Key Points
Tumor IDO inhibits CD19-CART activity, likely via induction of the kynurenine pathway, whose metabolites directly inhibit T cells.
Fludarabine and cyclophosphamide, frequently used before CART administration, downregulate IDO expression in lymphoma cells.
Abstract
Although T cells expressing CD19-specific chimeric antigen receptors (CARs) are a promising new therapy for B-cell malignancies, objective responses are observed at lower frequencies in patients with lymphoma than in those with acute B-cell leukemia. We postulated that the tumor microenvironment suppresses CAR-expressing T cells (CARTs) through the activity of indoleamine 2,3-dioxygenase (IDO), an intracellular enzyme that converts tryptophan into metabolites that inhibit T-cell activity. To investigate the effects of tumor IDO on CD19-CART therapy, we used a xenograft lymphoma model expressing IDO as a transgene. CD19-CARTs inhibited IDO-negative tumor growth but had no effect on IDO-positive tumors. An IDO inhibitor (1-methyl-tryptophan) restored IDO-positive tumor control. Moreover, tryptophan metabolites inhibited interleukin (IL)-2–, IL-7–, and IL-15–dependent expansion of CARTs; diminished their proliferation, cytotoxicity, and cytokine secretion in vitro in response to CD19 recognition; and increased their apoptosis. Inhibition of CD19-CARTs was not mitigated by the incorporation of costimulatory domains, such as 4-1BB, into the CD19-CAR. Finally, we found that fludarabine and cyclophosphamide, frequently used before CART administration, downregulated IDO expression in lymphoma cells and improved the antitumor activity of CD19-CART in vivo. Because tumor IDO inhibits CD19-CARTs, antagonizing this enzyme may benefit CD19-CART therapy.
Introduction
Recent clinical trials have shown that CD19-specific chimeric-antigen-receptor (CAR) T cells (CARTs) are a promising therapy for B-cell malignancies.1-7 CARs are fusion proteins combining the antigen-recognition fragment of a monoclonal antibody with T-cell activation domains from the T-cell receptor complex, such as the ζ chain, and costimulatory endodomains, from CD28, 4-1BB, or OX40.8 In clinical trials, up to 90% complete response rates have been seen after CD19-CART administration, even in chemotherapy-refractory acute lymphocytic leukemia.7 Results in other B-cell cancers, such as chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL), however, have been less striking.8,9 One explanation for the different response rates among tumor types is that CART functionality may be inhibited by an immunosuppressive tumor microenvironment. In a recent study, blockade of the programmed death-1 (PD-1) immunosuppressive pathway significantly enhanced the antitumor efficacy of CARTs in a preclinical mouse model,10 but it is likely that additional tumor immune evasion mechanisms are also exploited by resistant tumors.
Indoleamine 2,3-dioxygenase (IDO) is an intracellular enzyme that mediates the metabolism of the essential amino acid tryptophan11 into immunosuppressive metabolites, such as kynurenine and 3-hydroxyanthranilic acid (3-HAA). Accumulation of these tryptophan derivatives blocks antigen-specific T-cell proliferation and induces T-cell death through the aryl-hydrocarbon receptor (AHR), also known as the dioxin receptor.12-14 Because IDO is induced by inflammatory mediators, notably interferon (IFN)-γ, its expression is thought to be an endogenous feedback mechanism controlling excessive immune responses.15 IDO is known to be produced by tumor cells and by some immune cells, such as dendritic cells and macrophages, which reside in tumor-draining lymph nodes or are recruited to tumors.15-17 IDO is overexpressed in several human cancers, including prostate, breast, brain, and hematologic malignancies,16,18 and both IDO expression by tumor cells and high serum l-kynurenine levels correlate with poor prognosis in DLBCL patients.18,19 However, the effects of IDO on CD19-CART therapy are unknown.
Here, we show that tumor IDO activity can inhibit CD19-CART therapy through the action of tryptophan metabolites. We also demonstrate that fludarabine and cyclophosphamide, frequently administered before CD19-CART infusion to improve CART activity, downregulate IDO expression by B-cell malignancies. These data may provide an approach to enhancing the effectiveness of CD19-CART therapy in patients with otherwise resistant lymphoma.
Materials and methods
Cell lines
Raji, Daudi, BJAB, and Jeko-1 (CD19+ lymphoma lines), and K562 cells were maintained in RPMI-1640 (Hyclone Laboratories, Logan, UT), 10% fetal bovine serum, and 2 mM l-glutamine (Invitrogen). Raji cells were transduced with a retroviral vector encoding human IDO cDNA (Raji-IDO) or an empty vector (Raji-control) and a puromycin resistance gene. Transduced cells were single-cell cloned by limiting dilution.
CAR T-cell generation
Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteer donors and transduced with retroviral vectors encoding first-, second-, or third-generation CD19-CARs as previously described20 (supplemental Methods available on the Blood Web site). All experiments described used the second-generation CD19-CAR, except where noted. Experiments were done on protocols approved by the Baylor College of Medicine Institutional Review Board, in accordance with the Declaration of Helsinki.
Reagents
l-tryptophan, l-kynurenine, and 3-HAA (Sigma-Aldrich) were prepared in distilled water. 1-Methyl-d-tryptophan (1-MT; Sigma-Aldrich) was prepared in 0.1 N NaOH, which was then adjusted to pH 7.4. Mafosfamide (Santa Cruz Biotech, Santa Cruz, CA) and fludarabine (TOCRIS Bioscience, Minneapolis, MN) were prepared in dimethylsulfoxide.
Western blot analysis
Samples of total protein lysate were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Polyvinylidenefluoride membranes (Bio-Rad, Hercules, CA) were incubated after protein transfer with anti-IDO antibody (Adipogen, San Diego, CA) or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling, Beverly, MA) as a loading control. Jeko-1 and CLL PBMCs were incubated for 24 hours with mafosfamide (2 μg/mL), fludarabine (20 μM), or both. Cells were incubated with or without IFN-γ (50 U/mL) for 24 hours before proteins were extracted.
High performance liquid chromatography analysis
Concentrations of l-tryptophan and l-kynurenine in cell culture medium were determined by reverse-phase high performance liquid chromatography (HPLC) analysis.
Flow cytometry and antibodies
CARTs were stained with fluorochrome-labeled antibodies against CD3 (BD, Franklin Lakes, NJ), PD-1 (BD Biosciences), CTLA-4 (BD Biosciences), and immunoglobulin (Ig)G1-CH2CH3 (Jackson ImmunoResearch, West Grove, PA). Target lines were stained with CD19 antibodies (FMC63 clone; Millipore). Flow analysis used a FACSCalibur (Becton Dickinson) and CellQuest software (BD Biosciences).
Chromium-51 release assay
The cytotoxic activity of CARTs was evaluated in a standard 4-hour chromium-51 release assay.21
Transcriptional levels of AHR, CYP1A1, and CYP1B1
Real-time quantitative polymerase chain reaction was performed to determine expression of AHR, CYP1A1, and CYP1B1 (supplemental Methods).
Cell proliferation assay
Cell proliferation assays used the XTT cell proliferation kit (ATCC, Manassas, VA). To examine the effect of tryptophan metabolites on cell proliferation, we used a 1:1 mixture of l-kynurenine and 3-HAA (KHAA). CARTs were cultured in 0 to 50 μM KHAA, with interleukin (IL)-2, IL-7, or IL-15 (Peprotech, Rocky Hill, NJ) for 72 hours. Lymphoma cell lines were cultured in 0 to 50 μM KHAA.
Carboxylfluorescein diacetate succinimidyl ester dilution assay
Nontransduced T cells (NTs) and CARTs were labeled with carboxylfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR), as previously described.22 T cells were then stimulated with irradiated Raji cells (1:1 effector to target ratio [E:T]) in the absence or presence of KHAA without exogenous cytokines for 3 days and stained with CD3 antibody before assessing CFSE dilution.
CART expansion, apoptosis, and cytokine production
CARTs (1 × 106 cells) were stimulated once a week by irradiated Raji (1 × 106 cells) in the absence or presence of KHAA, without exogenous cytokines, and counted. CARTs were cultured in the absence or presence of KHAA for 3 days with IL-2 (50 U/mL), washed, and stained with Annexin-V/7-aminoactinomycin D (AAD) kit (BD). Seven days after expansion by irradiated Raji cells, CARTs (1 × 106 cells) were collected and recultured with Raji cells (1 × 106 cells) for 24 hours, and supernatant concentration of IL-2 and IFN-γ was quantified by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
In vitro coculture killing experiments
CARTs were cocultured with green fluorescent protein (GFP)-transduced wild-type Raji cells in the absence or presence of KHAA. On specific days after coculture, cells were counted and stained with CD3 antibody to distinguish tumor (GFP+CD3–) and T (GFP–CD3+) cells.
In vivo antitumor activity and trafficking of CARTs
Eight-week-old SCID-Beige mice (Charles River) were subcutaneously engrafted in opposite flanks with firefly luciferase (FFLuc)-transduced Raji-control and Raji-IDO cells (3 × 106). Seven days after tumor engraftment, CARTs or NTs (1 × 107) were infused intravenously. Tumor bioluminescence was monitored using the Xenogen In Vivo Imaging System (IVIS; Caliper Life Sciences, Hopkinton, MA). To evaluate an IDO inhibitor effect, FFLuc-transduced Raji-IDO or Jeko-1 cells were injected subcutaneously, and mice were treated with oral (drinking water) 1-MT (5 mg/mL), intravenous CARTs, or both. We observed no evidence of lower fluid intake in mice receiving 1-MT water (supplemental Figure 3). Tumor bioluminescence was monitored by IVIS. To study trafficking of CARTs, unlabeled Raji-control and Raji-IDO cells were injected subcutaneously and, 2 weeks later, FFLuc-transduced CARTs were infused intravenously. CART bioluminescence was monitored by IVIS. Institutional Animal Care and Use Committee approval was obtained from the Baylor College of Medicine.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Differences in means were tested using a 2-sided Student t test to evaluate continuous variables of 2 groups and with a 1-way analysis of variance to evaluate continuous variables of >2 groups, at a significance level of .05.
Results
IDO expression by B-cell lymphoma cell lines and primary B-cell leukemia cells
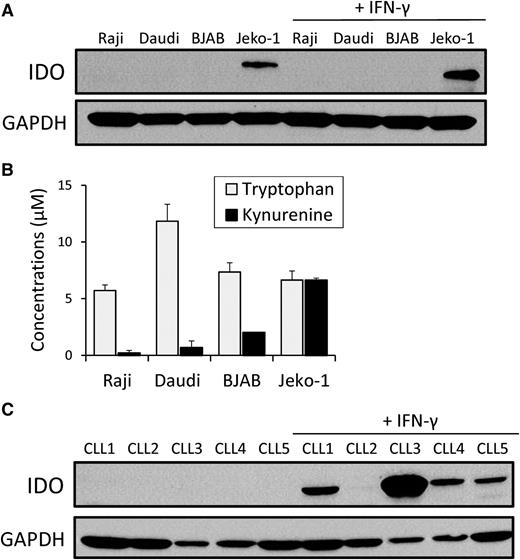
To study the effect of IDO on the antitumor activity of CD19-CARTs, we first examined IDO expression in a panel of B-cell lymphoma cell lines using immunoblot analysis. Jeko-1 naturally expressed IDO, whereas the enzyme was not expressed by Raji, Daudi, or BJAB, even after exposure to IFN-γ, a known inducer of IDO23 (Figure 1A). We confirmed the enzyme was active by HPLC analysis of cell culture supernatant, which showed that Jeko-1 produced higher l-kynurenine (6.6 ± 0.2 μM) from l-tryptophan than the other cell lines (Raji: 0.1 ± 0.2 μM, Daudi: 0.6 ± 0.5 μM, BJAB: 2.0 ± 0.1 μM; Figure 1B).
IDO in B-cell lymphoma lines and primary CLL cells. (A) Four B-cell lymphoma cell lines (Raji, Daudi, BJAB, and Jeko-1) and (C) 5 CLL patients’ PBMCs were cultured in the absence or presence of exogenous IFN-γ (50 U/mL) for 24 hours. Protein extracts were prepared for IDO western immunoblot analysis. Results are representative of 3 independent experiments. (B) IDO enzyme activity was evaluated by measuring the concentrations of l-tryptophan and l-kynurenine in the cell culture medium of the B-cell lymphoma lines using HPLC. Each bar represents the mean ± SD of the results from 3 independent experiments.
IDO in B-cell lymphoma lines and primary CLL cells. (A) Four B-cell lymphoma cell lines (Raji, Daudi, BJAB, and Jeko-1) and (C) 5 CLL patients’ PBMCs were cultured in the absence or presence of exogenous IFN-γ (50 U/mL) for 24 hours. Protein extracts were prepared for IDO western immunoblot analysis. Results are representative of 3 independent experiments. (B) IDO enzyme activity was evaluated by measuring the concentrations of l-tryptophan and l-kynurenine in the cell culture medium of the B-cell lymphoma lines using HPLC. Each bar represents the mean ± SD of the results from 3 independent experiments.
We next studied IDO expression by circulating leukemic cells from 5 B-CLL patients (supplemental Table). IDO could not be detected in the PBMCs of any of them, but, in 4 of the 5, IDO was dramatically upregulated when these cells were cultured with IFN-γ for 24 hours (Figure 1C).
CD19-CARTs inhibit Raji-control but not Raji-IDO tumors
To isolate the effects of IDO from other confounding variables, we chose a natively IDO-negative cell line, Raji, as a model and made an IDO-positive Raji clone (Raji-IDO) by retroviral transduction with human IDO cDNA (supplemental Figure 1A). A control cell line (Raji-control) was generated by retroviral transduction with an empty vector. Raji-IDO catabolized all of the l-tryptophan in RPMI-1640 medium within 72 hours into l-kynurenine (10.7 ± 1.4 μM), in contrast to Raji-control cells (0.6 ± 0.5 μM). When Raji-IDO was cultured with an IDO inhibitor (1-MT), the production of l-kynurenine decreased (1.6 ± 0.03 μM) and l-tryptophan levels remained (22.4 ± 1.8 μM) close to RPMI’s baseline of 25 μM (supplemental Figure 1B). CD19 and costimulatory ligand expression, and proliferation were identical in both cell types (supplemental Figures 1C-D and 2).
To evaluate the effect of tumor-derived IDO on CD19-CART therapy, we injected luciferase-transduced Raji-control and Raji-IDO cells subcutaneously in the opposite flanks of SCID-Beige mice. Seven days later, we injected human NTs or CD19-CARTs intravenously. Although IDO and control tumors grew rapidly and equally after infusion of NTs (Figure 2B), control tumors were inhibited by CD19-CARTs (5.5 ± 7.4 × 108 bioluminescence units [BU], photons per second, on day 7). By contrast, IDO tumors were resistant to CD19-CART inhibition (28.8 ± 18.5 × 108 BU, P = .009; Figure 2C).
Effect of tumor-derived IDO on CD19-CARTs. (A) Schematic representation of the experiments in SCID/Beige mice comparing the antitumor effects of CD19-CARTs on Raji-control (left flank) and Raji-IDO tumor (right flank). (B) Time course of tumor bioluminescence in mice treated with nontransduced (NT) T cells or CD19-CARTs. (C) Time course of bioluminescence of Raji-control and Raji-IDO tumors treated with NT and CARTs. Data represent mean ± SD of 8 mice per group from 2 independent experiments (*P < .05).
Effect of tumor-derived IDO on CD19-CARTs. (A) Schematic representation of the experiments in SCID/Beige mice comparing the antitumor effects of CD19-CARTs on Raji-control (left flank) and Raji-IDO tumor (right flank). (B) Time course of tumor bioluminescence in mice treated with nontransduced (NT) T cells or CD19-CARTs. (C) Time course of bioluminescence of Raji-control and Raji-IDO tumors treated with NT and CARTs. Data represent mean ± SD of 8 mice per group from 2 independent experiments (*P < .05).
IDO inhibitor (1-MT) protects CARTs against the deleterious effects of IDO-positive tumors
To evaluate the effect of inhibiting IDO in tumors treated with CD19-CARTs, we injected Raji-IDO cells subcutaneously and treated mice with 1-MT (a competitive IDO inhibitor), CARTs, or both agents (Figure 3A). We found that the combination treatment produced significantly better tumor control than either single therapy (1-MT: 14.8 ± 1.7 × 108 BU and CART: 16.8 ± 12.9 × 108 BU vs both: 7.0 ± 4.8×108 BU on day 7, P = .002 and 0.04, respectively; Figure 3B). We repeated the experiment using Jeko-1, a natively IDO-expressing cell line. We again observed similar results (1-MT: 31.1 ± 25.8 × 107 BU and CART: 13.1 ± 19.3 × 107 BU vs both: 1.2 ± 1.1 × 107 BU on day 7, P = .01 and 0.04, respectively; Figure 3C). Single therapy with 1-MT had no effect on Jeko-1 tumor growth (no treatment: 23.4 ± 21.7 × 107 BU vs 1-MT: 31.1 ± 25.8 × 107 BU on day 7; P = .54).
Combination of IDO inhibitor and CD19-CART therapy on IDO-positive tumors. (A) Schematic representation of the experiments in SCID/Beige mice comparing the antitumor effect of IDO inhibitor (1-MT), CD19-CARTs, or the combination of both on IDO-positive malignancies (Raji-IDO and Jeko-1). (B) Time course of Raji-IDO tumor bioluminescence. Data represent mean ± SD of 7 mice per group from 2 independent experiments (*P < .05). (C) Time course of Jeko-1 tumor bioluminescence. Data represent mean ± SD of 8 mice per group from 2 independent experiments (*P < .05). (D) CD19-CARTs retained similar cytotoxic activity against Raji-control and Raji-IDO in a 4-hour 51Cr-release assay. (E) Luciferase-labeled CD19-CARTs were injected intravenously in mice that had been subcutaneously inoculated with nonlabeled Raji-control (left flank) and Raji-IDO (right flank). The time course of T-cell bioluminescence at Raji-control and Raji-IDO tumor site is shown. Data represent mean ± SD of 4 mice.
Combination of IDO inhibitor and CD19-CART therapy on IDO-positive tumors. (A) Schematic representation of the experiments in SCID/Beige mice comparing the antitumor effect of IDO inhibitor (1-MT), CD19-CARTs, or the combination of both on IDO-positive malignancies (Raji-IDO and Jeko-1). (B) Time course of Raji-IDO tumor bioluminescence. Data represent mean ± SD of 7 mice per group from 2 independent experiments (*P < .05). (C) Time course of Jeko-1 tumor bioluminescence. Data represent mean ± SD of 8 mice per group from 2 independent experiments (*P < .05). (D) CD19-CARTs retained similar cytotoxic activity against Raji-control and Raji-IDO in a 4-hour 51Cr-release assay. (E) Luciferase-labeled CD19-CARTs were injected intravenously in mice that had been subcutaneously inoculated with nonlabeled Raji-control (left flank) and Raji-IDO (right flank). The time course of T-cell bioluminescence at Raji-control and Raji-IDO tumor site is shown. Data represent mean ± SD of 4 mice.
Tryptophan metabolites inhibit proliferation of CARTs in the presence of cytokines and tumor cells
We next investigated potential mechanisms of CART inhibition by IDO. Resistance of IDO tumors to CD19-CARTs was not explained by direct inhibition of cytotoxic function, because the cytotoxic activity of CD19-CARTs was identical on Raji-control and Raji-IDO cells when tested in a short-term, 4-hour cytotoxicity assay (Figure 3D).
To establish that the resistance of IDO tumors to CD19-CARTs was not a result of differential trafficking to tumor sites, we injected SCID mice subcutaneously in opposite flanks with nonlabeled Raji-control and Raji-IDO cells. Fourteen days later, we injected luciferase-transduced CD19-CARTs intravenously. CD19-CARTs were detected in the lungs, the spleen, and both tumor sites 16 hours after injection (Figure 3E). On days 2 and 3, CD19-CARTs were detected only at both tumor sites.
We then assessed the effects of tryptophan metabolites (KHAA) on the biology and proliferation of CARTs in vitro. KHAA activated AHR target genes in CARTs (Figure 4A), implicating pathways downstream of AHR in the effects observed. We also found that even low concentrations (6.25 µM) of KHAA significantly inhibited CART proliferation in response to a single T-cell growth cytokine (IL-2, or IL-7, or IL-15; Figure 4B). In contrast, there was no effect of KHAA on the proliferation of B-cell lymphoma cell lines until high concentrations (25 µM) were reached (Figure 4C).
Effect of tryptophan metabolites on CD19-CART proliferation. (A) AHR, CYP1A1, and CYP1B1 mRNA expression measured by real-time PCR on CD19-CARTs treated with or without KHAA (12.5 μM) for 24 hours. Data represent mean ± SD of 4 T-cell lines generated from 4 healthy donors and normalized to GAPDH expression. (B) CD19-CARTs were cultured in media containing increasing amounts of l-kynurenine and 3-HAA (KHAA) (0, 6.25, 12.5, 25, or 50 μM) with IL-2 (50 U/mL), IL-7 (10 ng/mL), or IL-15 (5 ng/mL) for 72 hours. (C) Four B-cell lymphoma lines (Raji, Daudi, BJAB, or Jeko-1) were cultured in media containing increasing amounts of KHAA for 72 hours, without exogenous cytokines. Cell proliferation was studied by the XTT assay. One representative set of 3 experiments is shown (*P < .05). (D) NTs or CD19-CARTs labeled with CFSE were cultured with irradiated Raji cells for 3 days in the absence or presence of KHAA (12.5 or 25 μM) without any added exogenous cytokines. CFSE dilution was assayed by flow cytometry, gating on CD3-positive cells. Controls (dotted line) were cultured without Raji cells. Data represent mean ± SD of CSFE dilution in 4 T-cell lines generated from 4 healthy donors (*P < .05). (E) The number of CD19-CARTs cultured in the presence or absence of KHAA (12.5 μM) was determined at the end of each 7-day expansion cycle. Data represent mean ± SD of 5 T-cell lines generated from 5 donors (*P < .01).
Effect of tryptophan metabolites on CD19-CART proliferation. (A) AHR, CYP1A1, and CYP1B1 mRNA expression measured by real-time PCR on CD19-CARTs treated with or without KHAA (12.5 μM) for 24 hours. Data represent mean ± SD of 4 T-cell lines generated from 4 healthy donors and normalized to GAPDH expression. (B) CD19-CARTs were cultured in media containing increasing amounts of l-kynurenine and 3-HAA (KHAA) (0, 6.25, 12.5, 25, or 50 μM) with IL-2 (50 U/mL), IL-7 (10 ng/mL), or IL-15 (5 ng/mL) for 72 hours. (C) Four B-cell lymphoma lines (Raji, Daudi, BJAB, or Jeko-1) were cultured in media containing increasing amounts of KHAA for 72 hours, without exogenous cytokines. Cell proliferation was studied by the XTT assay. One representative set of 3 experiments is shown (*P < .05). (D) NTs or CD19-CARTs labeled with CFSE were cultured with irradiated Raji cells for 3 days in the absence or presence of KHAA (12.5 or 25 μM) without any added exogenous cytokines. CFSE dilution was assayed by flow cytometry, gating on CD3-positive cells. Controls (dotted line) were cultured without Raji cells. Data represent mean ± SD of CSFE dilution in 4 T-cell lines generated from 4 healthy donors (*P < .05). (E) The number of CD19-CARTs cultured in the presence or absence of KHAA (12.5 μM) was determined at the end of each 7-day expansion cycle. Data represent mean ± SD of 5 T-cell lines generated from 5 donors (*P < .01).
We next studied the effect of tryptophan metabolites on the proliferation of CARTs in response to CD19-positive cells. CART proliferation was assessed by CFSE dilution. We found KHAA inhibited the proliferation of CD19-CARTs in response to Raji (percentage of CFSE dilution with KHAA 0 µM: 79.2 ± 5.0% vs 12.5 µM: 71.2 ± 7.5% and 25 µM: 44.5 ± 18.9%, P = .04 and 0.01, respectively; Figure 4D). Furthermore, CD19-CART expansion induced by weekly stimulation with irradiated Raji cells was significantly decreased in the presence of KHAA (after 14 days, expansion was 29.2 ± 3.4-fold vs 7.6 ± 2.2-fold, in the absence and presence of 12.5 µM KHAA, respectively; P = .001; Figure 4E).
Tryptophan metabolites reduce the cytotoxic activity of CD19-CARTs
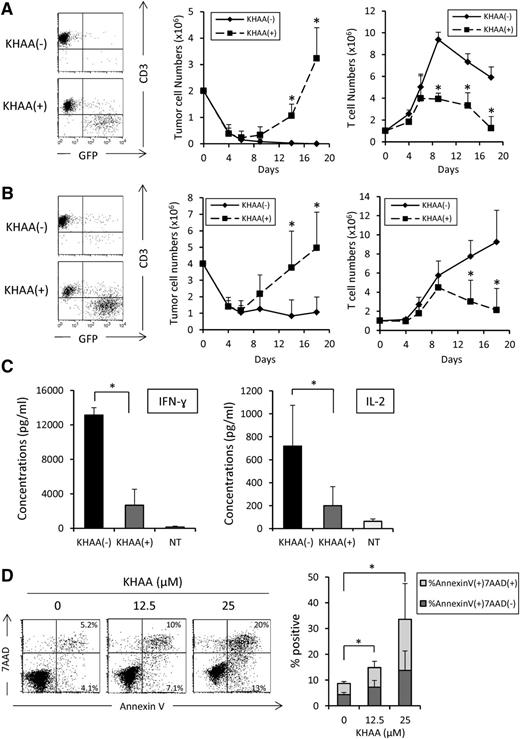
To assess the functional effects of tryptophan metabolites on the antitumor activity of CARTs, we cocultured CD19-CARTs (1 × 106) with GFP-transduced wild-type Raji cells (2 × 106). By day 9, tumor cells were almost eliminated in the absence of KHAA but were increased (and CARTs decreased) in the presence of KHAA (Figure 5A). On day 14, the percentage of tumor cells in culture in the absence and presence of KHAA was 0.3 ± 0.26% vs 22.1 ± 5.3% and their number was 0.02 ± 0.02 × 106 cells vs 1.05 ± 0.44 × 106 cells, respectively (P = .02). The number of CARTs in the absence and presence of KHAA was 7.3 ± 0.8 × 106 cells and 3.3 ± 1.2 × 106 cells, respectively (P = .008). Similar results were observed using a lower effector-to-target ratio (1:4 instead of 1:2; Figure 5B).
Effect of tryptophan metabolites on killing, apoptosis, and cytokine secretion by CD19-CARTs. GFP-transduced wild-type Raji cells were cocultured with CD19-CARTs at E:T ratios (A) 1:2 and (B) 1:4 in the presence or absence of KHAA (12.5 μM). The total number of tumor cells and CARTs in each culture condition was determined using flow cytometry analysis at the indicated time points. Dot plot shows representative data on day 14. Three experiments using CARTs generated from 3 different donors were analyzed and shown as the mean ± SD in the graph (*P < .05). (C) Seven days after the first expansion, CD19-CARTs (1 × 106) were cultured with Raji cells (1 × 106) in the presence or absence of KHAA (12.5 μM); supernatants were collected after 24 hours and analyzed for IL-2 and IFN-γ by ELISA. Data represent mean ± SD of 4 T-cell lines generated from 4 donors (*P < .05). (D) Apoptosis was determined by annexin V/7-AAD staining of CD19-CARTs after 3 days of culture in the absence or presence of KHAA (12.5 or 25 μM) and IL-2 (50 U/mL). Data represent mean ± SD of 4 T-cell lines generated from 4 donors (*P < .05).
Effect of tryptophan metabolites on killing, apoptosis, and cytokine secretion by CD19-CARTs. GFP-transduced wild-type Raji cells were cocultured with CD19-CARTs at E:T ratios (A) 1:2 and (B) 1:4 in the presence or absence of KHAA (12.5 μM). The total number of tumor cells and CARTs in each culture condition was determined using flow cytometry analysis at the indicated time points. Dot plot shows representative data on day 14. Three experiments using CARTs generated from 3 different donors were analyzed and shown as the mean ± SD in the graph (*P < .05). (C) Seven days after the first expansion, CD19-CARTs (1 × 106) were cultured with Raji cells (1 × 106) in the presence or absence of KHAA (12.5 μM); supernatants were collected after 24 hours and analyzed for IL-2 and IFN-γ by ELISA. Data represent mean ± SD of 4 T-cell lines generated from 4 donors (*P < .05). (D) Apoptosis was determined by annexin V/7-AAD staining of CD19-CARTs after 3 days of culture in the absence or presence of KHAA (12.5 or 25 μM) and IL-2 (50 U/mL). Data represent mean ± SD of 4 T-cell lines generated from 4 donors (*P < .05).
Mechanism of inhibition of CD19-CART proliferation and cytotoxicity by tryptophan metabolites
To investigate potential mechanisms of CART inhibition by tryptophan metabolites, we assessed the effect of KHAA on CART cytokine secretion and apoptosis. We found that KHAA inhibited the release of IFN-γ (13 143 ± 848 vs 2663 ± 1873 pg/mL in the absence and presence of KHAA, respectively) and IL-2 (718 ± 355 vs 199 ± 165 pg/mL) when exposed to Raji cells for 7 days (Figure 5C). KHAA was also associated with increased CART apoptosis, as assessed by Annexin-V staining (percent positive cells in 0, 12.5, and 25 µM KHAA was 8.6 ± 1.0%, 14.8 ± 4.2%, and 33.5 ± 21.3%, respectively; Figure 5D). However, we found no difference in the expression of exhaustion markers, such as PD-1 and CTLA-4, or in expression of granzyme B on CARTs irrespective of the presence of KHAA (data not shown).
Additional costimulatory domains do not overcome inhibition mediated by tryptophan metabolites
Second- and third-generation CARs contain additional costimulatory domains (such as those from CD28 and 4-1BB) apart from the ζ-chain. These later-generation CARs have improved expansion and persistence in vivo compared with first-generation CARs20 and seem to have better clinical activity.3,4 To learn whether KHAA-mediated inhibition can be modulated by the presence of additional costimulatory domains in the CD19-CAR, we compared CD19-CAR constructs that encoded the CD3ζ chain alone (first generation) with constructs that included costimulatory endodomains from CD28 (second generation) or CD28 and 4-1BB (third generation) (Figure 6A). After 14 days of weekly stimulation with irradiated Raji cells, KHAA still significantly decreased CART expansion irrespective of the construct used (first-generation CD19-CAR: 6.2 ± 5.4-fold vs 20.4 ± 9.9-fold, with and without KHAA, respectively, P = .02; second generation: 8.8 ± 6.2-fold vs 25.7 ± 4.2-fold, P = .001; third generation: 4.7 ± 3.9-fold vs 25.9 ± 8.9-fold; P = .001; Figure 6C).
Additional costimulatory domains are not able to mitigate the inhibitory effects of tryptophan metabolites. (A) Schematic representation of recombinant retrovirus vectors encoding CD19-CAR constructs (CD19.ζ, containing the CD3ζ chain alone; CD19.28.ζ, CD3ζ chain and CD28 endodomain; CD19.28.4-1BB.ζ, CD3ζ chain and CD28 and 4-1BB endodomains) (B) CD19-CAR surface expression on T cells transduced with each construct. (C) The number of CD19-CARTs cultured in the presence or absence of KHAA (12.5 μM) was determined at the end of each 7-day expansion cycle. Data represent the mean ± SD of 5 T-cell lines generated from 5 donors (*P < .05). (D) GFP-transduced wild-type Raji cells were cocultured with each CD19-CART type at an E:T ratio of 1:2 in the presence or absence of KHAA (12.5 μM). The total number of tumor cells and CARTs in each culture condition was determined by flow cytometry. Dot plot shows representative data on day 14. Five experiments using CARTs generated from 5 different donors were analyzed and represent mean ± SD in the graph (*P < .05). In the absence and presence of KHAA, the number of residual tumor was 0.28 ± 0.28 × 106 and 1.49 ± 0.84 × 106, respectively, for the first-generation CAR; 0.16 ± 0.13 × 106 and 0.83 ± 0.3 × 106, respectively, for the second-generation CAR; and 0.99 ± 0.33 × 106 and 1.79 ± 0.39 × 106, respectively, for the third-generation CAR.
Additional costimulatory domains are not able to mitigate the inhibitory effects of tryptophan metabolites. (A) Schematic representation of recombinant retrovirus vectors encoding CD19-CAR constructs (CD19.ζ, containing the CD3ζ chain alone; CD19.28.ζ, CD3ζ chain and CD28 endodomain; CD19.28.4-1BB.ζ, CD3ζ chain and CD28 and 4-1BB endodomains) (B) CD19-CAR surface expression on T cells transduced with each construct. (C) The number of CD19-CARTs cultured in the presence or absence of KHAA (12.5 μM) was determined at the end of each 7-day expansion cycle. Data represent the mean ± SD of 5 T-cell lines generated from 5 donors (*P < .05). (D) GFP-transduced wild-type Raji cells were cocultured with each CD19-CART type at an E:T ratio of 1:2 in the presence or absence of KHAA (12.5 μM). The total number of tumor cells and CARTs in each culture condition was determined by flow cytometry. Dot plot shows representative data on day 14. Five experiments using CARTs generated from 5 different donors were analyzed and represent mean ± SD in the graph (*P < .05). In the absence and presence of KHAA, the number of residual tumor was 0.28 ± 0.28 × 106 and 1.49 ± 0.84 × 106, respectively, for the first-generation CAR; 0.16 ± 0.13 × 106 and 0.83 ± 0.3 × 106, respectively, for the second-generation CAR; and 0.99 ± 0.33 × 106 and 1.79 ± 0.39 × 106, respectively, for the third-generation CAR.
We also found that the ability of CARTs to eliminate Raji cells in cocultures was equally inhibited by KHAA regardless of the costimulatory domains. Although levels of CAR expression are different for each construct (Figure 6B), 14 days after coculture of CARTs with wild-type Raji cells (1:2 E:T), the number of surviving tumor cells was consistently greater in the presence of KHAA, irrespective of the CAR construct used. The mean difference in tumor cell numbers in the presence and absence of KHAA was 1.21 ± 0.84 × 106 (95% confidence interval [CI], 0.16-2.25; P = .033), 0.68 ± 0.29 × 106 (95% CI, 0.31-1.04; P = .007), and 0.81 ± 0.29 × 106 (95% CI, 0.45-1.16; P = .003) for first-, second-, and third-generation CARs, respectively.
Fludarabine and cyclophosphamide downregulate IDO expression by B-cell malignancies
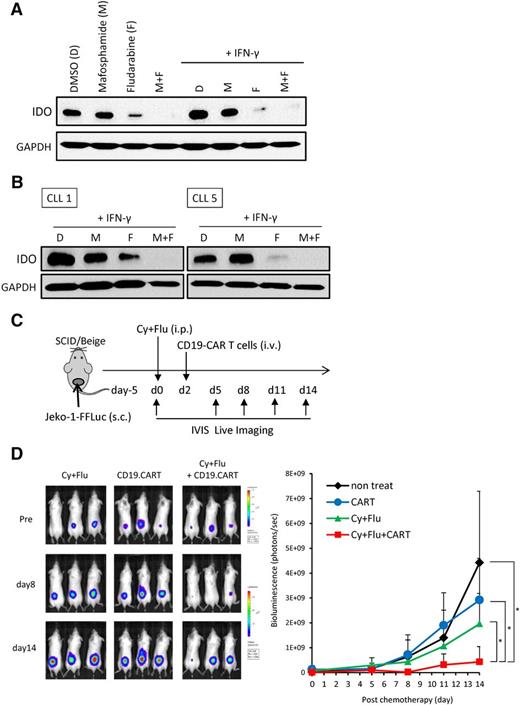
Fludarabine and cyclophosphamide (FluCy) are often used before CD19-CART infusion in clinical trials, because they may favorably affect the activity of CARTs.3,4,24 Fludarabine can inhibit expression of IDO in solid tumor cell lines.25 We therefore measured IDO expression in the B-cell lymphoma cell lines and primary malignant B cells after exposure to FluCy. IDO expression by Jeko-1 was reduced by fludarabine, and even further by both drugs, but not by mafosfamide alone (Figure 7A). IFN-γ was unable to upregulate IDO in Jeko-1 after treatment with fludarabine or both drugs. Strikingly, we observed the same downregulation of IFN-γ–induced IDO activity in primary CLL cells (Figure 7B). Exposure of Jeko-1 and primary CLL cells to FluCy did not reduce their viability before study of IDO expression (supplemental Figure 4). Finally, we evaluated the effects of FluCy before CD19-CART infusion in the Jeko-1 tumor model (Figure 7C). We found that FluCy significantly improved the efficacy of CARTs against IDO-positive tumors (chemotherapy: 1.96 ± 1.23 × 109 BU and CART: 2.78 ± 1.67 × 109 BU vs both: 0.43 ± 0.61 × 109 BU on day 14; P = .01 and 0.003, respectively; Figure 7D).
Fludarabine and cyclophosphamide downregulate IDO expression. (A) Jeko-1 cells were treated with mafosfamide (2 μg/mL), fludarabine (20 μM), or both for 24 hours. Cells were then washed and cultured with or without IFN-γ. Protein extracts were prepared for IDO immunoblot analysis. Results are representative of 3 independent experiments. (B) PBMCs from 2 CLL patients were treated with mafosfamide (2 μg/mL), fludarabine (20 μM), or both for 24 hours. Cells were then washed and incubated with IFN-γ. Twenty-four hours later, proteins were extracted, and IDO expression was revealed by immunoblot. (C) Schematic representation of the experiments in SCID/Beige mice comparing the antitumor effect of chemotherapy, CD19-CARTs, or their combination on Jeko-1 tumors. Mice were subcutaneously injected with luciferase-transduced Jeko-1 cells (3 × 106). Five days later, mice were treated with 0.75 mg fludarabine (Flu) and 0.75 mg cyclophosphamide (Cy) intraperitoneally. Two days after chemotherapy, CD19-CARTs (10 × 106) were infused intravenously. (D) Time course of Jeko-1 tumor bioluminescence. Data represent mean ± SD of 7 mice per group from 2 independent experiments (*P < .05).
Fludarabine and cyclophosphamide downregulate IDO expression. (A) Jeko-1 cells were treated with mafosfamide (2 μg/mL), fludarabine (20 μM), or both for 24 hours. Cells were then washed and cultured with or without IFN-γ. Protein extracts were prepared for IDO immunoblot analysis. Results are representative of 3 independent experiments. (B) PBMCs from 2 CLL patients were treated with mafosfamide (2 μg/mL), fludarabine (20 μM), or both for 24 hours. Cells were then washed and incubated with IFN-γ. Twenty-four hours later, proteins were extracted, and IDO expression was revealed by immunoblot. (C) Schematic representation of the experiments in SCID/Beige mice comparing the antitumor effect of chemotherapy, CD19-CARTs, or their combination on Jeko-1 tumors. Mice were subcutaneously injected with luciferase-transduced Jeko-1 cells (3 × 106). Five days later, mice were treated with 0.75 mg fludarabine (Flu) and 0.75 mg cyclophosphamide (Cy) intraperitoneally. Two days after chemotherapy, CD19-CARTs (10 × 106) were infused intravenously. (D) Time course of Jeko-1 tumor bioluminescence. Data represent mean ± SD of 7 mice per group from 2 independent experiments (*P < .05).
Discussion
In this study, we demonstrated that IDO expression by B-cell tumors inhibits CD19-CART activity and may explain the limited response of many CD19-positive B-cell lymphomas to this therapy, especially when used without lymphodepleting chemotherapy.1,5,20,26 Raji and Daudi are commonly used as CD19-positive target cell lines to evaluate the potency of CD19-CARTs in preclinical experiments,27-29 but because these cell lines lack IDO expression, they do not reflect the biology of IDO-positive B-cell malignancies. Therefore, we established a Raji clone expressing IDO to address the potential effects of tumor IDO on CD19-CARTs. We found that CD19-CARTs showed equivalent immediate killing of control and IDO-expressing Raji cells in vitro and could traffic both to sites of IDO-negative and positive tumors in vivo. Later, CART signals became progressively more intense at Raji-IDO sites than at Raji-control sites, suggesting that CARTs that expand at IDO-negative tumor sites can recirculate and accumulate in areas of IDO-positive tumor but are insufficient to kill these tumors. Hence, inhibition of antitumor activity was not immediately evident, relying instead on the gradual development of an inhibitory tumor microenviroment. We also found that inhibition of IDO production or activity protected CD19-CARTs against the inhibitory effects of IDO-expressing tumor cells.
Because IDO is an intracellular enzyme that mediates the catabolism of tryptophan along the kynurenine pathway, it is hard to mimic its in vivo effects during in vitro experiments. Both tryptophan depletion and accumulation of tryptophan metabolites, such as kynurenine, 3-hydroxykynurenine, and 3-HAA, exert immune regulatory effects leading to T-cell suppression.17,30 In vivo, local tryptophan depletion may rapidly be replenished by diffusion from surrounding tissues and blood circulation. Therefore, we decided to directly study the effect of KHAA on CD19-CARTs. In our experiments, we used concentrations of kynurenine ∼1 log higher than those measured in the serum of DLBCL patients with IDO-positive tumors, because local concentrations within tumors are likely to be far higher than those in serum.19 On the other hand, constitutive expression of IDO by Raji cells did not affect their proliferation compared with controls; thus, physiologic concentrations of KHAA produced by IDO activity are likely <25 µM. Therefore, we chose 12.5 µM as the concentration of KHAA for experiments intended to assess the inhibitory effects of IDO products.
We found that KHAA inhibited CD19-CART proliferation in response to the T-cell growth cytokines IL-2, IL-7, and IL-15. Consequently, these cytokines will likely fail to improve proliferation of adoptively transferred T cells at the site of IDO-positive tumors. Moreover, KHAA also inhibited CD19-CART proliferation in response to CD19 recognition, even when the CD19-CARs carried both CD28 and 4-1BB costimulatory signaling domains. This is particularly impressive because the inclusion of those costimulatory domains in CARs enhances the effector function of CD19-CARTs in preclinical models27,31 and improves expansion and persistence of CD19-CARTs in lymphoma patients.20 Our data also demonstrate that KHAA inhibits the expansion, cytokine secretion, and cytotoxic activity of CARTs and suggest that production of tryptophan metabolites by IDO may at least partially underlie the resistance of IDO-positive tumors to the antitumor activity of CARTs.
To confirm the potential clinical relevance of IDO expression, we examined expression of the enzyme in CLL patients, in whom malignant B cells comprise the majority of PBMCs. Our data agree with previous published results showing that expression of IDO in PBMCs from CLL patients was not increased compared with normal donors unless the cells were first exposed to IFN-γ.32 However, we confirmed that IDO expression was upregulated by exposure to IFN-γ for 24 hours in most patients. The IDO pathway may therefore play an important role in the biology of B-cell malignancy patients treated with immunotherapies, including CD19-CART, because clinical trials have shown that serum IFN-γ is dramatically elevated after CD19-CART infusion.4,6
Fludarabine has recently been shown to inhibit IDO expression by a breast cancer cell line (MDA231) even after exposure to IFN-γ through a proteasome-dependent pathway.25 It has also been observed that serum l-kynurenine was reduced and l-tryptophan was increased after chemotherapy in adult T-cell leukemia/lymphoma patients.33 We therefore investigated whether fludarabine and another chemotherapy drug, mafosfamide (a cyclophosphamide derivative), would have a similar inhibitory effect on IDO expression by B-cell malignancies. Mafosfamide was used instead of cyclophosphamide because the latter is inactive without liver metabolism.34 Our data demonstrate that a single treatment with fludarabine downregulates IDO expression by Jeko-1, and thus fludarabine could alter the concentration of kynurenine and tryptophan in the tumor microenvironment before CART infusion. Furthermore, we found that the combination of fludarabine and mafosphamide downregulates IDO expression better than fludarabine alone, even after exposure to IFN-γ, mirroring the benefits of these drugs when used alone or in combination prior to T-cell infusion.24
Although the major benefits of lymphodepleting chemotherapy before CART infusion are thought to result from higher availability of free serum IL-7 and IL-15 and from reduction of regulatory T cells, our data suggest that inhibition of IDO might also play an important role in these effects, because IL-7 and IL-15 were unable to promote CD19-CART proliferation in the presence of KHAA. The presence of IDO has also been shown to correlate with increased tumor-infiltrating regulatory T cells,35,36 likely because kynurenine binds to the AHR in T cells.37 This in turn leads to increased generation of CD25+ FoxP3+ T cells,38 which compromise the antitumor efficacy of CD19-CARTs in preclinical models.29 Therefore, preconditioning chemotherapy can modify the inhibitory microenvironment produced by tumor cells by downregulating IDO expression in B-cell tumors. About 30% of DLBCL express IDO constitutively18 (supplemental Figure 5), which may contribute to the poor response of this tumor to CD19-CART therapy, especially without preconditioning chemotherapy.20,24 IDO thus represents a therapeutic target for B-cell lymphoma patients who are resistant to chemotherapy and immunotherapy. A few clinical trials are evaluating the effects of IDO inhibitors (indoximod, INCB024360, and NLG919) that competitively block the degradation of tryptophan to kynurenine by the enzyme.39,40 Our study focused on the interaction between IDO in tumor cells and adoptively transferred CD19-CARTs. Nevertheless, IDO is produced not only by tumor cells but also by host immune cells, such as dendritic cells, tumor-associated macrophages, and myeloid-derived suppressor cells, which inhibit T-cell response and promote recruitment of regulatory T cells.15,18,41,42 Thus, systemic IDO inhibitors may have diverse benefits in combination with adoptive T-cell therapy in clinical practice. CTLA-4 blockade alone may be less effective against IDO-positive tumors than their negative counterparts.43 Hence, IDO inhibitors may also have implications for the use of checkpoint inhibitors in CART therapy.
Many chemotherapy-refractory patients with B-cell malignancies, who are potential candidates for CD19-CART therapy, are likely to have IDO-positive tumors.18 Because we have shown an inhibitory role of IDO in CD19-CART therapy, our data indicate that direct IDO inhibitors may augment the clinical activity of CART cells against otherwise resistant B-cell malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mariola Klis for technical assistance and Jessie Wu for statistical advice.
This work was supported in part by grants from the Leukemia and Lymphoma Society Specialized Center of Research (grant 7018), the National Institutes of Health National Cancer Institute (grant 3P50CA126752), and the Conquer Cancer Foundation of the American Society of Clinical Oncology (Career Development Award).
Authorship
Contribution: S.N., N.N., L.H., and S.Y. performed the experiments; S.N. and C.A.R. analyzed results and created the figures; S.N., B.S., G.D., M.K.B., C.M.R., and C.A.R. designed the research; S.N., H.E.H., M.K.B., C.M.R., and C.A.R. contributed to writing; and all authors approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Leslie Huye died on January 1, 2015.
Correspondence: Carlos A. Ramos, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates St, Suite 1760, Houston, TX 77030; e-mail: caramos@bcm.edu.