To the editor:
Allogeneic stem cell transplantation (SCT) for patients with acute lymphoblastic leukemia (ALL) is mostly undergone with myeloablative conditioning (MAC) and it could be the major cause of short- or long-term complications such as endocrinologic disorders including hypogonadism or growth hormone-deficient short stature.1,2 In recent years, SCT with reduced-intensity conditioning (RIC) regimens was introduced for children who have pretransplant morbidity or are unable to tolerate a MAC regimen.3-5 Although it has the possibility of reducing posttransplant late toxicities,6 the exact clinical implications of a RIC regimen are still unclear. Therefore, in this study, we retrospectively compared the transplant outcomes with RIC and MAC regimens for children with ALL to determine the feasibility of SCT with a RIC regimen using the Transplant Registry Unified Management Program (TRUMP), a nationwide database established by the Japan Society for Hematopoietic Cell Transplantation (JSHCT).
We analyzed 1334 children with ALL who underwent allogeneic SCT as the first transplant from January 2000 to December 2010 in Japan; they consisted of 1201 patients with a MAC regimen and 133 patients with a RIC regimen according to the intensity of the conditioning regimen. The definition of RIC or MAC was based on the internationally recognized criteria, in which MAC is defined as fractionated total body irradiation (TBI) of ≥8 Gy, a single TBI of ≥5 Gy, or busulfan of ≥8 mg/kg or ≥280 mg/m2, and other regimens are categorized as RIC.7 Patients were transplanted at first complete remission (CR1, n = 568), second complete remission (CR2, n = 374), or advanced stages (third or further remission and relapse, n = 371), and there was no significant difference between RIC and MAC in this regard (P = .125). The type of SCT according to the stem cell source was related bone marrow transplantation (n = 413), related peripheral blood stem cell transplantation (n = 89), unrelated bone marrow transplantation (n = 446), or unrelated cord blood transplantation (n = 386); the serological HLA disparity between donor and patient was none (n = 816) or mismatched (n = 486) for the graft-versus-host direction, and the number of mismatched transplants was significantly higher in RIC patients (P = .007).
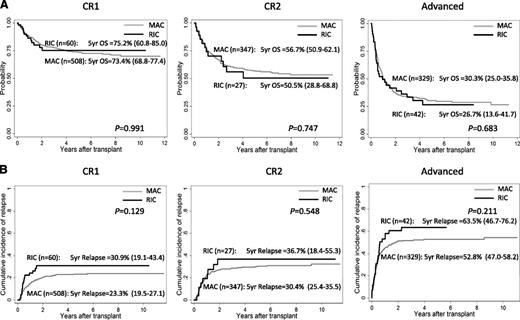
At a median follow-up of 765 days, 5-year overall survival (OS) rates of patients with RIC and MAC were 52.4% and 56.1% (P = .525) and they were 75.2% and 73.4% at CR1 (P = .991), 50.5% and 56.7% at CR2 (P = .747), and 26.7% and 30.3% at more advanced stages (P = .683), respectively (Figure 1A). Five-year relapse-free survival rates were 43.0% and 52.4% in RIC and MAC (P = .070) and were 62.3% and 68.2% at CR1 (P = .249), 46.6% and 54.0% at CR2 (P = .520), and 14.9% and 27.0% at more advanced stages (P = .295), respectively. Relapse was observed in 434 patients (54 with RIC and 380 with MAC), which included 125 in CR1, 110 in CR2, and 194 in advanced stages at SCT. The cumulative incidence of relapse rates at 5 years were 43.1% in RIC and 33.6% in MAC (P = .020). The 5-year relapse rates of RIC and MAC at each disease status of SCT were 30.9% and 23.3% at CR1 (P = .129), 36.7% and 30.4% at CR2 (P = .548), and 63.5% and 52.8% at more advanced stages (P = .211), respectively (Figure 1B). Treatment-related mortality (TRM) among all patients was observed in 196 patients (18 with RIC and 178 with MAC) and the cumulative incidences of TRM at 5 years were 15.7% and 15.3% with RIC and MAC, respectively (P = .953). Neutrophil engraftment with absolute neutrophil count of ≥500/mm3 was obtained in 944 patients (95 with RIC and 849 with MAC) and the cumulative incidence rates of neutrophil engraftment at day 100 were 91.7% with RIC and 96.2% with MAC (P = .498). After multivariate analysis adjusted by age at diagnosis, gender of patient, disease status at SCT, stem cell source, RIC/MAC, HLA compatibility, TBI, and cytogenetics, transplant outcomes with RIC and MAC regimens were not significantly different in OS (hazard ratio [HR] = 1.10, 95% confidence interval [CI] = 0.84-1.46, P = .488), relapse-free survival (HR = 1.25, 95% CI = 0.96-1.61, P = .093), relapse rates (HR = 1.11, 95% CI = 0.80-1.54, P = .530), TRM (HR = 0.89, 95% CI = 0.55-1.44, P = .621), and neutrophil engraftment (HR = 0.99, 95% CI = 0.79-1.26, P = .983).
Probability of OS and cumulative incidence of relapse of children who underwent transplantation at CR1, CR2, and advanced stages with RIC and MAC regimen. (A) Probability of OS. (B) Cumulative incidence of relapse.
Probability of OS and cumulative incidence of relapse of children who underwent transplantation at CR1, CR2, and advanced stages with RIC and MAC regimen. (A) Probability of OS. (B) Cumulative incidence of relapse.
In conclusion, the transplant outcomes of children with ALL who were given an RIC regimen in allogeneic SCT were not significantly different from those with an MAC regimen. Because this is a registry-based retrospective study and the number of patients with an RIC regimen is small, the results should be interpreted with caution. We need to proceed to a prospective study to prove the feasibility of SCT with an RIC regimen in children with ALL in order to reduce the transplant-related toxicities, especially in terms of the late effects after SCT.
Authorship
Contribution: Koji Kato designed the study; Koji Kato, M.K., and Y.A. analyzed and interpreted the data; M.K., D.H., H.K., H.I., Y.O., K. Koh, M.I., J.I., Keisuke Kato, and H.S. collected and assembled the data; Koji Kato, H.S., H.Y., and K. Kawa controlled patient registration; Koji Kato, M.K., R.S., and Y.A. critically reviewed the data; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koji Kato, Department of Hematology and Oncology, Children’s Medical Center, Japanese Red Cross Nagoya First Hospital, 3-35, Michishita-cho, Nakamura-ku, Nagoya 453-8511, Japan; e-mail: kokato@nagoya-1st.jrc.or.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal