Key Points
A real-time, integrated fluorescent Wnt reporter marks rare leukemia stem cells in T-ALL.
Deletion of β-catenin or Hif1α reduces LIC frequency in established tumors, but does not affect the growth of bulk cells.
Abstract
The Wnt signaling pathway has been shown to play important roles in normal hematopoietic stem cell biology and in the development of both acute and chronic myelogenous leukemia. Its role in maintaining established leukemia stem cells, which are more directly relevant to patients with disease, however, is less clear. To address what role Wnt signaling may play in T-cell acute lymphoblastic leukemia (T-ALL), we used a stably integrated fluorescent Wnt reporter construct to interrogate endogenous Wnt signaling activity in vivo. In this study, we report that active Wnt signaling is restricted to minor subpopulations within bulk tumors, that these Wnt-active subsets are highly enriched for leukemia-initiating cells (LICs), and that genetic inactivation of β-catenin severely reduces LIC frequency. We show further that β-catenin transcription is upregulated by hypoxia through hypoxia-inducible factor 1α (Hif1α) stabilization, and that deletion of Hif1α also severely reduces LIC frequency. Of note, the deletion of β-catenin or Hif1α did not impair the growth or viability of bulk tumor cells, suggesting that elements of the Wnt and Hif pathways specifically support leukemia stem cells. We also confirm the relevance of these findings to human disease using cell lines and patient-derived xenografts, suggesting that targeting these pathways could benefit patients with T-ALL.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignancy of immature T-cell progenitors. Over 80% of pediatric cases are cured by current therapies, whereas only 40% of adults survive beyond 5 years.1 Relapses in both patient populations are presumably due to ineffective targeting of so-called leukemia “stem” cells, which are thought to be primarily resistant to standard chemotherapy.2 Notwithstanding these properties, leukemia stem cells are operatively defined by their ability to propagate disease in naïve hosts at limiting dilution, typically referred to in the literature as leukemia-initiating cells (LICs). We and others, have shown that LICs in both mouse and human models of T-ALL reside asymmetrically within minor subpopulations of bulk tumors, although the precise markers used to identify these LIC-enriched populations are variable between models.3-9
A handful of genes/pathways have emerged as playing prominent roles in the self-renewal of normal hematopoietic and leukemia stem cells, including Notch, Wnt, and Sonic hedgehog.2 In T-ALL, NOTCH1 is activated by mutation in over 60% of patients,10 and Notch signaling has been linked to the maintenance of LICs.7,9,11 Constitutive Wnt/β-catenin signaling produces T-ALL in mice12 and leukemia stem cells from Ptennull mouse T-ALLs are characterized by elevated levels of β-catenin protein.8 As well, hematopoietic stem cells (HSCs) reside within specialized microenvironmental niches that provide for their particular metabolic needs, such as reliance on anaerobic glycolysis, a situation enforced at least in part by ambient hypoxia13 and mediated by hypoxia-inducible factor 1α (Hif1α).14
To address the role of Wnt signaling more directly in established T-ALL tumors, we first generated primary mouse T-ALL tumors using the well-characterized NOTCH1-ΔE bone marrow (BM) transduction/transplantation model,7,15,16 then introduced a stably integrated fluorescent Wnt reporter, 7x T-cell factor (Tcf)–enhanced green fluorescent protein (GFP), by lentiviral transduction.17-19 These Wnt reporter leukemias were then transplanted back into syngeneic recipient mice to interrogate their Wnt activation status in vivo. Here, we report on the properties and functional dependencies of leukemia stem cells in T-ALL with respect to Wnt and Hif signaling.
Methods
Mice
All NOTCH1 leukemia transplant donors were of C57BL/6 background. All transplant recipients were C57BL/6, B6.SJL-PtprcaPepcb/BoyJ (Ly5.1), or NOD/Prkdcscid/Il2rg−/− (NSG) mice. Mice harboring conditional Ctnnb120 and Hif1α21 alleles on B6 congenic backgrounds were obtained from The Jackson Laboratory. Animals were housed in specific pathogen-free facilities according to institutional guidelines and experiments were performed under approved institutional protocols.
Human samples
Primary human T-ALL samples were obtained with appropriate institutional approvals and informed consent under guidelines established by the Declaration of Helsinki. Patient-derived xenografts (PDX) were established by injection of primary patient biopsy material into irradiated NSG mice.22
Generation of primary mouse leukemias
BM cells from 5-fluorouracil–treated mice were transduced with NOTCH1-ΔE/truncated nerve growth factor receptor (NGFR) retrovirus by spinoculation.22 Three days later, 10 000 to 40 000 NGFR+ cells were injected by tail vein along with a rescue dose of normal marrow into lethally irradiated (810 rad) syngeneic recipient mice. Animals typically develop clinically morbid disease within 8 to 12 weeks following transplantation.
Serial transplantation
Varying numbers of total or fluorescence-activated cell sorter (FACS)-sorted mouse or human leukemia cells were injected by tail vein into non-irradiated C57BL/6 or sublethally irradiated (200 rad) NSG recipient mice, respectively. Animals were then monitored for engraftment/disease progression and euthanized when clinically morbid according to standard humane end point criteria.
Results
Minor subpopulations of leukemia cells exhibit active Wnt signaling in vivo
To explore the role of canonical Wnt/β-catenin signaling in established T-ALL tumors, we introduced a real-time fluorescent Wnt reporter construct into primary mouse T-cell leukemias by lentiviral transduction. The primary leukemias were first generated by transduction of mouse BM with a constitutively activated NOTCH1 construct termed ΔE7,15,16,23 and transplantation into syngeneic recipients. These primary leukemias were then explanted and transduced in vitro with a lentiviral Wnt signaling reporter consisting of 7 tandem Tcf/Lef consensus binding sites upstream of a minimal promoter driving expression of GFP and an SV40-puromycin selection (7TGP) or SV40-Cherry marker (7TGC) cassette17,19 (Figure 1A). After puromycin selection in vitro or FACS-sorting for the Cherry marker, 7TGP- or 7TGC-transduced cells, respectively, were injected by tail vein into secondary recipient mice, all of which subsequently developed clinically morbid disease. Flow cytometric analysis was performed on freshly explanted leukemia cells to detect GFP fluorescence indicative of endogenous Wnt signaling activity (“2° leukemia” in Figure 1B). Interestingly, only a small fraction of total leukemia cells in the marrow exhibited GFP fluorescence (median, 1.1%; mean, 1.6%; range, 0.15% to 5.6%; n = 26) (Figure 1C; see supplemental Table 1, available on the Blood Web site), suggesting that minor subpopulations of leukemia cells experience active Wnt signaling in vivo. We observed similarly low % GFP+ expression in leukemia cells harvested from the spleen (data not shown). Importantly, intracellular flow cytometry and immunofluorescence revealed higher levels of β-catenin and TCF7 in GFP+ than GFP− fractions, whereas western blots showed higher β-catenin protein levels in FACS-sorted GFP+ than GFP− fractions from tertiary leukemias with elevated GFP+ fraction (supplemental Figure 1).
LICs are enriched within a small tumor subpopulation with active Wnt signaling. (A) Schematic diagram of the integrated fluorescent Wnt reporters, 7TGP and 7TGC, used to mark leukemia cells with active Wnt signaling. The reporter element is composed of 7 Tcf/Lef-binding sites upstream of a minimal promoter and GFP, followed by a separate SV40-puromycin (7TGP) or SV40-Cherry (7TGC) selection marker. The construct backbone is a self-inactivating lentiviral vector. (B) Schematic diagram of experimental approach. Primary mouse NOTCH1-ΔE leukemias were explanted and transduced with 7TGP or 7TGC lentivirus. 7TGP-transduced cells were selected with 1 μg/ml puromycin on MS5-DL1 puro feeder cells for 5 days beginning on day 2 posttransduction. 7TGC-transduced cells were FACS-sorted on day 2 posttransduction. Transduced cells were then transplanted into recipient mice, all of which subsequently developed leukemia. These secondary 7TGP/7TGC-transduced leukemias were then analyzed by flow cytometry for GFP expression. GFP+ and GFP− subsets were then FACS sorted (also Cherry+ in 7TGC experiments) and transplanted at limiting dilution into tertiary recipients. (C) Summary of GFP+ cell abundance in Wnt reporter-transduced leukemias after passage in vivo. Freshly explanted BM from clinically morbid recipients of 7TGP or 7TGC-transduced primary leukemias was analyzed by flow cytometry for GFP expression within gated NGFR+ (7TGP) or NGFR+ Cherry+ (7TGC) leukemia cell populations (labeled “2° leukemia” in [B]). Results depicted are compiled from 10 independent primary leukemias (26 recipients in total; 11 × 7TGP and 15 × 7TGC). Each data point represents an individual mouse. Mean ± standard deviation (SD) values are indicated by horizontal lines. (D) Survival of recipient mice after transplantation with FACS-sorted Wnt active (GFP+) or Wnt inactive (GFP−) subsets from 7TGP and 7TGC-transduced secondary leukemias. Each of 4 recipient animals (n = 4) was injected with each of the cell doses as indicated in parentheses. Two of 3 transplant experiments using independent primary leukemias are depicted. ****P < .0001 (log-rank test). LTR, long terminal repeat; sinLTR, self-inactivating LTR.
LICs are enriched within a small tumor subpopulation with active Wnt signaling. (A) Schematic diagram of the integrated fluorescent Wnt reporters, 7TGP and 7TGC, used to mark leukemia cells with active Wnt signaling. The reporter element is composed of 7 Tcf/Lef-binding sites upstream of a minimal promoter and GFP, followed by a separate SV40-puromycin (7TGP) or SV40-Cherry (7TGC) selection marker. The construct backbone is a self-inactivating lentiviral vector. (B) Schematic diagram of experimental approach. Primary mouse NOTCH1-ΔE leukemias were explanted and transduced with 7TGP or 7TGC lentivirus. 7TGP-transduced cells were selected with 1 μg/ml puromycin on MS5-DL1 puro feeder cells for 5 days beginning on day 2 posttransduction. 7TGC-transduced cells were FACS-sorted on day 2 posttransduction. Transduced cells were then transplanted into recipient mice, all of which subsequently developed leukemia. These secondary 7TGP/7TGC-transduced leukemias were then analyzed by flow cytometry for GFP expression. GFP+ and GFP− subsets were then FACS sorted (also Cherry+ in 7TGC experiments) and transplanted at limiting dilution into tertiary recipients. (C) Summary of GFP+ cell abundance in Wnt reporter-transduced leukemias after passage in vivo. Freshly explanted BM from clinically morbid recipients of 7TGP or 7TGC-transduced primary leukemias was analyzed by flow cytometry for GFP expression within gated NGFR+ (7TGP) or NGFR+ Cherry+ (7TGC) leukemia cell populations (labeled “2° leukemia” in [B]). Results depicted are compiled from 10 independent primary leukemias (26 recipients in total; 11 × 7TGP and 15 × 7TGC). Each data point represents an individual mouse. Mean ± standard deviation (SD) values are indicated by horizontal lines. (D) Survival of recipient mice after transplantation with FACS-sorted Wnt active (GFP+) or Wnt inactive (GFP−) subsets from 7TGP and 7TGC-transduced secondary leukemias. Each of 4 recipient animals (n = 4) was injected with each of the cell doses as indicated in parentheses. Two of 3 transplant experiments using independent primary leukemias are depicted. ****P < .0001 (log-rank test). LTR, long terminal repeat; sinLTR, self-inactivating LTR.
To demonstrate more definitively that the GFP reporter was indeed responding to Wnt signaling rather than spurious expression due to random integration at permissive genomic loci, primary leukemias on either wild-type (WT) or Ctnnb1loxP/loxP background20 were transduced with the 7TGC reporter and transplanted into secondary recipients. Resulting secondary leukemias were then transduced with CreERT2 lentivirus and treated with 4-hydroxytamoxifen (4-OHT) in vitro to activate Cre-mediated deletion of β-catenin. Subsequent flow analysis confirmed that GFP expression was lost in Ctnnb1loxP/loxP leukemias after 4-OHT treatment, but not in WT leukemias (supplemental Figure 2A-B). A similar experiment was also performed using normal, whole BM cells that were doubly transduced with the 7TGP reporter and CreERT2 lentivirus, and yielded the same result (supplemental Figure 2C). Of note, enforced expression of a nondegradable β-catenin mutant (EβP) in 7TGP leukemia cells increased the intensity of GFP expression, but not the overall fraction of GFP+ cells, whereas enforced expression of TCF7 induced nearly all cells to become GFP+ (supplemental Figure 3), thereby not only confirming the responsiveness of the reporter to Wnt activation in our leukemia model but also suggesting that endogenous levels of TCF7 may be limiting in Wnt inactive cells. Taken together, these results support that the 7TGP/7TGC reporter accurately reflects Wnt signaling activity in our T-ALL model, and that only a minority of cells experience active Wnt signaling in vivo.
LICs are asymmetrically localized in populations with active Wnt signaling
Prior work has suggested that Wnt signaling plays important roles in self-renewal of normal hematopoietic and leukemia stem cells.24 To assess whether the GFP+ (Wnt signaling-active) subset of T-ALL cells might be enriched for LICs, GFP+ and GFP− fractions were FACS-sorted and transplanted into tertiary recipients at limiting dilution (Figure 1D and supplemental Figure 4). Strikingly, LICs were enriched in the GFP+ fraction ∼2.5-fold over total cells, and 200-fold over the GFP− fraction (supplemental Figure 5), suggesting Wnt signaling may contribute to stem-like self-renewal properties. Of note, GFP+ cells did not exhibit a distinctive CD4/CD8 phenotype as compared with the GFP subset or that was consistent across different tumors (supplemental Figure 6).
One relevant criterion to distinguish stem from nonstem cells is the ability of stem cells to give rise to stem and nonstem progeny.25 Although serial transplantation of FACS-sorted GFP− populations seldom produced T-ALL disease in recipient animals, it is notable that leukemias arising following transplantation of FACS-sorted GFP+ subsets were composed of both GFP+ and GFP− cells (supplemental Table 2). Interestingly, there was wide variation in the % GFP+ in recipients for 2 of 3 leukemias tested, occurring at both high and low injected cell doses and with both types of Wnt reporter. Yet, serial transplantation of total (unsorted) cells from “high” % GFP+ leukemias showed further diminution in the GFP+ fraction (supplemental Figure 7A). Of note, these “high” % GFP+ leukemias only arose after transplantation of FACS-sorted GFP+ cells and were not observed following transplantation of unsorted cells (<5% GFP+), which always yielded leukemias with similarly low GFP+ percentages (supplemental Figure 7B), suggesting this may represent the “steady state” level of Wnt activation. These findings thus support the notion that GFP+ cells are competent to give rise to leukemias comprised of both GFP+ and GFP− cells, but that GFP− populations are essentially devoid of LICs. Further studies will be required to define the variables that affect the rate of extinction of Wnt signaling after transplantation of sorted GFP+ cells.
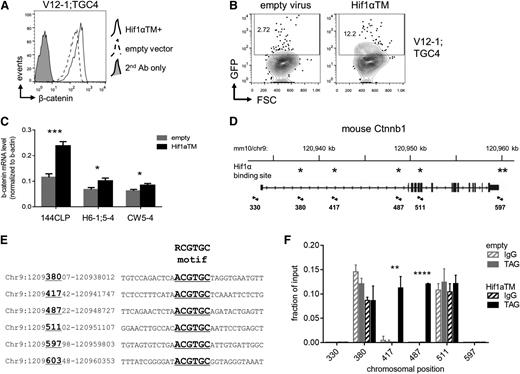
LICs are dependent on Wnt signaling
To test whether there was a causal relationship between Wnt signaling and LIC frequency, mouse NOTCH1-ΔE leukemias were transduced with lentivirus encoding dominant negative TCF (dnTCF) lacking the N-terminal β-catenin binding domain,26 and thus blocks canonical activation of target genes.27 Compared with empty virus controls, dnTCF prolonged survival (Figure 2A) and reduced the measured LIC frequency by at least fourfold (supplemental Figure 8); however, dnTCF-transduced cells cultured in vitro also showed reduced proliferation/survival in bulk (Figure 2B) and virtually no clonogenic activity by colony forming cell (CFC) assay (Figure 2C), suggesting that dnTCF potently restricts transit amplifying cells. Since bulk leukemia cells must accumulate to the extent that they overwhelm the organism in order to readout positively in our survival-end point LIC assay, this limits any potential conclusions about the effects of dnTCF on leukemia stem cells.
Inhibition of Wnt signaling eliminates LICs. (A) Survival of recipient mice after transplantation with leukemias transduced by dnTCF lentivirus to block β-catenin signaling. Primary mouse NOTCH1-ΔE leukemias were explanted and transduced with dnTCF/GFP or empty virus control. Two days later, GFP+ leukemia cells were FACS-sorted and injected into each of 4 recipient animals (n = 4) at a dose of 1 × 105 cells per mouse. Results depicted are compiled from 2 separate transplant experiments using independent primary leukemias (#V10-1 and #V12-1). **P < .01 (log-rank test). (B) Proliferation of mouse leukemias following transduction with dnTCF lentivirus vs nontransduced cells present in the same culture as measured by 5-bromo-2′-deoxyuridine incorporation. G1/G0, S, G2/M, and subG1 populations are gated as shown. Results depicted are representative of 2 independent leukemias analyzed (#V10-4 and #V12-1;3). (C) CFC assay for clonogenic activity. Primary mouse leukemias were transduced with dnTCF or empty control lentiviruses, FACS-sorted for the linked GFP marker, and plated in methylcellulose-containing media. *P < .05; **P < .01; ***P < .001 (Student t test). (D) Survival of recipient mice after transplantation with leukemias deleted of β-catenin. Primary mouse NOTCH1-ΔE leukemias on a Ctnnb1loxP/loxP background were explanted, transduced with CreERT2/GFP lentivirus, and then treated with 4-OHT or vehicle (mock) control in vitro for 2 days. GFP+ leukemia cells were then FACS-sorted and injected into each of 4 recipient mice (n = 4) at each of the cell doses indicated in parentheses. Two separate experiments are depicted using independent primary leukemias. ****P < .0001 (log-rank test). (E-F) Cell growth/viability assays. Primary mouse NOTCH1-ΔE leukemias generated on a Ctnnb1loxP/loxP background were transduced with CreERT2/GFP lentivirus, and then treated with 4-OHT or vehicle (mock) in vitro. In (E), relative cell growth was assessed by tracking the % GFP+ cells over time in culture by flow cytometry. A decreasing GFP+ fraction indicates transduced (GFP+) cells are growth disadvantaged compared with nontransduced (GFP−) cells in the same culture. In (F), absolute viable cell numbers were tracked over a 3-day culture period by flow cytometry with admixed polystyrene counting beads. Mean ± SD values are plotted.
Inhibition of Wnt signaling eliminates LICs. (A) Survival of recipient mice after transplantation with leukemias transduced by dnTCF lentivirus to block β-catenin signaling. Primary mouse NOTCH1-ΔE leukemias were explanted and transduced with dnTCF/GFP or empty virus control. Two days later, GFP+ leukemia cells were FACS-sorted and injected into each of 4 recipient animals (n = 4) at a dose of 1 × 105 cells per mouse. Results depicted are compiled from 2 separate transplant experiments using independent primary leukemias (#V10-1 and #V12-1). **P < .01 (log-rank test). (B) Proliferation of mouse leukemias following transduction with dnTCF lentivirus vs nontransduced cells present in the same culture as measured by 5-bromo-2′-deoxyuridine incorporation. G1/G0, S, G2/M, and subG1 populations are gated as shown. Results depicted are representative of 2 independent leukemias analyzed (#V10-4 and #V12-1;3). (C) CFC assay for clonogenic activity. Primary mouse leukemias were transduced with dnTCF or empty control lentiviruses, FACS-sorted for the linked GFP marker, and plated in methylcellulose-containing media. *P < .05; **P < .01; ***P < .001 (Student t test). (D) Survival of recipient mice after transplantation with leukemias deleted of β-catenin. Primary mouse NOTCH1-ΔE leukemias on a Ctnnb1loxP/loxP background were explanted, transduced with CreERT2/GFP lentivirus, and then treated with 4-OHT or vehicle (mock) control in vitro for 2 days. GFP+ leukemia cells were then FACS-sorted and injected into each of 4 recipient mice (n = 4) at each of the cell doses indicated in parentheses. Two separate experiments are depicted using independent primary leukemias. ****P < .0001 (log-rank test). (E-F) Cell growth/viability assays. Primary mouse NOTCH1-ΔE leukemias generated on a Ctnnb1loxP/loxP background were transduced with CreERT2/GFP lentivirus, and then treated with 4-OHT or vehicle (mock) in vitro. In (E), relative cell growth was assessed by tracking the % GFP+ cells over time in culture by flow cytometry. A decreasing GFP+ fraction indicates transduced (GFP+) cells are growth disadvantaged compared with nontransduced (GFP−) cells in the same culture. In (F), absolute viable cell numbers were tracked over a 3-day culture period by flow cytometry with admixed polystyrene counting beads. Mean ± SD values are plotted.
As a further test of Wnt dependence, primary mouse NOTCH1-ΔE leukemias were generated on a conditional Ctnnb1loxP/loxP background,20 transduced with lentiviral CreERT2,28 and treated in vitro with 4-OHT. Transduced cells were then FACS-sorted and transplanted at limiting dilution into recipient mice (Figure 2D). Deletion of β-catenin was highly efficient and resulted in a marked decrease in LIC frequency (at least 85-fold) (supplemental Figure 9). Of note, leukemias that developed in mice injected with mock-treated cells were positive for the viral Cre marker, whereas the uncommon leukemia that developed in mice injected with 4-OHT–treated cells was negative, consistent with outgrowth of nontransduced cells with intact β-catenin (data not shown). Importantly, activation of CreERT2 with 4-OHT in WT leukemias (Ctnnb1+/+) had no significant effect on LIC frequency (supplemental Figure 10), excluding nonspecific genotoxicity by Cre recombinase in this context.
In contrast to dnTCF, the deletion of β-catenin had no apparent detrimental effect on the short-term growth or viability of bulk leukemia cells cultured in vitro (Figure 2E-F), suggesting that β-catenin supports cellular phenotypes specific to minor LIC-enriched subpopulations. Of note, this is not to say that β-catenin effects are restricted to leukemia stem cells only, but rather that its deletion does not lead to obvious effects on bulk cells and thus the LIC assay is not limited in the way that it is for dnTCF. Taken together, these findings support the conclusion that intact Wnt signaling is required for maintenance of LICs in T-ALL. Further study will be required to determine why dnTCF has broader effects than β-catenin deficiency in this setting.
Leukemia cells with active Wnt signaling reside in interstitial hypoxic BM niches
Normal HSCs are thought to reside in hypoxic niches within endosteal and other regions of the marrow.29,30 To determine whether leukemia cells with active Wnt signaling differentially resided within similar hypoxic niches in vivo, mice previously transplanted with 7TGP-transduced leukemias were injected with pimonidazole, a 2-nitroimidazole that forms adducts with peptide thiol groups under hypoxic conditions.31 Animals were euthanized 1 hour later and BM cells explanted, stained with antibodies against pimonidazole, and analyzed by flow cytometry. The GFP+ subset exhibited increased pimonidazole staining compared with the GFP− subset (Figure 3A), consistent with preferential occupancy within hypoxic regions of the marrow. To determine if this corresponded to a discrete anatomic compartment within the marrow, we performed immunohistochemical staining for GFP to highlight Wnt-active populations. Unexpectedly, GFP+ cells were distributed throughout the interstitial space without apparent localization to any particular anatomic site, such as adjacent to blood vessels or bony trabeculae (Figure 3B and supplemental Figure 11). This suggests that under conditions where the marrow cavity is essentially replaced by leukemia, localization of Wnt-active cells within hypoxic microenvironments is not likely based on occupancy within normal anatomic niches. It remains possible that the earliest of marrow-engrafting leukemia cells could localize initially within such preexisting normal anatomic niches; however, our results suggest that as the disease progresses, there may be substantial remodeling of the marrow cavity to create new hypoxic compartments that are permissive to Wnt activation and can support an expanding leukemia stem cell population.
Hypoxia potentiates Wnt signaling and supports clonogenic activity. (A) Flow cytometric analysis of pimonidazole staining, a marker of hypoxia. Mice were transplanted with 7TGP-transduced leukemia cells and, after developing clinically morbid disease, were injected by tail vein with pimonidazole (60 mg/kg). One hour later, mice were euthanized and BM cells harvested, fixed, and stained with anti-pimonidazole antibody. Gated NGFR+ leukemia cells are shown. The marrow contained >90% tumor cells (of which 40% to 70% were GFP+). Results depicted are representative of 2 different leukemic animals. (B) Immunohistochemical staining for GFP in BM sections of mice following transplantation with 7TGP-transduced leukemia cells. Mice were clinically morbid with disease at the time they were euthanized and contained >90% tumor cells (of which 40% to 70% were GFP+) within the marrow. Red arrowheads indicate individual GFP+ cells. Images depicted are representative of 2 different leukemic animals. Scale bar = 60 μm. (C-D) Analysis of β-catenin protein expression level in NOTCH1-ΔE mouse leukemia cells cultured in vitro under reduced oxygen conditions. Cells were cultured in complete growth media under 20% or 5% oxygen for 3 days in plastic tissue culture dishes, then harvested for analysis by western blot (C) and flow cytometry (D). The numbers below the β-catenin blot panel in (C) indicate relative band intensities after normalization to the β-actin loading control. Gated leukemia cells are depicted in (D). Results from at least 2 independent leukemias are shown. (E-F) CFC assay for in vitro clonogenic activity. Primary leukemia cells were plated in methylcellulose-containing media supplemented or not with Wnt3a ligand or Dickkopf-related protein 1 and cultured in either 20% or 5% oxygen. Each colored data point depicted represents an individual culture dish. Mean ± SD values are indicated by horizontal lines. Results in (F) are representative of 2 different leukemias. *P < .05; **P < .01; ****P < .0001 (Student t test in [E], 2-way analysis of variance [ANOVA] with Bonferroni multiple comparisons test in [F]). ns, not significant.
Hypoxia potentiates Wnt signaling and supports clonogenic activity. (A) Flow cytometric analysis of pimonidazole staining, a marker of hypoxia. Mice were transplanted with 7TGP-transduced leukemia cells and, after developing clinically morbid disease, were injected by tail vein with pimonidazole (60 mg/kg). One hour later, mice were euthanized and BM cells harvested, fixed, and stained with anti-pimonidazole antibody. Gated NGFR+ leukemia cells are shown. The marrow contained >90% tumor cells (of which 40% to 70% were GFP+). Results depicted are representative of 2 different leukemic animals. (B) Immunohistochemical staining for GFP in BM sections of mice following transplantation with 7TGP-transduced leukemia cells. Mice were clinically morbid with disease at the time they were euthanized and contained >90% tumor cells (of which 40% to 70% were GFP+) within the marrow. Red arrowheads indicate individual GFP+ cells. Images depicted are representative of 2 different leukemic animals. Scale bar = 60 μm. (C-D) Analysis of β-catenin protein expression level in NOTCH1-ΔE mouse leukemia cells cultured in vitro under reduced oxygen conditions. Cells were cultured in complete growth media under 20% or 5% oxygen for 3 days in plastic tissue culture dishes, then harvested for analysis by western blot (C) and flow cytometry (D). The numbers below the β-catenin blot panel in (C) indicate relative band intensities after normalization to the β-actin loading control. Gated leukemia cells are depicted in (D). Results from at least 2 independent leukemias are shown. (E-F) CFC assay for in vitro clonogenic activity. Primary leukemia cells were plated in methylcellulose-containing media supplemented or not with Wnt3a ligand or Dickkopf-related protein 1 and cultured in either 20% or 5% oxygen. Each colored data point depicted represents an individual culture dish. Mean ± SD values are indicated by horizontal lines. Results in (F) are representative of 2 different leukemias. *P < .05; **P < .01; ****P < .0001 (Student t test in [E], 2-way analysis of variance [ANOVA] with Bonferroni multiple comparisons test in [F]). ns, not significant.
Hypoxic conditions enhance in vitro clonogenic activity in a Wnt-dependent manner
To determine if there was a causal association between hypoxia and Wnt signaling, primary mouse leukemia cells were cultured in vitro in either 20% or 5% oxygen. Cells cultured in 5% oxygen showed consistently greater levels of β-catenin protein than cells cultured in 20% oxygen, as measured by western blot (Figure 3C). The fact that this difference was observable using bulk cell protein lysates suggested this effect was occurring in a substantial proportion of the cells as opposed to only within a minor subpopulation. In fact, flow cytometric and immunofluorescence assays confirmed that the majority of cells for any given tumor showed greater levels of β-catenin protein expression when cultured in 5% vs 20% oxygen (Figure 3D and supplemental Figure 12). Interestingly, leukemia cells cultured in vitro under reduced oxygen also exhibited greater clonogenic activity by CFC assay (Figure 3E), and this response to hypoxia was antagonized by inhibition of Wnt signaling with the soluble antagonist Dickkopf-related protein 1 (Figure 3F). These data support the idea that hypoxia promotes clonogenic activity in T-ALL and that upregulation of Wnt signaling contributes to this effect.
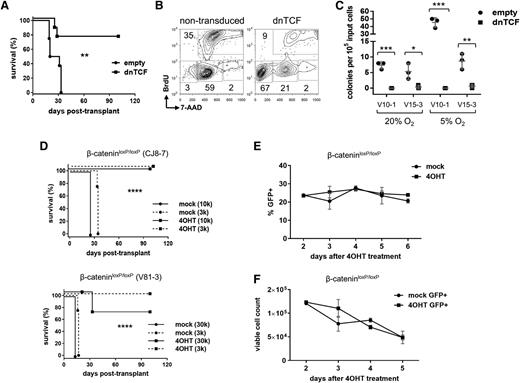
Hif1α supports Wnt signaling by contributing to β-catenin transcription
One of the major effectors of HSC gene programs under hypoxic conditions is Hif, which is a heterodimer consisting of Hif1α and Hif1β. Hif function is primarily regulated by levels of the Hif1α protein, which is ubiquitinated by the von Hippel–Lindau protein under normoxic conditions and subsequently degraded by the 26S proteasome, but under hypoxic conditions is allowed to accumulate.32 To determine if Hif1α was capable of contributing to Wnt signaling in our context, leukemia cells were transduced with a normoxia-stable, active Hif1α triple mutant (P402A/P577A/N813A),33 or “Hif1αTM”, and cultured in vitro under 20% oxygen. Indeed, transduction with Hif1αTM resulted in increased levels of β-catenin protein (Figure 4A) and also increased the number of GFP+ cells in 7TGC-transduced leukemias (Figure 4B). This regulation was apparent at the messenger RNA (mRNA) level (Figure 4C), and inspection of the Ctnnb1 genomic locus revealed six putative Hif1α binding sites matching the consensus RCGTGC motif34 (Figure 4D-E). Chromatin immunoprecipitation (ChIP) of epitope-tagged Hif1αTM revealed significant enrichment at two of these six positions (#417 and #487; Figure 4F). These results show that Hif1α promotes accumulation of β-catenin protein that is mediated, at least in part, by contributing to β-catenin transcription.
Hif1α contributes to β-catenin transcription directly. (A) Flow cytometric analysis of β-catenin protein levels. 7TGC-transduced mouse NOTCH1-ΔE leukemias were transduced with Hif1αTM (normoxia-stable, active Hif1α P402A/P577A/N813A triple mutant) or empty virus control, and cultured in vitro for 2 days on MS5 stromal feeders. Leukemia cells carrying the Hif1αTM or empty lentivirus were FACS-sorted by the linked hCD8 marker, then stained for intracellular β-catenin protein and analyzed by flow cytometry. Results depicted are representative of 3 different leukemias. (B) Flow cytometric analysis for Wnt reporter GFP expression. 7TGC-transduced leukemia cells were transduced with Hif1αTM/hCD8 or empty lentivirus and cultured in vitro for 2 days on MS5 stromal feeders. Cells were then harvested and assayed by flow cytometry. Gated Cherry+ hCD8+ leukemia cells are shown. Results depicted are representative of 3 different leukemias. (C) Real-time quantitative reverse transcription PCR analysis of β-catenin mRNA levels. The 144CLP mouse T-ALL cell line and 2 independent mouse leukemias were transduced with Hif1αTM or empty virus control, FACS-sorted for the linked GFP marker 2 days later, and then lysed for RNA. Mean ± SD values of triplicate assays are plotted. *P < .05; ***P < .001 (Student t test). (D) Map of the mouse Ctnnb1 locus with putative Hif1α binding sites indicated with asterisks. Primer pairs used for quantitative local ChIP assays are indicated by arrowheads with associated map coordinates. (E) Alignment of putative Hif1α binding sites within the mouse Ctnnb1 locus. Six regions containing Hif1α consensus binding sites are shown with the core RCGTGC motif indicated by underlined text in large font. Map coordinates corresponding to the positions assayed by local ChIP-PCR in (F) are indicated by underlined numbers in large font. Locus coordinates are from the GRCm38/mm10 genome assembly. (F) ChIP for Hif1α occupancy over the mouse Ctnnb1 locus. The 144CLP cell line was transduced with Myc epitope-tagged Hif1αTM or empty virus control, and FACS-sorted for the linked GFP marker 3 days later. Sheared chromatin was immunoprecipitated with anti-Myc tag (TAG) or IgG control antibody. Quantitative PCR was performed using primer pairs flanking putative Hif1α binding sites as indicated in (C). Data were normalized to their respective input DNA controls. Mean ± SD values of triplicate assays are plotted. **P < .01; ****P < .0001 (Student t test). FSC, forward light scatter.
Hif1α contributes to β-catenin transcription directly. (A) Flow cytometric analysis of β-catenin protein levels. 7TGC-transduced mouse NOTCH1-ΔE leukemias were transduced with Hif1αTM (normoxia-stable, active Hif1α P402A/P577A/N813A triple mutant) or empty virus control, and cultured in vitro for 2 days on MS5 stromal feeders. Leukemia cells carrying the Hif1αTM or empty lentivirus were FACS-sorted by the linked hCD8 marker, then stained for intracellular β-catenin protein and analyzed by flow cytometry. Results depicted are representative of 3 different leukemias. (B) Flow cytometric analysis for Wnt reporter GFP expression. 7TGC-transduced leukemia cells were transduced with Hif1αTM/hCD8 or empty lentivirus and cultured in vitro for 2 days on MS5 stromal feeders. Cells were then harvested and assayed by flow cytometry. Gated Cherry+ hCD8+ leukemia cells are shown. Results depicted are representative of 3 different leukemias. (C) Real-time quantitative reverse transcription PCR analysis of β-catenin mRNA levels. The 144CLP mouse T-ALL cell line and 2 independent mouse leukemias were transduced with Hif1αTM or empty virus control, FACS-sorted for the linked GFP marker 2 days later, and then lysed for RNA. Mean ± SD values of triplicate assays are plotted. *P < .05; ***P < .001 (Student t test). (D) Map of the mouse Ctnnb1 locus with putative Hif1α binding sites indicated with asterisks. Primer pairs used for quantitative local ChIP assays are indicated by arrowheads with associated map coordinates. (E) Alignment of putative Hif1α binding sites within the mouse Ctnnb1 locus. Six regions containing Hif1α consensus binding sites are shown with the core RCGTGC motif indicated by underlined text in large font. Map coordinates corresponding to the positions assayed by local ChIP-PCR in (F) are indicated by underlined numbers in large font. Locus coordinates are from the GRCm38/mm10 genome assembly. (F) ChIP for Hif1α occupancy over the mouse Ctnnb1 locus. The 144CLP cell line was transduced with Myc epitope-tagged Hif1αTM or empty virus control, and FACS-sorted for the linked GFP marker 3 days later. Sheared chromatin was immunoprecipitated with anti-Myc tag (TAG) or IgG control antibody. Quantitative PCR was performed using primer pairs flanking putative Hif1α binding sites as indicated in (C). Data were normalized to their respective input DNA controls. Mean ± SD values of triplicate assays are plotted. **P < .01; ****P < .0001 (Student t test). FSC, forward light scatter.
Hif1α is required for LIC maintenance
Since we have shown that Hif1α is upstream of β-catenin, and that Wnt signaling is critical for the maintenance of LICs, we next tested the role of Hif1α in LICs using a conditional Hif1αloxP allele with loxP sites flanking exon 2 that encodes a helix-loop-helix motif required for heterodimerization with Hif1β and subsequent transcriptional activation.21 Primary mouse leukemias were generated by transduction of Hif1αloxP/loxP BM progenitors with NOTCH1-ΔE virus and transplantation into recipient animals. The resulting leukemias were explanted, transduced with lentiviral CreERT2/GFP, and treated with 4-OHT in vitro to delete the loxP-flanked region. GFP+ cells were then FACS-sorted from parallel 4-OHT and mock-treated cultures, and after confirming efficient deletion by polymerase chain reaction (PCR) assay (supplemental Figure 13), equal numbers were injected into secondary recipient mice. Strikingly, all animals receiving mock-treated cells succumbed to leukemia (confirmed at necropsy to be GFP+, data not shown), whereas those receiving 4-OHT–treated cells all remained viable throughout the study period (Figure 5A), equating to an approximately threefold difference in LIC frequency (supplemental Figure 14). Importantly, transduction with Hif1αTM/Cherry virus restored disease in 4-OHT–treated, Hif1αΔ/Δ cells (confirmed at necropsy to be GFP+ Cherry+, data not shown) (Figure 5A), excluding the possibility that Cre-associated genotoxicity was responsible for loss of LICs in 4-OHT–exposed cells. Furthermore, 4-OHT–treated, Hif1αΔ/Δ leukemia cells performed comparably to mock-treated, Hif1αloxP/loxP controls on short-term culture in vitro (Figure 5B-C), demonstrating that Hif1α deficiency is not associated with a generalized loss of cellular viability. We did attempt to rescue LICs in Hif1α-deficient leukemias by enforced expression of β-catenin; however, we were unable to obtain cultures of reasonable viability suggesting that excessive Wnt signaling is not tolerated in this context. These findings thus demonstrate that Hif1α is essential for the propagation of T-ALL disease in vivo, but is not required generally for cell growth/viability.
Deletion of Hif1α eradicates LICs. (A) Survival of recipient mice after transplantation with leukemias deleted of Hif1α. Two independent mouse NOTCH1-ΔE leukemias on Hif1αloxP/loxP background were explanted, transduced with CreERT2/GFP only or CreERT2/GFP and Hif1αTM/Cherry viruses, and then treated with 4-OHT or vehicle (mock) control in vitro. GFP+ or GFP+ Cherry+ leukemia cells, respectively, were then FACS-sorted and injected into each of 4 recipient animals (n = 4) at a dose of 1 × 105 cells per mouse. ***P < .001 (log-rank test). (B-C) Growth/survival of Hif1αΔ/Δ leukemia cells cultured in vitro. Primary mouse Hif1αloxP/loxP leukemias were explanted, transduced with CreERT2/GFP virus, and then treated with 4-OHT or vehicle (mock) in vitro. In (B), relative cell growth was assessed by tracking the % GFP+ cells over time, in culture by flow cytometry. A decreasing GFP+ fraction indicates transduced (GFP+) cells are growth disadvantaged compared with nontransduced (GFP−) cells in the same culture. In (C), transduced (GFP+) cells were FACS-sorted 2 days after initiation of 4-OHT treatment, then overall culture viability was assessed by flow cytometry for propidium iodide (PI) exclusion.
Deletion of Hif1α eradicates LICs. (A) Survival of recipient mice after transplantation with leukemias deleted of Hif1α. Two independent mouse NOTCH1-ΔE leukemias on Hif1αloxP/loxP background were explanted, transduced with CreERT2/GFP only or CreERT2/GFP and Hif1αTM/Cherry viruses, and then treated with 4-OHT or vehicle (mock) control in vitro. GFP+ or GFP+ Cherry+ leukemia cells, respectively, were then FACS-sorted and injected into each of 4 recipient animals (n = 4) at a dose of 1 × 105 cells per mouse. ***P < .001 (log-rank test). (B-C) Growth/survival of Hif1αΔ/Δ leukemia cells cultured in vitro. Primary mouse Hif1αloxP/loxP leukemias were explanted, transduced with CreERT2/GFP virus, and then treated with 4-OHT or vehicle (mock) in vitro. In (B), relative cell growth was assessed by tracking the % GFP+ cells over time, in culture by flow cytometry. A decreasing GFP+ fraction indicates transduced (GFP+) cells are growth disadvantaged compared with nontransduced (GFP−) cells in the same culture. In (C), transduced (GFP+) cells were FACS-sorted 2 days after initiation of 4-OHT treatment, then overall culture viability was assessed by flow cytometry for propidium iodide (PI) exclusion.
Antagonizing WNT signaling in human T-ALL
To assess the relevance of the WNT pathway in human T-ALL, PDX samples were transduced by lentiviruses carrying dnTCF to block WNT signaling. Transduced cells were then sorted by flow cytometry for the linked GFP marker and transplanted into immunodeficient NSG recipient mice. Blockade of WNT signaling with dnTCF significantly prolonged survival (Figure 6A). The measured LIC frequency was also impacted negatively (supplemental Figure 15); however, this was significant only for the M71 sample and approached, but did not reach significance for the F1313 sample (P = .06, χ2 test). Based on our mouse data above, we expect that effects of dnTCF on transit amplifying cells likely limit our ability to draw conclusions with respect to leukemia stem cells in this circumstance. Nonetheless, these data still demonstrate that dnTCF has a strong antitumor effect on human PDX samples and provide genetic evidence that antagonizing Wnt signaling may have clinical utility in T-ALL.
Effects of WNT inhibition on human T-ALL. (A) Survival of recipient NSG mice after transplantation with xenograft-expanded primary human T-ALL leukemias transduced by lentivirus encoding dnTCF or empty vector control. Leukemia cells were transduced with virus, cultured on MS5-DL1 feeders for 3 days, FACS-sorted for the viral GFP marker, and then injected into each of 4 recipient animals (n = 4) at a dose of 1 × 104 cells per mouse. Results are depicted for 2 different patient leukemias (M71 and F1313) in each experiment. *P < .05; **P < .01 (log-rank test). (B-D) Cell growth as measured by resazurin reduction. Human T-ALL cell lines (HPBALL and RPMI 8402) or PDX tumor cells (M71) were treated in vitro with XAV-939 and/or the γ-secretase inhibitor (GSI) compound E at the doses indicated for 3 to 5 days prior to assay. For the PDX culture, results were normalized against MS5-DL1 feeder-only wells. Mean ± SD values of triplicate assays are plotted. **P < .01; ****P < .0001 (one-way ANOVA). In (D), GSI accounted for 51% of the variation (P < .0001), XAV for 39% of the variation (P < .0001), and their interaction for 3% of the variation (P = .0125) by 2-way ANOVA.
Effects of WNT inhibition on human T-ALL. (A) Survival of recipient NSG mice after transplantation with xenograft-expanded primary human T-ALL leukemias transduced by lentivirus encoding dnTCF or empty vector control. Leukemia cells were transduced with virus, cultured on MS5-DL1 feeders for 3 days, FACS-sorted for the viral GFP marker, and then injected into each of 4 recipient animals (n = 4) at a dose of 1 × 104 cells per mouse. Results are depicted for 2 different patient leukemias (M71 and F1313) in each experiment. *P < .05; **P < .01 (log-rank test). (B-D) Cell growth as measured by resazurin reduction. Human T-ALL cell lines (HPBALL and RPMI 8402) or PDX tumor cells (M71) were treated in vitro with XAV-939 and/or the γ-secretase inhibitor (GSI) compound E at the doses indicated for 3 to 5 days prior to assay. For the PDX culture, results were normalized against MS5-DL1 feeder-only wells. Mean ± SD values of triplicate assays are plotted. **P < .01; ****P < .0001 (one-way ANOVA). In (D), GSI accounted for 51% of the variation (P < .0001), XAV for 39% of the variation (P < .0001), and their interaction for 3% of the variation (P = .0125) by 2-way ANOVA.
Several pharmacologic compounds have been developed to block WNT signaling,35 and in fact others have shown previously that modulating WNT signaling affects growth/survival of human T-ALL cells.36-38 Here, we employed the small molecule tankyrase inhibitor XAV-939 that enhances β-catenin turnover by stabilizing AXIN.39 XAV-939 showed efficacy against human T-ALL cell lines in vitro in a dose-dependent manner and modest synergy in combination with a γ-secretase inhibitor that blocks Notch signaling (Figure 6B-C). XAV-939 also showed activity against human PDX cells in vitro (Figure 6D). Importantly, we confirmed that XAV-939 indeed reduced β-catenin levels in this cellular context (supplemental Figure 16). Of note, these pharmacologic effects of WNT inhibition were apparent at the bulk cell level and thus recapitulate the effects of dnTCF seen on mouse leukemia cells (Figure 2). Thus, these findings suggest that pharmacologic WNT inhibitors may prove to be clinically useful in treating patients with T-ALL.
Antagonizing HIF signaling in human T-ALL
To assess the relevance of the HIF pathway in human T-ALL, PDX samples were also transduced with lentiviruses carrying short hairpin RNA (shRNA) against HIF1α to block HIF signaling. Transduced cells were then sorted by flow cytometry for the linked GFP marker and transplanted into immunodeficient NSG recipient mice. Knock-down of HIF1α was efficient (supplemental Figure 17) and significantly prolonged survival (Figure 7A), although the F1313/shHIF1α-11 cohort approached, but did not reach significance (P = .08, log-rank test). Knock-down of HIF1α also significantly reduced LIC frequency in the M71 sample, but only approached significance in the F1313/shHIF1α-10 cohort (P = .06, χ2 test) (supplemental Figure 18). Of note, leukemias that developed from F1313/shHIF1α cells retained the viral GFP marker (data not shown), but had managed to restore expression of HIF1α mRNA to levels comparable to control cells (supplemental Figure 19), suggesting counter-selection had occurred in vivo to maintain HIF signaling. In agreement with results seen following Hif1α deletion in mouse leukemia cells (Figure 5), shHIF1α-transduced human cells performed comparably to controls during short-term culture in vitro (Figure 7B-C), suggesting HIF1α is specifically required within minor LIC-enriched subpopulations. Overall, these results corroborate the mouse leukemia experiments and confirm the requirement for intact HIF signaling in human T-ALL.
Effects of HIF inhibition on patient-derived T-ALL xenografts. (A) Survival of recipient NSG mice after transplantation with xenograft-expanded primary human T-ALL leukemias transduced by lentivirus encoding shRNAs against HIF1α or scrambled shRNA control. Leukemia cells were transduced with virus, cultured on MS5-DL1 feeders for 3 days, FACS-sorted for the viral GFP marker, and then injected into each of 4 recipient animals (n = 4) at a dose of 1 × 104 cells per mouse. Results are depicted for 2 different patient leukemias (M71 and F1313) in each experiment. **P < .01 (log-rank test). (B-C) Cell growth/viability assays. Xenograft-expanded primary human T-ALL cells were transduced with 2 different lentiviral shRNAs against HIF1α or scrambled shRNA control. In (B), transduced (GFP+) cells were FACS-sorted 2 days after transduction and cultured in vitro on MS5-DL1 feeders. Cell viability was tracked daily by flow cytometry for PI dye exclusion. In (C), cells were cultured in vitro on MS5-DL1 feeders following viral transduction. Absolute numbers of viable GFP+ cells from the unsorted culture were tracked daily by flow cytometry for PI dye exclusion with admixed polystyrene counting beads. Mean ± SD values are plotted. ns, not significant.
Effects of HIF inhibition on patient-derived T-ALL xenografts. (A) Survival of recipient NSG mice after transplantation with xenograft-expanded primary human T-ALL leukemias transduced by lentivirus encoding shRNAs against HIF1α or scrambled shRNA control. Leukemia cells were transduced with virus, cultured on MS5-DL1 feeders for 3 days, FACS-sorted for the viral GFP marker, and then injected into each of 4 recipient animals (n = 4) at a dose of 1 × 104 cells per mouse. Results are depicted for 2 different patient leukemias (M71 and F1313) in each experiment. **P < .01 (log-rank test). (B-C) Cell growth/viability assays. Xenograft-expanded primary human T-ALL cells were transduced with 2 different lentiviral shRNAs against HIF1α or scrambled shRNA control. In (B), transduced (GFP+) cells were FACS-sorted 2 days after transduction and cultured in vitro on MS5-DL1 feeders. Cell viability was tracked daily by flow cytometry for PI dye exclusion. In (C), cells were cultured in vitro on MS5-DL1 feeders following viral transduction. Absolute numbers of viable GFP+ cells from the unsorted culture were tracked daily by flow cytometry for PI dye exclusion with admixed polystyrene counting beads. Mean ± SD values are plotted. ns, not significant.
Discussion
Prior work has demonstrated that NOTCH19,11 and its downstream targets including HES1,40 IGF1R,15 MYC,41 and RUNX/PKCθ7 each play contributing roles in propagation of T-ALL disease in vivo (mediated by so-called “leukemia-initiating” cells). Based on the established importance of Wnt signaling in stem cells from various tissue contexts27 and known genetic interactions between Notch and Wnt pathways in multiple systems,42 we have explored here whether Wnt signaling may play a similar role in T-ALL, a human leukemia that frequently shows dysregulated Notch signaling. Using an integrated fluorescent reporter of Wnt signaling, we have characterized the properties of leukemia cells exhibiting Wnt activation in vivo. We found that Wnt-active cells comprise only a small minority of the bulk leukemia cell population resident in the BM of mice with clinically morbid T-ALL disease, and moreover, that these cells were highly enriched for LICs. Deletion of β-catenin resulted in the loss of LICs, demonstrating their functional dependence on canonical Wnt signaling. Interestingly, the Wnt-active cells were localized in regions of relative hypoxia and deletion of Hif1α also resulted in loss of LICs. We found β-catenin was upregulated in bulk leukemia cell populations by either culture in hypoxia or enforced expression of a normoxia-stable and activated Hif1α construct. Taken together, these findings suggest a model in which hypoxic niches in vivo support Wnt signaling, in part through increased Hif1α-dependent transcription of β-catenin, which drives the expression of a complement of genes required for self-renewal of leukemia stem cells (supplemental Figure 20). We also confirmed the relevance of these processes in human T-ALL using both cell lines and PDX.
The integrated Wnt signaling reporter has allowed us for the first time to document specific and real-time localization of Wnt signaling activity to a minor subpopulation of cells that demonstrate features characteristic of leukemia stem cells, including asymmetric enrichment for LICs, recapitulation of phenotypic heterogeneity, and relative rarity.25 Since the LIC assay requires propagation of both leukemia stem and transit amplifying cell populations to score positively, it can be ambiguous, particularly in gene modification experiments, whether loss of LICs is due to effects on the former, latter, or both cell populations. For example, although the deletion of β-catenin and enforced expression of dnTCF both reduced LIC numbers, dnTCF also affected the growth/survival of bulk cells (Figure 2). These results suggest the component of Wnt signaling that is sensitive to β-catenin deletion (and detected by the Wnt reporter) includes downstream target genes specifically relevant to leukemia stem cell behavior, whereas dnTCF likely impacts a broader spectrum of targets including those specific to LICs, but also others that support cell growth/survival, more generally among transit amplifying cell populations. Of note, Premsrirut et al used reversible adenomatous polyposis coli knock-down to show that propagation of T-ALL in vivo required continued adenomatous polyposis coli suppression; however, the rapidity of disease clearance would suggest that bulk cells were affected in their model.43 In this regard, our results are particularly noteworthy because they bring together (1) direct evidence of localized Wnt activation in a minor tumor subpopulation with (2) genetic demonstration of functional dependence that is restricted to the cellular subpopulation in question and is not confounded by broad effects on the bulk tumor cell population. Further studies are currently underway to identify Wnt target genes responsible for leukemia stem cell-specific vs bulk cell phenotypes.
Our observation that hypoxic niches are dispersed throughout interstitial areas would fit with other work suggesting that infiltrating leukemia cells actively remodel the local stromal microenvironment as opposed to simply passively occupying preexisting, anatomically defined compartments. For example, in myeloproliferative neoplasia it has been shown that leukemia cells stimulate multipotent stromal cells to produce abnormal osteoblastic lineage cells that favor leukemia stem cells over normal HSCs.44 Similar findings of leukemic infiltration-induced expansion of interstitially localized hypoxic niches have been reported in models of acute lymphoblastic leukemia45 and that are populated by clusters of HIF-expressing cells.46 Of note, HIF transcription factors have been shown to be required for maintenance of cancer stem cells in the mouse TGB lymphoma model/human AML47 and human glioma.48
Wnt signaling supports self-renewal of normal HSCs,24 and in its absence (modeled using Ctnnb1null progenitors), the initial transformation of otherwise normal progenitor cells by BCR-ABL,49 Hoxa9/Meis1a, MLL-AF9,50 or PTEN loss8 is impaired. In the present study, we assessed the effect of acute loss of Wnt signaling in established T-ALL by deleting β-catenin after having generated leukemias with activated NOTCH1. Accordingly, our results, though noninformative on the role of Wnt signaling in T-ALL disease initiation, are ultimately more relevant to identifying functional dependencies that can be targeted therapeutically in patients with established disease. Overall, our results demonstrate that Hif and Wnt/β-catenin signaling pathways support leukemia stem cell function in T-ALL, and thus suggest that pharmacologic inhibitors of these pathways may improve clinical outcomes, particularly for those with disease that is refractory to, or relapses after conventional chemotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Celeste Simon (University of Pennsylvania) for providing the triple mutated Hif1α construct and the MacDougald Laboratory (University of Michigan) for the dnTCF4e construct.
This study was supported by research funding from the Terry Fox Foundation, Canadian Institutes of Health Research, and the Leukemia & Lymphoma Society of Canada (A.P.W.).
Authorship
Contribution: V.G., C.J., S.H.L, C.H., M.B., X.W., S.G., and D.G. generated the data; and V.G. and A.P.W. designed the experiments, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew P. Weng, BC Cancer Agency, 675 West 10th Ave, CRC 12-111, Vancouver, BC V5Z 1L3, Canada; e-mail: aweng@bccrc.ca; and Vincenzo Giambra, BC Cancer Agency, 675 West 10th Ave, Vancouver, BC V5Z 1L3, Canada; e-mail: vgiambra@bccrc.ca.

![Figure 1. LICs are enriched within a small tumor subpopulation with active Wnt signaling. (A) Schematic diagram of the integrated fluorescent Wnt reporters, 7TGP and 7TGC, used to mark leukemia cells with active Wnt signaling. The reporter element is composed of 7 Tcf/Lef-binding sites upstream of a minimal promoter and GFP, followed by a separate SV40-puromycin (7TGP) or SV40-Cherry (7TGC) selection marker. The construct backbone is a self-inactivating lentiviral vector. (B) Schematic diagram of experimental approach. Primary mouse NOTCH1-ΔE leukemias were explanted and transduced with 7TGP or 7TGC lentivirus. 7TGP-transduced cells were selected with 1 μg/ml puromycin on MS5-DL1 puro feeder cells for 5 days beginning on day 2 posttransduction. 7TGC-transduced cells were FACS-sorted on day 2 posttransduction. Transduced cells were then transplanted into recipient mice, all of which subsequently developed leukemia. These secondary 7TGP/7TGC-transduced leukemias were then analyzed by flow cytometry for GFP expression. GFP+ and GFP− subsets were then FACS sorted (also Cherry+ in 7TGC experiments) and transplanted at limiting dilution into tertiary recipients. (C) Summary of GFP+ cell abundance in Wnt reporter-transduced leukemias after passage in vivo. Freshly explanted BM from clinically morbid recipients of 7TGP or 7TGC-transduced primary leukemias was analyzed by flow cytometry for GFP expression within gated NGFR+ (7TGP) or NGFR+ Cherry+ (7TGC) leukemia cell populations (labeled “2° leukemia” in [B]). Results depicted are compiled from 10 independent primary leukemias (26 recipients in total; 11 × 7TGP and 15 × 7TGC). Each data point represents an individual mouse. Mean ± standard deviation (SD) values are indicated by horizontal lines. (D) Survival of recipient mice after transplantation with FACS-sorted Wnt active (GFP+) or Wnt inactive (GFP−) subsets from 7TGP and 7TGC-transduced secondary leukemias. Each of 4 recipient animals (n = 4) was injected with each of the cell doses as indicated in parentheses. Two of 3 transplant experiments using independent primary leukemias are depicted. ****P < .0001 (log-rank test). LTR, long terminal repeat; sinLTR, self-inactivating LTR.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/25/10.1182_blood-2014-10-609370/4/m_3917f1.jpeg?Expires=1770765145&Signature=qs1l3KLs3VHgFRxMpnqpbRE61vvG9hK3J~5iaNqXANTFBbdYausPoE9HTjGakuSKAZA6W3ub0RqqQTrJYVJfRJmLZOxwKok~1scGP9nGTbReklZOUZgA8DPxsD7FFbmCxz5ufHeYNZgUbp25HVcbfO9iEskpGdVLqgPHRj2HY4IwZyUGUabuVGxmzrfTDQ7kcq2oo77YnnO3lL2rO6EeW8sljEwi86kc3ivZAnxGdYp1XgA49RHO3RrH3rhNOAksfccYZZEO3Sd8vGGfejnRG7dWso8XP2p87tiPb5nkT1ZMbj25XgWzjQBhXJPNoS5vlckCrilkuHvhCjK0d12yaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Hypoxia potentiates Wnt signaling and supports clonogenic activity. (A) Flow cytometric analysis of pimonidazole staining, a marker of hypoxia. Mice were transplanted with 7TGP-transduced leukemia cells and, after developing clinically morbid disease, were injected by tail vein with pimonidazole (60 mg/kg). One hour later, mice were euthanized and BM cells harvested, fixed, and stained with anti-pimonidazole antibody. Gated NGFR+ leukemia cells are shown. The marrow contained >90% tumor cells (of which 40% to 70% were GFP+). Results depicted are representative of 2 different leukemic animals. (B) Immunohistochemical staining for GFP in BM sections of mice following transplantation with 7TGP-transduced leukemia cells. Mice were clinically morbid with disease at the time they were euthanized and contained >90% tumor cells (of which 40% to 70% were GFP+) within the marrow. Red arrowheads indicate individual GFP+ cells. Images depicted are representative of 2 different leukemic animals. Scale bar = 60 μm. (C-D) Analysis of β-catenin protein expression level in NOTCH1-ΔE mouse leukemia cells cultured in vitro under reduced oxygen conditions. Cells were cultured in complete growth media under 20% or 5% oxygen for 3 days in plastic tissue culture dishes, then harvested for analysis by western blot (C) and flow cytometry (D). The numbers below the β-catenin blot panel in (C) indicate relative band intensities after normalization to the β-actin loading control. Gated leukemia cells are depicted in (D). Results from at least 2 independent leukemias are shown. (E-F) CFC assay for in vitro clonogenic activity. Primary leukemia cells were plated in methylcellulose-containing media supplemented or not with Wnt3a ligand or Dickkopf-related protein 1 and cultured in either 20% or 5% oxygen. Each colored data point depicted represents an individual culture dish. Mean ± SD values are indicated by horizontal lines. Results in (F) are representative of 2 different leukemias. *P < .05; **P < .01; ****P < .0001 (Student t test in [E], 2-way analysis of variance [ANOVA] with Bonferroni multiple comparisons test in [F]). ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/25/10.1182_blood-2014-10-609370/4/m_3917f3.jpeg?Expires=1770765145&Signature=zU6NBSzrBCy9M5976YMuRYY7AsVywHJcdoTviIj1m1bo77UhtTfYIaFx61g3JqQdsXYMiRtxKOo3EERN6ESRH~B79Kr6aQlFN7sPk7b8H7HqZx2HlNxL6A8OGZNo1tfRkRF1UGXSZn9gJracfoPD2ZrrnBzfzIktuAPAIztlgh8qcZoJUxj8bH~U17EHrYAdum6pF2m-~yX64l1yWia205fQ2FJ3AFrbPbIYIRYVZbYU3Ag0oUfE0Du7WWcLGLSfLCnemtFjv2nza7QcwG8w-qtlGQ7BlMGtCcWMVwg5hAxMJAlD7UWKLa9D5LZeb18L5G3KCBZpwsOp5-5lNTpoCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal