To the editor:
Treatment of the glycosylphosphatidylinositol (GPI) anchor deficiency paroxysmal nocturnal hemoglobinuria (PNH) has been revolutionized by the use of the anti-C5 antibody eculizumab, which blocks complement-mediated hemolysis and the associated pathology.1,2 In addition to complement susceptibility, GPI anchor deficiency alters cellular function with the potential to further contribute to disease. For example, defective natural killer (NK) cell activity in PNH was first described >3 decades ago.3,4 NK cell deficiencies are associated with susceptibility to infection,5,6 suggesting that NK function in PNH should be analyzed in more detail. Here we show that functional defects in NK cell activity in PNH result from reduced NK cell numbers rather than cell intrinsic defects.
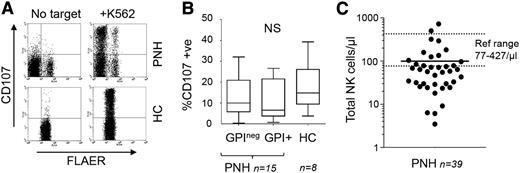
Early PNH studies showed that impaired NK cell activity was associated with reduced large granular lymphocyte (LGL) counts.3,4 However, these reports preceded the definition of NK cells and our understanding of the molecular basis of PNH, prompting reassessment. Mosaicism in PNH1 allows side-by-side functional comparisons of GPI+ and GPIneg NK cells within individual patients, enabling the assessment of NK cell activity on a per cell basis (Figure 1; supplemental Figure 1, available on the Blood Web site). Despite reports of impaired activity,3,4 the GPI-deficient NK cells were proficient at target cell-induced granule exocytosis (Figure 1A-B; supplemental Figure 1). Thus, early findings associating reduced NK cell activity with reduced LGL numbers rather than intrinsic cellular activity are correct.4 The absolute number of NK cells (and more variably, other lymphocytes) is indeed reduced in PNH7 ; in our cohort of 39 patients, two thirds had NK cell counts below the reference range (Figure 1C), and NK cell numbers were not significantly correlated with neutrophil, monocyte, or platelet counts (supplemental Figure 2). The basis for reduced NK cell numbers in PNH is unclear, although this might be related to impaired chemotactic or homeostatic mechanisms, as we recently reported.8 Although the activity of GPI-deficient NK cells is unimpaired, a reduction in absolute numbers of NK cells will reduce NK cell activity in the blood as a whole.
NK cell activity in PNH. (A) NK cell granule exocytosis in PNH. NK cells were purified from blood samples from healthy controls (HCs) or PNH patients using indirect selection reagents from Miltenyi. NK cell degranulation was assayed using a modification of a standard method to allow detection of GPI+ and GPI-deficient NK cells using fluorescent aerolysin (FLAER) (supplemental Text).8 Briefly, purified NK cells were cocultured with K562 target cells (for 4 hours). Cocultures were then stained with anti-CD56 antibody to identify NK cells, FLAER to distinguish GPI-deficient and GPI+ cells, and anti-CD107 to identify degranulated NK cells. This analysis is from 1 PNH patient and 1 HC (gating on the purified CD56+ NK cells) with the data from the cohort shown in panel B. We also compared the mean fluorescence intensity of CD107 staining in paired GPI-deficient and GPI+ cells within each patient. This demonstrated a significant increase in CD107 display on the GPI-deficient NK cells (supplemental Figure 1). (B) Summary of NK cell degranulation activity from 15 PNH patients and 8 HCs. The box plot shows the percentage of GPI-deficient (GPIneg) and GPI+ NK cells that have degranulated in response to target cells. The range (whiskers), median (horizontal line), and interquartile range (box) are shown. The percentage of CD107+ NK cells was not statistically significant (NS) between any 2 groups according to the Mann-Whitney test. Comparison of the percentage of CD107+ NK cells within individual patients showed no significant differences between matched GPI+ and GPIneg NK cells (supplemental Figure 1). (C) Number of total NK cells in 39 PNH patients (cells per microliter). Patient values ranged from 3 to 725 cells per microliter (mean, 100 cells/μL). A European reference range (77-427 cells/μL) is shown by the dotted lines. The patients with NK cell counts within the normal range were unremarkable in terms of gender, age, or treatment (cyclosporin or eculizumab). Furthermore, there was no correlation between the NK cell counts and the absolute numbers of other cell types (supplemental Figure 2). Peripheral blood samples used in this work were collected after informed consent in accordance with the Declaration of Helsinki.
NK cell activity in PNH. (A) NK cell granule exocytosis in PNH. NK cells were purified from blood samples from healthy controls (HCs) or PNH patients using indirect selection reagents from Miltenyi. NK cell degranulation was assayed using a modification of a standard method to allow detection of GPI+ and GPI-deficient NK cells using fluorescent aerolysin (FLAER) (supplemental Text).8 Briefly, purified NK cells were cocultured with K562 target cells (for 4 hours). Cocultures were then stained with anti-CD56 antibody to identify NK cells, FLAER to distinguish GPI-deficient and GPI+ cells, and anti-CD107 to identify degranulated NK cells. This analysis is from 1 PNH patient and 1 HC (gating on the purified CD56+ NK cells) with the data from the cohort shown in panel B. We also compared the mean fluorescence intensity of CD107 staining in paired GPI-deficient and GPI+ cells within each patient. This demonstrated a significant increase in CD107 display on the GPI-deficient NK cells (supplemental Figure 1). (B) Summary of NK cell degranulation activity from 15 PNH patients and 8 HCs. The box plot shows the percentage of GPI-deficient (GPIneg) and GPI+ NK cells that have degranulated in response to target cells. The range (whiskers), median (horizontal line), and interquartile range (box) are shown. The percentage of CD107+ NK cells was not statistically significant (NS) between any 2 groups according to the Mann-Whitney test. Comparison of the percentage of CD107+ NK cells within individual patients showed no significant differences between matched GPI+ and GPIneg NK cells (supplemental Figure 1). (C) Number of total NK cells in 39 PNH patients (cells per microliter). Patient values ranged from 3 to 725 cells per microliter (mean, 100 cells/μL). A European reference range (77-427 cells/μL) is shown by the dotted lines. The patients with NK cell counts within the normal range were unremarkable in terms of gender, age, or treatment (cyclosporin or eculizumab). Furthermore, there was no correlation between the NK cell counts and the absolute numbers of other cell types (supplemental Figure 2). Peripheral blood samples used in this work were collected after informed consent in accordance with the Declaration of Helsinki.
Clearly, PNH should not be classified as a functional NK cell deficiency (NKD). Classical NKD is characterized by ∼1/10 the normal number of NK cells, and counts in most of our PNH patients exceeded this (Figure 1C). Furthermore, the term NKD is reserved for where “the impact upon NK cells need represent the major immunological abnormality in the patient.”6 In PNH, all hematopoietic lineages are affected because of the presence of PIGA mutations in hematopoietic stem cells.1 More compelling is the clinical phenotype; the defining feature of NKD is the heightened susceptibility to viruses,5,6 which has not been observed in PNH.7,9,10 Instead, infection in PNH is bacterial in origin10 and likely to be associated with neutropenia secondary to underlying bone marrow failure or associated with use of eculizumab, which increases the risk of infection with encapsulated bacteria normally eliminated by terminal complement components.1 In summary, the low numbers of NK cells in PNH affect overall cytotoxicity, but this defect is not severe enough to manifest as heightened susceptibility to viral infection as seen in NKD.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Darren Newton and Christopher Parrish for help and valued discussion.
Contribution: R.J.K., A.H., and P.H. manage the PNH clinic and recruited the patient cohort; Y.M.E.-S. performed the experimental work; G.P.C. and Y.M.E.-S. designed the study; Y.M.E.-S., G.M.D., R.J.K., and G.P.C. analyzed the data; and G.P.C. wrote the article with input from all authors.
Conflict-of-interest disclosure: R.J.K., A.H., and P.H. have received speaker fees from and sat on advisory committees for Alexion Pharmaceuticals. P.H. has received research funding from Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Graham P. Cook, Leeds Institute of Cancer and Pathology, University of Leeds, Wellcome Brenner Building, St James's University Hospital, Leeds LS9 7TF, United Kingdom; e-mail: g.p.cook@leeds.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal