiments were approved by and performed in accordance with the QIMR Berghofer Animal Ethics Committee.
Stem cell/bone marrow transplantation
Recipient mice received 900 (Balb/c), 1000 (bm1, B6), or 1100 (B6D2F1, CD11c.DOG.F1) cGy total-body irradiation (137Cs source at 84.6cGy/min) split over 2 doses (d−1). Grafts composed of either 10 × 106 G-CSF–treated splenocytes (B6D2F1, B6, CD11c.DOGF1) or 5 × 106 bone marrow + 0.2 × 106 Biomag purified T cells (Bm1) were injected (d0). Recombinant human G-CSF was given subcutaneously to donors at 10 µg/dose per animal (4-6 days).15 T-cell depletion (TCD) was performed by anti-CD4 (RL172.4), anti-CD8 (TIB211), and anti-Thy1.2 (HO-13-4) treatment, followed by rabbit complement.17 Cell suspensions contained <1% viable CD3+ T cells. Leukemic cells B6D2F1.BCR/ABL-NUP98/HOXA9 (GFP+, H-2Dd/b, CD45.2+) were generated as described.18 Mice that developed clinical scores ≥6 and/or high leukemia burdens (white blood cell count >100 × 106) were sacrificed. In CD8+ T-cell mixing transplants, mice received either 0.5 × 106 purified CD8+ T cells and 10 × 106 splenocytes derived from CD8+ T cell depleted, G-CSF treated donors (B6D2F1 recipients), 0.1 × 106 purified CD8+ T cells and 5 × 106 TCD bone marrow (Bm1 recipients) or 0.1 × 106 CD4+ T cells, 0.2 × 106 CD8+ T cells and 10 × 106 TCD bone marrow (Balb/c recipients). G-CSF–treated donor mice were given anti-CD8β–depleting mAbs (53.5.8) on d−5 and d−2. CD8+ T cells were isolated from donor mice treated with G-CSF in parallel (B6D2F1 recipients). For in vivo depletion of inducible diphtheria toxin receptor (iDTR) expressing cells, diphtheria toxin from Corynebacterium diphtheriae was administered intraperitoneally (IP) at 250 ng per dose on d4, d5, and d6. In long-term experiments, mice were additionally treated with diphtheria toxin at 100 ng per dose on d10, d13, d17, and d20 posttransplant (Sigma-Aldrich, St. Louis, MO). Non-GVHD control groups were injected with TCD grafts. Mice were monitored daily and systemic GVHD assessed weekly using a cumulative scoring system.19 Mice with GVHD scores ≥6 were culled and the date of death registered as the next day. For histologic analysis, tissue was fixed in paraformaldehyde and embedded in paraffin before tissue sectioning and hematoxylin and eosin staining.
Monoclonal antibodies
A list of antibodies used is provided in supplemental Table 1. Rat anti-mouse IL-6R mAb (MR16-1, provided by Chugai Pharmaceutical Co, Japan) or Rat IgG (Sigma-Aldrich) was given IP at 500 μg per dose on d−1 and d3 post-SCT as described.20 Mouse anti-transforming growth factor-β (TGFβ) mAb (1D11) and matched IgG isotype 13C4 (provided by Genzyme Corporation, Framingham, MA) was administered IP at 500 μg per dose on d0 and 100 μg per dose on d2, d4, and d6 posttransplant. Anti-IL-12/23p40 (C17.8) and matched-control IgG (Anti-AGP3 [cIg, 4D2]) were administered IP at 500 μg per dose on d−2, d0, d2, d4, and d6 pre-/posttransplant (AMGEN Inc., Thousand Oaks, CA).
Cell preparation, culture, and cytokine analysis
Cells were isolated by mechanical disruption and treated with lysis buffer to remove contaminating erythrocytes. For intracellular cytokine staining, cells were cultured with phorbol 12-myristate 13-acetate (5 μg/mL) and ionomycin (50 μg/mL) (Sigma-Aldrich) for 4 hours with Brefeldin A (BioLegend, San Diego, CA) included in the final 3 hours of incubation. Cells were surface-labeled and processed for intracellular staining, cytokines were assessed via cytofix/cytoperm kit (BD Biosciences, Franklin Lakes, NJ), and nuclear staining performed via fixation and permeablization (eBioscience, San Diego, CA). All samples were acquired on BD LSR Fortessa (BD Biosciences) using BD FACSDiva (v7.0) and analyzed with FlowJo software (v9.7, Ashland, OR).
Gene expression analysis
Total RNA was extracted with the RNeasy Micro kit (QIAGEN, The Netherlands) from sort-purified (>95% purity) cells and gene expression determined using TaqMan GE assays (Applied Biosystems, Waltham, MA). All measurements were run in parallel with the housekeeping gene Hprt. mRNA was amplified and biotinylated with the Illumina TotalPrep RNA Amplification Kit (Life Technologies, Beverly, MA) before hybridization to Mouse Ref-8 v2.0 Expression Bead Chip arrays. Chips were read via iScan Microarray Scanner and analyzed using GenomeStudio (Illumina) and GeneSpring GX v12.5 (Agilent Technologies, Santa Clara, CA). Pathway analyses were performed using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, QIAGEN). All microarray analyses have been deposited into the GEO public database under accession #GSE 70931.
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. An unpaired 2-tailed Mann-Whitney U test was used to evaluate differences in cytokine and mRNA studies. Data are mean ± standard error of the mean (SEM) and P < .05 is considered significant. Gene expression by microarray was assessed after Benjamini-Hochberg false discovery rate correction by unpaired Student t test (P < .05 cutoff) via GeneSpring GX v12.5 software, and then supervised hierarchical clustering performed. Seven-hundred two differentially expressed probes with >1.2-fold change were identified by unpaired Student t test (P < .05 cutoff) and this dataset was used in IPA analysis.
Results
Tc17 development after allo-SCT is an early and transient phenomenon
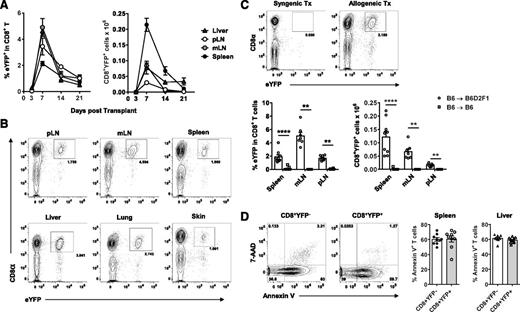
To track Tc17 induction and differentiation we used an IL-17A “fate-mapping” reporter donor IL-17CreRosa26eYFP, which permanently induces YFP expression in IL-17A−producing cells regardless of ongoing IL-17A gene expression.16 In time course experiments, CD8+YFP+ Tc17 cells were rapidly induced in all lymphoid tissues and GVHD target organs examined (Figure 1A-B). CD8+YFP+ expansion peaked at d7 posttransplant and contracted significantly over the following weeks, with up to 100-fold fewer CD8+YFP+ cells present at d21 relative to d7. Tc17 development was clearly an alloantigen-induced phenomenon because a CD8+YFP+ population did not develop in syngeneic recipients (Figure 1C). Given the relative enrichment of CD8+YFP+ T cells in the liver, which is a known site of apoptotic clearance,21 we examined CD8+ T-cell apoptosis in both liver and spleen via Annexin V staining. We observed high levels of Annexin V binding in all CD8+ T cells (∼60%), which likely explains the overall decrease in organ cellularity normally observed between d7 and d21 in GVHD (data not shown). However, we did not detect any significant differences in apoptosis between CD8+YFP+ and CD8+YFPneg counterparts in either spleen or liver T cells (Figure 1D). Because CD8+YFP+ T cell numbers appear to preferentially decline between d7 and d14, these data suggest that differential levels of expansion may contribute to the observed shift in CD8+YFP+ frequencies after d7.
Tc17 cells develop early post–allo-SCT in both central lymphoid and GVHD target organs. (A-D) CD8+YFP+ population development in lethally irradiated allogeneic (B6.IL-17CreRosa26eYFP→ B6D2F1) or (C) syngeneic (B6.IL-17CreRosa26eYFP→B6) mice transplanted with G-CSF–mobilized grafts. (A) Time-course analysis of CD8+YFP+ frequencies and absolute numbers within the CD8+ T-cell compartment 3, 7, 14, and 21 days posttransplant (mean ± SEM, n = 4-15 pooled mice/group from 2-4 independent experiments). pLN, peripheral (axillary and inguinal) lymph node; mLN, mesenteric lymph node. (B) Representative FACS analyses of CD8+YFP+ T cells 7 days after allo-SCT. (C) Representative FACS analyses (mLN) and CD8+YFP+ absolute numbers 7 days after syngeneic or allogeneic SCT (mean ± SEM, n = 6-10 pooled mice/group from 2 independent experiments; **P < .01, ****P < .0001). (D) Representative FACS analyses and frequencies of CD8+ T cells 7 days after allo-SCT costained with Annexin V and 7-AAD viability dye (mean ± SEM, n = 9-10 mice/group).
Tc17 cells develop early post–allo-SCT in both central lymphoid and GVHD target organs. (A-D) CD8+YFP+ population development in lethally irradiated allogeneic (B6.IL-17CreRosa26eYFP→ B6D2F1) or (C) syngeneic (B6.IL-17CreRosa26eYFP→B6) mice transplanted with G-CSF–mobilized grafts. (A) Time-course analysis of CD8+YFP+ frequencies and absolute numbers within the CD8+ T-cell compartment 3, 7, 14, and 21 days posttransplant (mean ± SEM, n = 4-15 pooled mice/group from 2-4 independent experiments). pLN, peripheral (axillary and inguinal) lymph node; mLN, mesenteric lymph node. (B) Representative FACS analyses of CD8+YFP+ T cells 7 days after allo-SCT. (C) Representative FACS analyses (mLN) and CD8+YFP+ absolute numbers 7 days after syngeneic or allogeneic SCT (mean ± SEM, n = 6-10 pooled mice/group from 2 independent experiments; **P < .01, ****P < .0001). (D) Representative FACS analyses and frequencies of CD8+ T cells 7 days after allo-SCT costained with Annexin V and 7-AAD viability dye (mean ± SEM, n = 9-10 mice/group).
Donor Tc17 are highly inflammatory and plastic in their cytokine profile
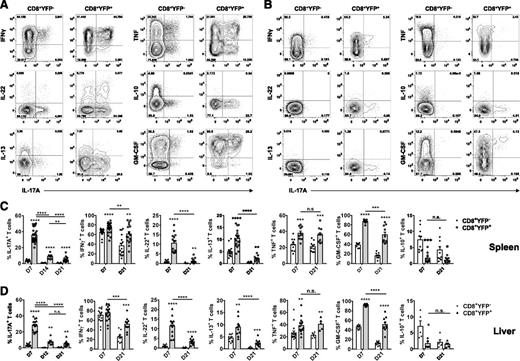
CD8+YFP+ Tc17 cells were the dominant IL-17A−producing T cells (Figure 2). At d7 after allo-SCT, ∼35% of CD8+YFP+ cells coexpressed IL-17 and IFNγ after restimulation, whereas the remaining CD8+YFP+ cells no longer expressed IL-17A but maintained IFNγ production. By d21, only 5% of remaining CD8+YFP+ cells continued to produce IL-17A, whereas IFNγ production was largely maintained, demonstrating significant plasticity within Tc17 (Figure 2A-D). Thus Tc17 differentiation is an early and transient phenomenon, with most remaining cells ultimately gaining a Tc1-like cytokine profile. To elaborate on the cytokine profile and plasticity observed in IL-17A production within the CD8+YFP+ population, we examined expression of a range of cytokines at d7 and d21 posttransplant. CD8+YFP+ T cells displayed considerable cytokine heterogeneity at d7, regardless of ongoing IL-17A expression. In addition to IL-17A, a significantly higher frequency of splenic CD8+YFP+ T cells also expressed the proinflammatory cytokines IFNγ, IL-22, IL-13, tumor necrosis factor (TNF) and granulocyte macrophage colony-stimulating factor (GM-CSF) relative to CD8+YFPneg T cells and YFP expression was inversely correlated with the immunoregulatory cytokine IL-10 (Figure 2A-C). A similar pattern was also observed in GVHD target tissue, whereby liver CD8+YFP+ T cells displayed significantly higher frequencies of IL-22, IL-13, TNF, and GM-CSF, and reduced IL-10 relative to CD8+YFPneg T cells (Figure 2D). By d21, in contrast to the relatively consistent cytokine profile observed in CD8+YFPneg T cells, CD8+YFP+ T cells had transitioned toward a more restricted IFNγ, TNF, and GM-CSF cytokine profile (Figure 2B-D). However, the proportion of CD8+YFP+ T cells expressing these cytokines remained significantly higher compared with CD8+YFPneg counterparts; thus, the CD8+YFP+ T cells maintained a proinflammatory profile (Figure 2C-D). Considerable plasticity was also observed in cytokine production within the CD8+YFP+ compartment, whereby IL-17A, IFNγ, IL-22, IL-13, and GM-CSF production significantly decreased over time in both spleen and liver (Figure 2C-D).
Posttransplant Tc17 cells are highly proinflammatory and plastic in cytokine profile. (A-D) Donor CD8+ T-cell cytokine expression was examined after allogeneic (B6.IL-17CreRosa26eYFP→B6D2F1) transplant. Representative FACS analysis showing splenic IL-17A, IFNγ, IL-22, IL-13, TNF, IL-10, and GM-CSF cytokine expression by CD8+YFPneg or CD8+YFP+ populations after short-term in vitro restimulation (A) d7 or (B) d21 posttransplant. (C-D) Frequencies of cytokine-expressing CD8+ T cells isolated from (C) spleen or (D) liver and assessed 1 to 3 weeks post–allo-SCT as indicated (mean ± SEM, n = ≥5 mice/group; *P < .05, **P < .01, ***P < .001, ****P < .0001).
Posttransplant Tc17 cells are highly proinflammatory and plastic in cytokine profile. (A-D) Donor CD8+ T-cell cytokine expression was examined after allogeneic (B6.IL-17CreRosa26eYFP→B6D2F1) transplant. Representative FACS analysis showing splenic IL-17A, IFNγ, IL-22, IL-13, TNF, IL-10, and GM-CSF cytokine expression by CD8+YFPneg or CD8+YFP+ populations after short-term in vitro restimulation (A) d7 or (B) d21 posttransplant. (C-D) Frequencies of cytokine-expressing CD8+ T cells isolated from (C) spleen or (D) liver and assessed 1 to 3 weeks post–allo-SCT as indicated (mean ± SEM, n = ≥5 mice/group; *P < .05, **P < .01, ***P < .001, ****P < .0001).
Alloantigen-induced Tc17 cells express a unique pattern of genes associated with inflammation and leukocyte, recruitment, and migration
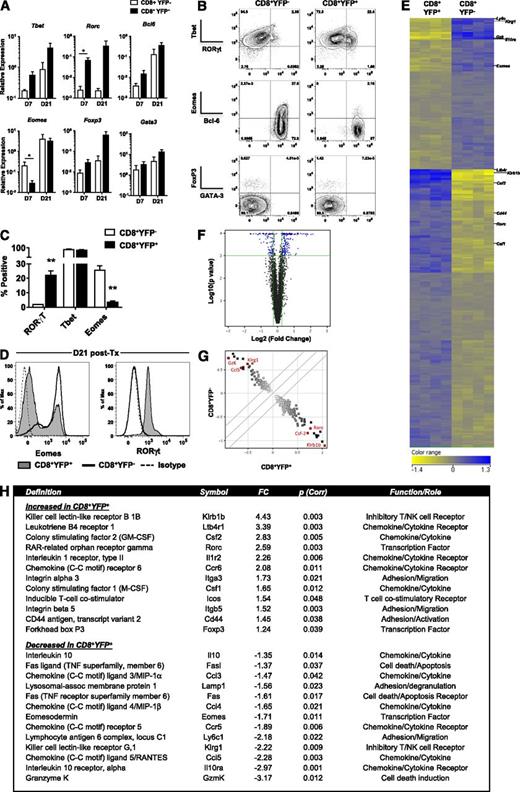
Given the observed cytokine heterogeneity and plasticity, we next investigated the expression of lineage-defining transcription factors to address differences in T-cell polarization between the CD8+YFP+ and YFPneg populations. Quantitative polymerase chain reaction (qPCR) of CD8+ T cells sorted from allograft recipients revealed that CD8+YFP+ T cells express high levels of both Rorc and Tbet mRNA, the transcription factors associated with Th17/Tc17 and Th1/Tc1 differentiation, respectively (Figure 3A). In addition to increased expression of Rorc and Tbet in the CD8+YFP+ population, a general trend toward increased expression of Bcl6 and Foxp3 was observed. Notably, however, at d7 when these cells were most abundant, the Tbet-related transcription factor Eomesodermin (Eomes) was significantly reduced in CD8+YFP+ cells. The differential expression of both RORγt and Eomes proteins were confirmed via intracellular staining of sort-purified YFP+ and YFPneg CD8+ T cells (Figure 3B-D) and demonstrated an inverse correlation between the expression of RORγt and Eomes. Interestingly, although the CD8+YFP+ T-cell proinflammatory cytokine profile was significantly altered over time, the transcription factor profile remained strikingly diverse (Figure 3A,D). Despite significantly reduced IL-17A expression in CD8+YFP+ T cells d21 posttransplant, RORγt protein expression increased over time. Similarly, Eomes protein expression also increased over this same period, but remained low compared with CD8+YFPneg T cells. Taken together, cytokine and transcription factor profiling highlight distinct differentiation programs in the CD8+YFP+ and YFPneg populations, with CD8+YFPneg cells exhibiting classical and fixed Tc1 polarization in contrast to YFP+ T cells, which exhibited a unique Tc17 differentiation profile characterized by secretion of multiple inflammatory cytokines.
Posttransplant Tc17 cells express a distinct transcription factor and gene expression profile. (A-C) CD8+YFP+ and CD8+YFPneg T-cell transcription factor expression after allogeneic (B6.IL-17CreRosa26eYFP→B6D2F1) transplant. (A) YFP+ and YFPneg CD8+ T cells isolated from spleen, mLNs, and liver by FACS on d7 and d21 followed by qPCR (mean ± SEM, n = 3-4 independent experiments each consisting of 10 pooled mice/group; *P < .05). (B) Representative flow cytometry data from transcription factor staining of CD8+ T-cell populations isolated from pooled spleen, mLNs, and liver d7 posttransplant. (C) Frequencies of transcription factor–expressing CD8+ T cells gated on YFP expression 7 days posttransplant (mean ± SEM, n = 4 independent experiments each consisting of 10 pooled mice/group; **P < .01). (D) Eomes and RORγt expression 21 days posttransplant in YFP+ and YFPneg CD8+ T cells isolated from pooled spleen, mLNs, and liver. (E-H) Microarray analysis of CD8+YFP+ and CD8+YFPneg T-cell gene expression after allogeneic (B6.IL-17CreRosa26eYFP→ B6D2F1) transplant. (E) Gene expression heat map d7 posttransplant showing 299 gene probes differentially expressed between CD8+YFP+ and CD8+YFPneg T cells sorted from 4 independent experiments consisting of 10 mice pooled/group. Genes were identified by unpaired Student t test (P < .05) after false discovery rate (Benjamini-Hochberg) P value correction. (F) Volcano plot showing the distribution of expression in all genes expressed by either CD8+YFP+ or CD8+YFPneg T cells. Blue data points represent genes with a >1.2 fold differential expression with an uncorrected P value < .001. (G) Scatter plot highlighting the 299 gene probes shown in (D). Red scatter data points highlight differentially expressed genes of interest. (H) Twenty-five selected immunologically relevant genes differentially regulated in CD8+YFP+ and CD8+YFPneg T cells. FC, fold change. See also supplemental Tables 2 and 3).
Posttransplant Tc17 cells express a distinct transcription factor and gene expression profile. (A-C) CD8+YFP+ and CD8+YFPneg T-cell transcription factor expression after allogeneic (B6.IL-17CreRosa26eYFP→B6D2F1) transplant. (A) YFP+ and YFPneg CD8+ T cells isolated from spleen, mLNs, and liver by FACS on d7 and d21 followed by qPCR (mean ± SEM, n = 3-4 independent experiments each consisting of 10 pooled mice/group; *P < .05). (B) Representative flow cytometry data from transcription factor staining of CD8+ T-cell populations isolated from pooled spleen, mLNs, and liver d7 posttransplant. (C) Frequencies of transcription factor–expressing CD8+ T cells gated on YFP expression 7 days posttransplant (mean ± SEM, n = 4 independent experiments each consisting of 10 pooled mice/group; **P < .01). (D) Eomes and RORγt expression 21 days posttransplant in YFP+ and YFPneg CD8+ T cells isolated from pooled spleen, mLNs, and liver. (E-H) Microarray analysis of CD8+YFP+ and CD8+YFPneg T-cell gene expression after allogeneic (B6.IL-17CreRosa26eYFP→ B6D2F1) transplant. (E) Gene expression heat map d7 posttransplant showing 299 gene probes differentially expressed between CD8+YFP+ and CD8+YFPneg T cells sorted from 4 independent experiments consisting of 10 mice pooled/group. Genes were identified by unpaired Student t test (P < .05) after false discovery rate (Benjamini-Hochberg) P value correction. (F) Volcano plot showing the distribution of expression in all genes expressed by either CD8+YFP+ or CD8+YFPneg T cells. Blue data points represent genes with a >1.2 fold differential expression with an uncorrected P value < .001. (G) Scatter plot highlighting the 299 gene probes shown in (D). Red scatter data points highlight differentially expressed genes of interest. (H) Twenty-five selected immunologically relevant genes differentially regulated in CD8+YFP+ and CD8+YFPneg T cells. FC, fold change. See also supplemental Tables 2 and 3).
To further investigate Tc1 and Tc17, we performed a genome-wide microarray study of sorted CD8+YFP+ and CD8+YFPneg T cells. This analysis demonstrated that the 2 populations differed by ∼300 genes (Figure 3E-H), including increased Rorc and decreased Eomes transcription factor expression in CD8+YFP+ T cells, as observed in both qPCR and fluorescence-activated cell sorting (FACS) analysis. In confirmation of our earlier findings, multiple genes associated with inflammatory pathways were significantly upregulated in the CD8+YFP+ population, including leukocyte adhesion/migration (Itga3, Integrin α3; Itgb4, Integrin β4), chemotactic factor/cytokines (Csf2, GM-CSF; Csf1, CSF-1), and chemokine/cytokine receptors (Ltb4r1, Leukotriene B4 receptor; Il1r2, IL-1R; Ccr6) (Figure 3H). The most highly upregulated gene in the CD8+YFP+ population was Klrb1b, the murine homolog of CD161, whose expression in humans is a marker of IL-17−producing T cells.22 In addition, we observed that the expression of a number of genes associated with cytolytic function were downregulated in CD8+YFP+ cells, including those encoding the degranulation marker CD107a (Lamp1) and the CTL effector molecules FasL and Granzyme K. In agreement with intracellular cytokine staining, gene expression of the immunoregulatory cytokine IL-10 was also downregulated in the CD8+YFP+ population, as was the IL-10 receptor (IL10ra) (Figure 3H), suggesting that not only are Tc17 poor immune regulators themselves, but they may also be less responsive to IL-10 from other sources. Ingenuity Pathway Analysis identified canonical pathways associated with differential gene expression between CD8+YFP+ and CD8+YFPneg populations (supplemental Tables 2 and 3). These data confirmed that these genes were significantly associated with inflammatory pathways and CTL activity and highlighted a number of potential upstream regulators including IL-10Ra, Eomes, FASL, and SOCS3.
Tc17 are a functionally distinct subset with reduced levels of CTL effector protein expression
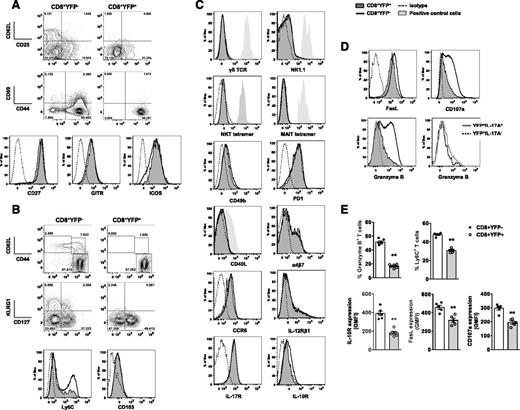
To confirm differences observed at the gene level, we assessed phenotypic markers associated with T-cell activation/memory, specialized T-cell subsets, chemokine/cytokine receptors, and CTL effector function after allo-SCT. Irrespective of YFP expression, the activation markers CD25, CD69, CD44, and costimulatory molecules CD27 and GITR were similarly expressed in all CD8+ T cells 7 days after transplant (Figure 4A); however, reduced frequencies of CD62L+CD44neg (naïve) and CD62L+CD44+ (central memory/TCM) populations were detected in CD8+YFP+ T cells in spleen and liver, both early and late posttransplant (Figure 4B and supplemental Figure 1). Similarly, expression of the TCM-associated protein Ly6C23 was significantly lower in CD8+YFP+ T cells in concordance with microarray analyses (Figure 4B,E). Given the reduced Eomes and Ly6C expression observed in Tc17 cells, we examined other molecules associated with T-cell differentiation and memory. CD8+YFP+ T cells displayed significantly reduced expression of the short-lived effector cell−associated protein KLRG1 and increased expression of the memory-precursor effector cell marker CD127 (IL-7Rα).24 Furthermore, differences were observed in the development of tissue resident memory T cells (TRM) in lung on d21 posttransplant (supplemental Figure 1). No preferential expression was observed in CD8+YFP+ T cells of markers associated with known IL-17−producing, functionally distinct T cell populations, including γδ T cells (γδ TCR), NKT (NK1.1, CD49b, murine CD1d-α-gal tetramer), MAIT (murine MR1 tetramer), CD8+ “helper” cells (CD40L), and Th17 (α4β7, CCR6) (Figure 4C).25-31 IL-12, IL-23, IL-17, and IL-10 have each have been reported to contribute to the regulation of IL-17A production,32-34 and although IL-12Rβ1 (involved in both IL-12 and IL23 recognition) and IL-17R were expressed at equal levels in YFP+ and YFPneg CD8+ T-cell subsets (Figure 4C), IL-10R expression was significantly lower in CD8+YFP+ T cells, as suggested by microarray analysis (Figure 4C,E). We next assessed cytolytic molecule expression (Figure 4D-E) and noted reduced expression of the cell death−inducing effector molecule FasL and the degranulation marker CD107a (Lamp1). Furthermore, we observed strikingly limited Granzyme B expression in CD8+YFP+ T cells, which was independent of ongoing IL-17A expression within the Tc17 population. Given this inflammatory, noncytolytic phenotype, we have defined this cell population as inflammatory Tc17 (iTc17) cells.
Early posttransplant Tc17 cells express altered T-cell memory markers and reduced CTL effector molecules. Phenotypic analysis of splenic CD8+YFP+ and CD8+YFPneg T cells d7 posttransplant (B6.IL-17CreRosa26eYFP→B6D2F1). (A-D) Representative flow cytometry analysis of markers associated with (A) T-cell activation, (B) T-cell memory, (C) specialized T-cell functions and chemokine/cytokine receptor expression, and (D) CTL effector function. CD8+YFP+ (filled dark gray), CD8+YFPneg (thick black), isotype (thin dashed), positive control cells (filled light gray), CD8+YFP+IL-17+ (thick gray), and CD8+YFP+IL-17neg (black dotted). (E) Quantitative analysis of Granzyme B, Ly6C, IL-10R, FasL, and CD107a expression in CD8+ T cells (mean ± SEM, n = 5-6 mice/group; **P < .01). (C) Positive controls used: γδ TCR+ CD4–CD8– naïve T cells, NK1.1+ naïve NK cells, NKT tetramer+CD4+ naïve NKT-cells, MR1 tetramer+CD4–CD8– naïve T cells, and CD40L+CD4+ naïve T-cells.
Early posttransplant Tc17 cells express altered T-cell memory markers and reduced CTL effector molecules. Phenotypic analysis of splenic CD8+YFP+ and CD8+YFPneg T cells d7 posttransplant (B6.IL-17CreRosa26eYFP→B6D2F1). (A-D) Representative flow cytometry analysis of markers associated with (A) T-cell activation, (B) T-cell memory, (C) specialized T-cell functions and chemokine/cytokine receptor expression, and (D) CTL effector function. CD8+YFP+ (filled dark gray), CD8+YFPneg (thick black), isotype (thin dashed), positive control cells (filled light gray), CD8+YFP+IL-17+ (thick gray), and CD8+YFP+IL-17neg (black dotted). (E) Quantitative analysis of Granzyme B, Ly6C, IL-10R, FasL, and CD107a expression in CD8+ T cells (mean ± SEM, n = 5-6 mice/group; **P < .01). (C) Positive controls used: γδ TCR+ CD4–CD8– naïve T cells, NK1.1+ naïve NK cells, NKT tetramer+CD4+ naïve NKT-cells, MR1 tetramer+CD4–CD8– naïve T cells, and CD40L+CD4+ naïve T-cells.
iTc17 development is promoted by IL-6 and recipient dendritic cells and is regulated by IFNγ
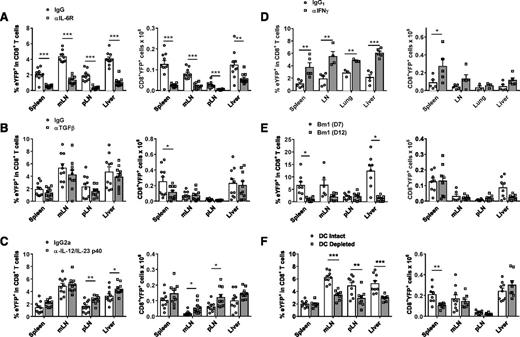
We next investigated the cytokine and antigen presentation requirements for iTc17 differentiation after allogeneic transplantation. Because both IL-6 and TGFβ are known to drive Th17 polarization, we examined the effect of genetic ablation/antibody neutralization of these cytokines on iTc17 development. The absence of IL-6 had the most prominent effect, whereby mice that received IL-6 receptor–blocking mAb after allo-SCT displayed significantly reduced CD8+YFP+ frequencies and cell numbers in multiple tissues posttransplant (Figure 5A). Similarly, a significant reduction in CD8+YFP+ numbers was also observed when IL-6−/− recipient mice were transplanted (data not shown), demonstrating that the source of IL-6 required for iTc17 induction is mainly host-derived. In contrast, administration of TGFβ-neutralizing mAb had little effect on iTc17 development in this system, with only the spleen exhibiting a significant reduction in CD8+YFP+ numbers after TGFβ inhibition (Figure 5B). Neutralization of the shared p40 subunit of IL-12 and IL-23 resulted in a partial enhancement of iTc17 development, with increases in CD8+YFP+ numbers in mesenteric and peripheral lymph nodes (Figure 5C). IFNγ is the dominant cytokine downstream of IL-12 and its neutralization also resulted in a significant increase in CD8+YFP+ frequencies in multiple tissues posttransplant (Figure 5D). CD8+YFP+ development was robust in the B6→bm1 model of CD8-dependent GVHD induced by an isolated major histocompatibility complex class I mismatch (Figure 5E). This confirms that iTc17 can develop in a class I restricted manner in the absence of an alloreactive CD4+ T-cell response, and therefore CD4+ T-cell help. Finally, CD8+YFP+ iTc17 development was partially dependent on the presence of recipient dendritic cells (DCs) because CD8+YFP+ frequencies were significantly reduced when host DCs were depleted using diphtheria toxin (DT)-treated CD11c-DTR (CD11c.DOG.F1) recipients (Figure 5F). Given that iTc17 appear within the first week of BMT, before the reconstitution of donor DCs,35 it would seem less likely that these cells initiate iTc17 development. These data demonstrate that primarily host-derived IL-6 and DCs drive the development of iTc17 cells after allo-SCT, and this process is inhibited by the presence of IFNγ.
iTc17 development posttransplant is primarily driven by IL-6 and regulated by IFNγ and IL-12p40. (A-D) iTc17 development was assessed by enumeration of CD8+YFP+ T cells 7 days post–allo-SCT (B6.IL-17CreRosa26eYFP→B6D2F1). Mice were treated with either isotype control mAbs or blocking mAbs targeting (A) IL-6R, (B) TGFβ, (C) IL-12/IL-23p40, and (D) IFNγ. (E-F) CD8+YFP+ T-cell frequencies 7 days posttransplant in (E) a B6.IL-17CreRosa26eYFP→bm1 model, wherein only donor CD8+ T cells react to alloantigen, or (F) a B6.IL-17CreRosa26eYFP→CD11c.DOG-F1 model, wherein recipient mice were treated with DT to deplete DCs before transplant. Data are pooled from 2 independent experiments (3-10 mice/group total ± SEM; *P < .05, **P < .01, ***P < .001).
iTc17 development posttransplant is primarily driven by IL-6 and regulated by IFNγ and IL-12p40. (A-D) iTc17 development was assessed by enumeration of CD8+YFP+ T cells 7 days post–allo-SCT (B6.IL-17CreRosa26eYFP→B6D2F1). Mice were treated with either isotype control mAbs or blocking mAbs targeting (A) IL-6R, (B) TGFβ, (C) IL-12/IL-23p40, and (D) IFNγ. (E-F) CD8+YFP+ T-cell frequencies 7 days posttransplant in (E) a B6.IL-17CreRosa26eYFP→bm1 model, wherein only donor CD8+ T cells react to alloantigen, or (F) a B6.IL-17CreRosa26eYFP→CD11c.DOG-F1 model, wherein recipient mice were treated with DT to deplete DCs before transplant. Data are pooled from 2 independent experiments (3-10 mice/group total ± SEM; *P < .05, **P < .01, ***P < .001).
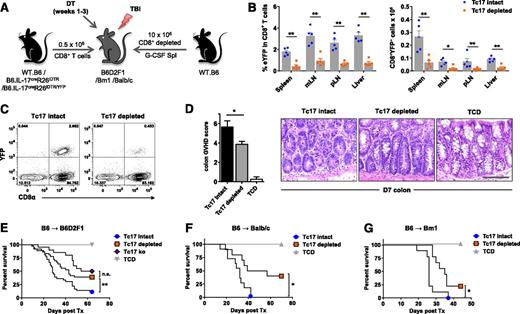
Targeted deletion of iTc17 protects against lethal GVHD
Given the proinflammatory phenotype exhibited by iTc17 (Figures 2 and 3), we hypothesized that by depleting these cells posttransplant, the severity of GVHD may be reduced. To test this, we used a second “deletable” fate-mapping system (IL-17CreRosa26iDTR), in which the DT receptor is induced on IL-17–producing cells regardless of ongoing IL-17 gene expression.36 By transplanting mice with CD8+ T cell–depleted G-CSF–mobilized allografts supplemented with either wild-type (WT) or IL-17CreRosa26iDTR–derived CD8+ T cells, iTc17 cells could be targeted for depletion, and other IL-17–producing cells, such as Th17, remained intact. Therefore, by treating mice early posttransplant with DT and providing ongoing treatment over a period of 3 weeks, we depleted iTc17 cells independent of their continued IL-17 production (Figure 6A). Successful depletion was confirmed in IL-17CreRosa26iDTR/YFP “reporter-deleter” mice at d7 posttransplant, when we detected significantly reduced CD8+YFP+ T cells in all organs examined (Figure 6B-C). In line with their proinflammatory phenotype, blinded histologic analysis demonstrated significantly reduced GVHD pathology in the colon tissue d7 posttransplant. Furthermore, we observed significant protection from lethal GVHD after iTc17 depletion in B6D2F1 recipients (Figure 6D-E), similar to that of Tc17-deficient grafts (Tc17 ko), where IL-17A−/− CD8 T cells were transplanted. In addition, we also demonstrated significant protection in an alternate major histocompatibility complex mismatch model of allo-SCT using Balb/c recipients (Figure 6F) and in a CD8+ T cell–dependent model of GVHD (Figure 6G). These data confirm that iTc17 cells are a pathogenic population and represent a potential target for the prevention of GVHD.
iTc17 depletion protects mice from lethal aGVHD. Lethally irradiated (A-E) B6D2F1, (F) Balb/c, or (G) Bm1 mice received CD8+ T cell–depleted, G-CSF–mobilized WT.B6 allografts supplemented with purified CD8+ T cells derived from WT.B6 (Tc17 intact), IL-17CreRosa26iDTR, IL-17CreRosa26iDTR (Tc17 depleted), or IL-17A–deficient (Tc17 ko) mice as indicated. WT.B6 G-CSF–mobilized WT allografts depleted of both CD4+ and CD8+ T cells were used as no-GVHD controls (TCD). All groups received continued DT treatment during weeks 1 to 3 as described in Materials and methods. (B-C) CD8+YFP+ T-cell depletion was assessed d7 posttransplant in B6D2F1 recipients of grafts containing IL-17CreRosa26eYFP/iDTR heterozygous CD8+ T cells. (B-C) CD8+YFP+ T-cell frequencies after Tc17 depletion were enumerated by flow cytometry (mean ± SEM, n = 5 mice/group; *P < .05, **P < .01). (D) Quantitative GVHD histopathology analysis was performed on colon tissue isolated d7 posttransplant from B6D2F1 recipients of allografts as described in (A) (Tc17 intact, 6 mice/group; Tc17 depleted, 8 mice/group; TCD, 4 mice/group; *P < .05). Representative d7 colon histology images are shown (scale bar = 0.1 mm). (E-G) Survival indices by Kaplan-Meier analyses are shown for (E) B6D2F1, (F) Balb/c, and (G) Bm1 recipients of allo-SCT as described before. (E) Data are pooled from 4 independent experiments (Tc17 intact; Tc17 depleted, 36 mice/group; Tc17 ko, 20 mice/group; TCD, 16 mice/group; **P < .01). (F) Data are pooled from 2 independent experiments (Tc17 intact; Tc17 depleted, 10 mice/group; TCD, 6 mice/group; *P < .05). (G) Data are derived from 1 experiment (Tc17 intact; Tc17 depleted, 9 mice/group; TCD, 3 mice/group; *P < .05).
iTc17 depletion protects mice from lethal aGVHD. Lethally irradiated (A-E) B6D2F1, (F) Balb/c, or (G) Bm1 mice received CD8+ T cell–depleted, G-CSF–mobilized WT.B6 allografts supplemented with purified CD8+ T cells derived from WT.B6 (Tc17 intact), IL-17CreRosa26iDTR, IL-17CreRosa26iDTR (Tc17 depleted), or IL-17A–deficient (Tc17 ko) mice as indicated. WT.B6 G-CSF–mobilized WT allografts depleted of both CD4+ and CD8+ T cells were used as no-GVHD controls (TCD). All groups received continued DT treatment during weeks 1 to 3 as described in Materials and methods. (B-C) CD8+YFP+ T-cell depletion was assessed d7 posttransplant in B6D2F1 recipients of grafts containing IL-17CreRosa26eYFP/iDTR heterozygous CD8+ T cells. (B-C) CD8+YFP+ T-cell frequencies after Tc17 depletion were enumerated by flow cytometry (mean ± SEM, n = 5 mice/group; *P < .05, **P < .01). (D) Quantitative GVHD histopathology analysis was performed on colon tissue isolated d7 posttransplant from B6D2F1 recipients of allografts as described in (A) (Tc17 intact, 6 mice/group; Tc17 depleted, 8 mice/group; TCD, 4 mice/group; *P < .05). Representative d7 colon histology images are shown (scale bar = 0.1 mm). (E-G) Survival indices by Kaplan-Meier analyses are shown for (E) B6D2F1, (F) Balb/c, and (G) Bm1 recipients of allo-SCT as described before. (E) Data are pooled from 4 independent experiments (Tc17 intact; Tc17 depleted, 36 mice/group; Tc17 ko, 20 mice/group; TCD, 16 mice/group; **P < .01). (F) Data are pooled from 2 independent experiments (Tc17 intact; Tc17 depleted, 10 mice/group; TCD, 6 mice/group; *P < .05). (G) Data are derived from 1 experiment (Tc17 intact; Tc17 depleted, 9 mice/group; TCD, 3 mice/group; *P < .05).
iTc17 cells do not contribute to GVL effects
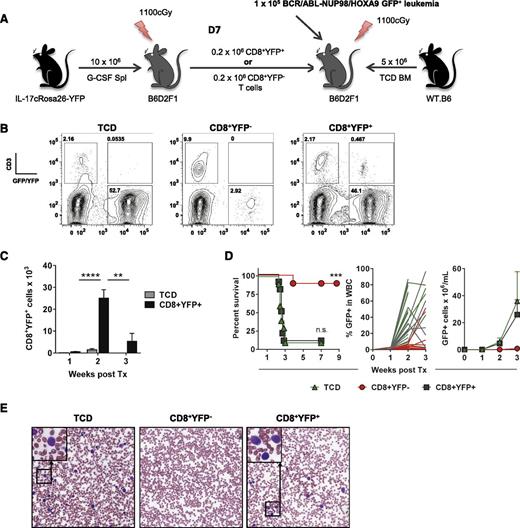
Because CD8+ T cells are critical mediators of both GVHD and GVL posttransplant, and the quality of antitumor responses positively correlate with the incidence of GVHD,37,38 we assessed the contribution of iTc17 cells to GVL using a primary blast-crisis chronic myeloid leukemia (CML) model. Initial transplants were performed using B6.IL-17CreRosa26YFP donors to generate alloantigen-induced CD8+YFP+ or CD8+YFPneg cells, which were isolated by FACS sorting on d7 after transplantation. Subsequently, WT.B6 T cell–depleted BM was supplemented with the purified Tc1 or iTc17 populations and transplanted along with primary leukemia cells18 into B6D2F1 recipients (Figure 7A). Leukemia burdens and CD8+YFP+ T-cell expansion were monitored in peripheral blood via leukemia expression of green fluorescent protein and yellow fluorescent protein expression in CD3+ T cells, respectively (Figure 7B).
iTc17 cells do not directly contribute to posttransplant tumor clearance. (A) CD8+YFP+ or CD8+YFPneg T cells were isolated 7 days posttransplant (B6.IL-17CreRosa26eYFP→B6D2F1) and combined with WT.B6 T cell–depleted (TCD) BM for secondary transplant into lethally irradiated B6D2F1 recipients. Mice receiving TCD BM only were included as controls and all grafts supplemented with 1 × 105 B6D2F1-derived BCR/ABL-NUP98/HOXA9 GFP+ CML leukemic cells before transplantation. (B) Representative flow cytometry analysis of leukemia progression and CD8+YFP+ T-cell expansion 14 days posttransplant. (C) CD8+YFP+ T cells were enumerated on d7, d14, and d21 posttransplant (± SEM, n = 10 mice/group; ****P < .0001, **P < .01). (D) Survival indices by Kaplan-Meier analysis and tumor growth posttransplant are shown. Data are pooled from 2 independent experiments (10 mice/group total; ***P < .001). (E) Representative images of blood smears collected 3 weeks posttransplant (Wright-Giemsa stain, original magnification ×200).
iTc17 cells do not directly contribute to posttransplant tumor clearance. (A) CD8+YFP+ or CD8+YFPneg T cells were isolated 7 days posttransplant (B6.IL-17CreRosa26eYFP→B6D2F1) and combined with WT.B6 T cell–depleted (TCD) BM for secondary transplant into lethally irradiated B6D2F1 recipients. Mice receiving TCD BM only were included as controls and all grafts supplemented with 1 × 105 B6D2F1-derived BCR/ABL-NUP98/HOXA9 GFP+ CML leukemic cells before transplantation. (B) Representative flow cytometry analysis of leukemia progression and CD8+YFP+ T-cell expansion 14 days posttransplant. (C) CD8+YFP+ T cells were enumerated on d7, d14, and d21 posttransplant (± SEM, n = 10 mice/group; ****P < .0001, **P < .01). (D) Survival indices by Kaplan-Meier analysis and tumor growth posttransplant are shown. Data are pooled from 2 independent experiments (10 mice/group total; ***P < .001). (E) Representative images of blood smears collected 3 weeks posttransplant (Wright-Giemsa stain, original magnification ×200).
CD8+YFP+ T cell numbers increased significantly between d7 and d14, demonstrating the viability of iTc17 cells and their expansion after secondary transplantation; however, this expansion was not maintained during week 3, when tumor burdens were very high (Figure 7C). Importantly, in mice receiving CD8+YFP+ T cells, leukemia was uncontrolled and both survival and tumor growth curves directly overlapped with that of the recipients receiving no donor T cells. In contrast, the majority of recipients of CD8+YFPneg donor T cells cleared leukemia within 3 weeks of transplantation, and antileukemic immunity was maintained long term (Figure 7D-E). Taken together, these data demonstrate that iTc17, which develop during GVHD, represent a unique, highly plastic, hyperinflammatory, noncytolytic CD8+ T-cell effector population.
Discussion
Antitumor immunity and GVHD after allo-SCT are directly linked to donor T-cell differentiation. Although the role of T cell–derived IL-17 in GVHD is increasingly recognized, the focus has been predominantly on IL-17 production by CD4+ T cells, whereas donor CD8+ T-cell polarization in this context has been largely unappreciated. We have previously shown that in a murine model of allo-SCT, G-CSF mobilization promotes donor Th17/Tc17 differentiation and that IL-17A–deficient allografts protect recipients from CD8+ T cell–mediated sclerodermatous GVHD.15 Recent studies in other disease models have demonstrated significant plasticity within both Th17 and Tc17 lineages12,32,34,39-41 ; however, plasticity studies of Tc17 have been limited to the use of in vitro polarized CD8+ T cells. To circumvent this limitation, we used a murine IL-17A “fate-mapping” reporter model to characterize an inflammatory Tc17 lineage that develops during GVHD, and to our knowledge, we are the first to do so. We found that iTc17 development is both an early and transient phenomenon after allo-SCT dependent on host IL-6 and DCs, which is regulated by the presence of IL-12 and IFNγ. The iTc17 subset displays considerable lineage promiscuity in transcriptional profile and cytokine production and targeted depletion of iTc17 cells early after transplant was protective in lethal models of acute GVHD. In contrast, iTc17 are significantly impaired in cytolytic and GVL activity and provide little or no direct antitumor immunity in vivo.
iTc17 numbers declined dramatically in weeks 2 to 3 after transplant, although there was some evidence for their persistence in the liver. This Tc17 contraction is concordant with a human study in which CD161 was used as a surrogate marker of IL-17–producing T cells,42 whereby a decline in CD8+CD161+ T-cell frequencies in peripheral blood was observed over time after allo-SCT. Although previous attempts have been made to address the persistence of Tc17 cells in vivo, the significant level of plasticity observed in this population has hampered these questions. There are some data to suggest that in vitro, polarized Tc17 may be more persistent after adoptive transfer than Tc1-polarized CD8+ T cells41,43 ; however, this was not the case in our in vivo murine system, where we observed a preferential decline in CD8+YFP+ frequencies over time after transplant.
iTc17 plasticity was particularly striking early after transplant, whereby the majority of CD8+YFP+ T cells had already downregulated IL-17 production as early as d7, highlighting the importance of a fate-mapping model to monitor this population. In addition, iTc17 also displayed considerable heterogeneity in cytokine expression. Notably, coexpression of both IL-17A and IFNγ was observed early posttransplant, which appears to be a general feature of Tc17 cells.15,32,34 This dual expression has been attributed to epigenetic suppression of the regulatory protein SOCS3,32 whose absence in donor T cells we have shown to also result in polyfunctional T-cell responses and exacerbated GVHD.44 In this study, we found that Socs3 gene expression was downregulated in CD8+YFP+ T cells posttransplant (fold-change −1.306, P = .001, unpaired moderated Student t test with false discovery rate correction), and was highlighted by microarray analysis as a potential upstream regulator of CD8+YFP+ cells (supplemental Table 3). These data suggest that differential expression of Socs3 may also drive the hyper–proinflammatory T-cell phenotype observed in iTc17. Multiple studies have looked at the mechanisms behind in vitro Tc17 plasticity, finding that IL-12 signaling via Stat4 is required for IFNγ induction in both CD4+ and CD8+ populations during inflammation.40,45 This is also likely to be the case in the context of allo-SCT, because IL-12 is upregulated early posttransplant and is a well-described mediator of aGVHD.46
Our comprehensive characterization of the CD8+YFP+ T-cell population posttransplant revealed a distinct subset of proinflammatory CD8+ T cells, with diminished capacity for “classical” effector T-cell function regardless of ongoing IL-17A expression. This unique phenotype appears to be driven by dual expression of the transcription factors Tbet and RORγt, previously associated with an inflammatory profile likely to be protective during host-pathogen interactions in innate lymphoid cells and CD4+ T cells.47 However, the Tbet+RORγt+ T-cell differentiation pathway may carry an increased risk of autoimmune disease pathologies,47-49 which share many of the clinical and histologic features of GVHD. Similarly, expression of the transcription factor Eomes is negatively correlated with IL-17 expression8,50,51 and has been reported to be necessary for full CTL effector function and is therefore likely to explain the poor cytotoxicity observed in the iTc17 population.52 Because these iTc17 are difficult to identify after BMT based on surface marker and cytokine profiles (given their promiscuity), it may be that transcription factor expression instead represents a more useful approach to their identification. Given that these cells are in fact highly functional with regard to proinflammatory cytokine function, we propose that the iTc17 population represents a unique population of nonclassical effector CD8+ T cells whose primary function is to contribute to inflammation and recruitment of both lymphoid and myeloid effector cells, resulting in GVHD pathology. In agreement with this, we observed significantly elevated Csf1 gene expression by CD8+YFP+ T cells, which we have recently demonstrated in IL-17–dependent models of lung and sclerodermatous GVHD is a critical factor driving macrophage tissue infiltration and pathology.53
The signaling pathways that contribute to type-17 differentiation are relatively well characterized, with IL-6, TGF-β, IL-21, and IL-23 promoting IL-17A production and both IFNγ and IL-12 acting as regulators.39 We have previously demonstrated that type-17 differentiation in G-CSF–mobilized donor T cells is highly IL-21–dependent,15 and we now show that IL-6R signaling is also required for iTc17 differentiation, coinciding with the presence of elevated IL-6 levels in plasma early posttransplant.20 These data are in line with previously published observations that IL-6R blockade significantly protects from lethal GVHD in murine models of GVHD.20,54 Furthermore, we have recently demonstrated in phase 1/2 clinical trials that IL-6R inhibition after allo-SCT is a promising therapeutic approach for GVHD intervention in humans.55 In contrast, TGFβ and IL-12/IL-23p40 were minor contributors to iTc17 development in our systems, with little to no effect observed on iTc17 differentiation when neutralized in vivo. IFNγ neutralization significantly enhanced type-17 differentiation, consistent with the known crossregulation of lineage differentiation by IFNγ and IL-17A. Importantly, iTc17 cells continue to produce high levels of IFNγ in the absence of IL-17 production; thus, without a fate reporter, iTc17 are indistinguishable from Tc1 cells based on IFNγ and IL-17 expression, and this likely contributes to our early observations regarding the disappearance of CD8+IL-17+ T cells after transplantation.
Because GVHD arises as a complication to therapy designed to provide GVL effects, the identification of factors that contribute to GVHD but not GVL is highly desirable. The proinflammatory phenotype and impaired CTL effector function observed in iTc17 posttransplant suggested that this population may represent a suitable target for therapeutic intervention after allo-SCT. There are conflicting reports in the literature regarding the potential for cytolytic activity in Tc17 in vivo, particularly with regard to Tc1-shifted cells derived from the Tc17 subset. Reports of Tc17 CTL function range from enhanced in some studies34,40,43 to diminished in others.41,56 In addition, other studies have suggested that Tc17 contribute to host protection via inflammatory mediators (such as IFNγ) as opposed to cytotoxic mechanisms.12,57 Our studies demonstrate that CD8+YFP+ iTc17 cells cannot eradicate leukemia in vivo, which is concordant with their uniformly poor expression of factors including Eomes (previously linked with poor antitumor immunity)58 and the cytolytic effector molecule Granzyme B.59
This study provides new insights into donor T-cell polarization after allo-SCT, demonstrating that iTc17 differentiation is an early and highly plastic differentiation program culminating in a poorly cytolytic, hyperinflammatory donor T cell. Intervention to prevent this differentiation program, putatively via either IL-6 or RORγt inhibition, would thus be predicted to reduce GVHD while maintaining or enhancing GVL effects.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lars Kier-Nielson for providing reagents and acknowledge the assistance of Andrea Henden, Kelly Locke, and the Flow Cytometry & Imaging facility at QIMR Berghofer; Paula Hall; Nigel Waterhouse; Michael Rist; and Grace Chojnowski.
G.R.H. and M.J.S. are National Health and Medical Research Council (NHMRC) Fellows. K.P.A.M. is a Cancer Council Queensland Senior Research Fellow. M.W.L.T. is an NHMRC Career Development Fellow. K.A.M. is an NHMRC Early Career Fellow. M.K. is a Leukemia Foundation Postdoctoral Fellow.
This work was supported by grants from the Rio Tinto Ride to Conquer Cancer, Leukemia Foundation of Australia and the National Health and Medical Research Council.
Authorship
Contribution: K.H.G. designed and performed experiments and wrote the manuscript; K.A.M., A.V., M.D.B., M.K., and G.M.B. performed experiments and provided useful discussion; R.D.K., N.C.R., S.D.O., K.E.L., M.C., B.E.T., M.L., and I.C. performed experiments; S.W.L., M.W.L.T., M.J.S., J.M., J.R., and B.S. provided essential reagents; A.D.C. performed blinded histological analysis; S.R.M. provided useful discussion and experimental advice; K.P.A.M. designed and performed experiments and helped write the manuscript; and G.R.H. designed experiments and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kate H. Gartlan, Immunology Department, QIMR Berghofer Medical Research Institute, Level 9 CBCRC, 300 Herston Rd, Herston, QLD 4006, Australia; e-mail: kate.gartlan@qimrberghofer.edu.au.
References
Author notes
K.P.A.M. and G.R.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal