Key Points
Increasing FVIIIa by stabilizing the A2 domain association enhances its function in vitro and in vivo in hemophilia.
Stabilized FVIIIa improved efficacy in several vascular injury models, including laser injury, in which it was particularly effective.
Abstract
An important negative regulator of factor VIIIa (FVIIIa) cofactor activity is A2 subunit dissociation. FVIII molecules with stabilized activity have been generated by elimination of charged residues at the A1-A2 and A2-A3 interfaces. These molecules exhibited reduced decay rates as part of the enzymatic factor Xa generation complex and retained their activities under thermal and chemical denaturing conditions. We describe here the potency and efficacy of 1 such stability variant, D519V/E665V, derived from B domain–deleted FVIII (BDD-FVIII). The major effect of A2 stabilization was on cofactor activity. D519V/E665V potency was increased twofold by the 2-stage chromogenic assay relative to BDD-FVIII. D519V/E665V demonstrated enhanced thrombin generation responses (fivefold by peak thrombin) relative to BDD-FVIII. In vivo consequences of enhanced cofactor activity of D519V/E665V included >fourfold increased maximal platelet-fibrin deposition after laser injury and twofold increased protection from bleeding in acute and prolonged vascular injury model in hemophilia A mice. These results demonstrate that noncovalent stabilization of the FVIII A2 subunit can prolong its cofactor activity, leading to differential enhancement in clot formation over protection from blood loss in hemophilia. The FVIII molecule described here is the first molecule with clear efficacy enhancement resulting from noncovalent stabilization of the A2 domain.
Introduction
Hemophilia A (HemA) is an X-linked recessive disorder caused by a deficiency of factor VIII (FVIII), a procofactor whose conversion to the cofactor factor VIIIa (FVIIIa) is required for the efficient amplification of factor Xa (FXa) generation.1 FXa is required for the generation of thrombin and fibrin, which are needed to limit blood loss during vascular injury.2
A focus of drug development for HemA is to increase the effectiveness of FVIII treatment. This has been achieved by prolongation of half-life3,4 to decrease the frequency of infusion. An alternative approach is to increase the stability of FVIIIa. Cofactor subunit dissociation, particularly dissociation of the A2 domain, is thought to limit FVIIIa activity.1 Stabilization can be achieved by a variety of means, including covalent A2-A3 subunit disulfide linkage alone5 or removal of cleavage sites between A2 and A3 in combination with mutations conferring resistance to activated protein C.6 Additional approaches include point mutations in the B domain,7 in the activated protein C cleavage site(s),8-10 and in the interfaces between the A2 and A1 or A3 domains.11 The predicted outcome of FVIIIa stabilization would be sustained cofactor activity, increased FXa and thrombin generation, and, ultimately, enhanced clot formation and protection from bleeding.
Mutations in the interfaces between A2 and A1 or A3 have been shown to reduce FVIIIa decay,11,12 presumably by reducing the A2 dissociation and retaining its association with the A1/A3C1C2 dimer. This report describes the further characterization of the variant D519V/E665V, which yields a potent noncovalent A2 domain–stabilized FVIII variant, with regard to in vitro potency and in vivo efficacy. The results of our studies indicate that noncovalent stabilization of the A2 domain in the D519V/E665V variant yields an FVIII molecule with enhanced cofactor activity, clot formation, and protection from bleeding in different vascular injury models in hemophilia mice. Such a molecule may function as the basis of an improved therapeutic for the treatment of hemophilia.
Materials and methods
Generation of FVIII constructs
The FVIII variant D519V/E665V was transferred from the ReNeo expression vector12 to the pSS207 expression vector13 following polymerase chain reaction amplification. Briefly, forward (5′-cattaggctagccaccatggaaatagagc-3′) and reverse (5-acactggtttaaactcagtagaggtcctg-3′) primers containing an NheI or PmeI site, respectively, were used to amplify and clone the FVIII variants into the NheI/PmeI-digested pSS207 vector. Transfection of the pSS207 expression vectors containing B domain–deleted (BDD)-FVIII genes to produce the corresponding FVIII proteins from stably transfected HKB11 cell lines has been described previously.13
Purification of FVIII molecules
Chemicals and reagents used for FVIII purification were purchased from Sigma-Aldrich (St. Louis, MO). The A3-specific monoclonal antibody used for the immunoaffinity column, C7F7, was provided by Bayer HealthCare Pharmaceuticals (Berkeley, CA). FVIII proteins from conditioned media of stably transfected baby hamster kidney cells were purified by chromatography from sequential C7F7, Sephadex G25, and Q HP Sepharose columns.4
Physical measurement of A2 stability of FVIIIa variants
Stability of A2 subunit association with the A1/A3C1C2 dimer of FVIIIa was determined by monitoring the dissociation of FVIIIa subunits using surface plasmon resonance (Biacore 3000; GE HealthCare, Buckinghamshire, United Kingdom).5 The A2-specific monoclonal antibody R8B12 (GMA-012; Green Mountain Antibodies, Burlington, VT) or a non–FVIII-specific isotype control antibody (50 µg/mL) was coupled to a CM5 chip using amine coupling following the manufacturer’s protocol and reagents (Biacore; GE Healthcare). The antibodies were coupled to the chip surface at approximately 1400 resonance units. BDD-FVIII and variants (0.2-0.5 µg/mL) were injected for 30 to 90 seconds with a flow rate of 30 µL/min, followed by a 300-second dissociation phase. Thrombin (Haematologic Technologies, Inc., Essex Junction, VT) at 6 U/mL was then injected for 60 seconds followed by a 1500-second dissociation phase. A total of 10 mM glycine pH2.5 was used to regenerate the chip surface. A single-phase exponential decay equation was used to fit the dissociation curve initiated by thrombin cleavage.
In vitro cofactor activity of FVIII variants
A chromogenic substrate assay was performed to assess the cofactor activity of FVIII proteins. The chromogenic substrate assay was performed using the Coatest kit as specified by its manufacturer (Chromogenix, Milan, Italy). The calibrator used to define FVIII molecule activity was the chromogenic 02/122 reference (National Institute for Biological Standards and Controls, Hertfordshire, United Kingdom).
Enzyme kinetic studies of FVIII variants
All enzymes and cofactors were generated using standard methods in-house or purchased from Haematologic Technologies. Chemicals were purchased from Sigma-Aldrich. Lipid formulation containing phosphatidylcholine:phosphatidylethanolamine:phosphatidylserine in a 40:40:20 (weight percentage) ratio was purchased from Avanti Polar Lipids (Alabaster, AL).
Kinetic assays were performed in a buffer containing 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid pH 7.4, 150 mM NaCl, 5 mM CaCl2, 0.01% Tween-80, 0.01% bovine serum albumin, and 10 µM liposomes. FX activation complex (FXase) kinetics experiments were designed based on published methods.14 Briefly, FVIII (0.16-10 nM) or activated factor IX (FIXa) (0.08-10 nM) was combined and incubated at room temperature for 1 minute. Thrombin (20 nM) was added for 1 minute to activate FVIII. FX (5-300 nM) was added; aliquots were removed from the reaction at 20-second intervals, and the reactions stopped in 25 mM ethylenediaminetetraacetic acid. The chromogenic activity of the generated FXa was assayed using a mixture containing the chromogenic substrate S2765 and the thrombin inhibitor I2581 (Diapharma, West Chester, OH). An FXa standard curve was used to determine the FXa generated and the data were fit to a standard Michaelis-Menten model using nonlinear regression.
In vitro procoagulant activity of FVIII variants
Procoagulant activity of FVIII was determined by activated partial thromboplastin time (aPTT) and thrombin generation assay (TGA). The 1-stage aPTT assay for FVIII was performed on the ACL Advance (Instrumentation Laboratory, Bedford, MA) using an APTT-SP activator (Instrumentation Laboratory). The FVIII-deficient plasma pool (HRF Inc., Raleigh, NC), needed for the FVIII assay, contained <0.4% FVIII. The aPTT assay was calibrated against BDD-FVIII, whose activity referenced back to the European Pharmacopoeia Biological Reference Preparation Batch 3/Mega-2 (US Food and Drug Administration) for FVIII.15 The TGA was performed with the PPP-Low reagent containing 1 pM tissue factor–4 µM phospholipid vesicles (Diagnostica Stago, Parsippany, NJ). The same FVIII-deficient plasma pool (HRF Inc.) used for the aPTT was also used in the TGA.
Pharmacokinetic assessment
Pharmacokinetic (PK) assessment was performed as described by Mei et al.4 FVIII knockout HemA mice (The Jackson Laboratory, Bar Harbor, ME) were dosed with 200 U/kg D519V/E665V or the parental BDD-FVIII intravenously through the tail veins (3 mice per time point; a total of 30 mice for each test group). Blood samples were collected in 3.8% sodium citrate at 0.083, 0.5, 1, 2, 4, 8, 16, 24, 32, and 48 hours after administration. Plasma FVIII was assessed with the Coatest chromogenic activity assay (Chromogenix, Milan, Italy). PK parameter estimates were calculated using a noncompartmental analysis model (WinNonLin version 5.2; Pharsight, Centara, St. Louis, MO).
Hemostatic activity of A2 domain–stabilized FVIII in a laser-induced cremaster muscle arteriole injury model
Animals
Eight- to 10-week-old male FVIII knockout HemA mice (exon 16 knockout [B6.129S4-F8tm1Kaz/J]) were used in these studies.16 Mice were housed at a pathogen-free facility using static caging in a room with 12-hour light/dark cycle and access to water and food ad libitum. All study protocols were approved by the Bayer HealthCare Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
The effect of D519V/E665V on hemostasis was assessed by intravital microscopy following laser injury. Six- to 8-week-old HemA mice were treated with 10 µg/kg BDD-FVIII or D519V/E665V 24 hours before vascular injury. HemA mice were anesthetized with a ketamine-medetomidine cocktail by intraperitoneal administration and placed on a heating pad to maintain their body temperature at 37°C. The jugular vein was cannulated for the delivery of Alexa 647–labeled anti-mouse CD41 Fab (BD BioScience, San Jose, CA) and Alexa 488-labeled anti-fibrin II β chain (Accurate Chemical, Westbury, NY). The cremaster muscle was exposed by exteriorizing a testicle through an incision on the scrotum. The cremaster muscle was incised and mounted over a glass slide with the testicle pushed to the side for intravital microscopy. The cremaster muscle preparation was placed under the Nikon Eclipse FN-1 fluorescent microscope with a ×40 water immersion lens. Bicarbonate-buffered saline (37°C) was superfused on the exposed muscle during the experiment. Four to 9 mice were used in each experimental group.
After the cremaster microvasculature of the mouse was exposed, injury was induced by applying a pulsed nitrogen dye laser at 440 nm through the microscope objective using the Micropoint laser system (Photonics Instruments, St. Charles, IL). Platelet accumulation and fibrin deposition to the injury site following laser ablation was recorded continuously for 5 minutes using a QuantEM512SC digital camera (Photometrics, Tucson, AZ). The total thrombus fluorescence in each frame of the video was analyzed using ImageJ software. The maximum platelet accumulation and fibrin deposition were compared using the Mann-Whitney U test.
Tail clip and tail vein transection efficacy assessment
The ability of D519V/E665V to offer protection from systemic bleeding was assessed by the murine tail clip and tail vein transection (TVT) models following vascular injury. The murine tail clip model was performed as described by Mei et al.4 Briefly, HemA mice were anesthetized with isoflurane and the tails were prewarmed to 37°C in 0.9% saline for 10 minutes. Animals were then injected with 100 µL/mouse of the appropriate concentration of BDD-FVIII or D519V/E665V variant (n = 9-20 mice/group) via the right jugular vein. Five minutes later, the tail was cut at 4 mm from the tip and immediately placed into a new tube containing 10 mL of saline (prewarmed to 37°C). The volume of blood loss over a period of 40 minutes was quantified gravimetrically.
The TVT studies were performed as described previously.4 HemA mice were dosed with BDD-FVIII or D519V/E665V by tail vein injection at 24 hours before the transection of 1 lateral tail vein. HemA mouse survival was monitored hourly until ∼8 hours postinjury (n = 12 mice/dose group). A final 24-hour survival assessment postinjury was determined for each FVIII molecule at various doses.
Results
Reduced A2 subunit dissociation in D519V/E665V
Surface plasmon resonance was used to show that the D519V/E665V variant possessed increased physical association of the A2 subunit in FVIIIa. For this experiment, BDD-FVIII or the D519V/E665V variant was captured onto a Biacore chip by an A2 domain–specific monoclonal antibody and was subsequently activated by flowing thrombin over the chip. Subunit dissociation of the A1/A3C1C2 dimer from the immobilized A2 subunit in the thrombin-activated BDD-FVIII was evident as a decrease in signal as buffer flowed over the chip for 30 minutes (Figure 1). Under these conditions, the BDD-FVIII showed a dissociation rate of 0.25 minutes−1 (Figure 1), as determined by a 1-phase exponential decay fit to dissociation curves. In contrast, the D519V/E665V variant demonstrated a decreased dissociation rate under the same conditions (0.07 minutes−1). Inasmuch as both FVIII forms are cleaved and activated at equivalent rates by thrombin, these results are consistent with earlier results showing a reduced rate of FVIIIa activity decay for the variant relative to wild-type FVIIIa.11
A representative sensogram (Biacore analysis) showing A2 domain dissociation from FVIIIa after activation of FVIII by thrombin. The top solid line represents D519V/E665V; the dashed line represents BDD-FVIII. The lower lines (dashed and solid) are the respective nonspecific antibody control channels. All data have been subtracted from a blank channel and are representative of 2 independent experiments.
A representative sensogram (Biacore analysis) showing A2 domain dissociation from FVIIIa after activation of FVIII by thrombin. The top solid line represents D519V/E665V; the dashed line represents BDD-FVIII. The lower lines (dashed and solid) are the respective nonspecific antibody control channels. All data have been subtracted from a blank channel and are representative of 2 independent experiments.
D519V/E665V-specific activity reflects enhanced FXase activity
The functional consequences of reduced A2 subunit dissociation were initially assessed in vitro by measurement of aPTT and chromogenic assay specific activities. By aPTT, D519V/E665V had comparable specific activity relative to BDD-FVIII. In contrast, its chromogenic assay specific activity was twofold greater than that of BDD-FVIII (Table 1). Greater sensitivity of the chromogenic assay compared with the aPTT assay is consistent with enhanced FVIIIa stability as the mode of action for the D519V/E665V variant because the 2-stage chromogenic assay is more sensitive to changes in FVIIIa stability.
Effect of A2 domain stabilization on specific activities and kinetic parameters
| Protein . | aPTT, U/µg . | Chromogenic assay, U/µg . | K1/2 FVIIIa, nM* . | K1/2 FIXa, nM* . | Km, nM* . | Kcat, s-1* . |
|---|---|---|---|---|---|---|
| BDD-FVIII | 7.00 ± 2.35 (n = 3) | 6.87 ± 1.58 (n = 4) | 2.08 ± 0.40 | 1.62 ± 0.62 | 9.95 ± 0.65 | 1.48 ± 0.02 |
| D519V/E665V | 6.55 ± 1.34 (n = 2) | 12.58 ± 2.55 (n = 6) | 0.74 ± 0.10 | 0.43 ± 0.09 | 11.77 ± 0.82 | 1.87 ± 0.03 |
| Protein . | aPTT, U/µg . | Chromogenic assay, U/µg . | K1/2 FVIIIa, nM* . | K1/2 FIXa, nM* . | Km, nM* . | Kcat, s-1* . |
|---|---|---|---|---|---|---|
| BDD-FVIII | 7.00 ± 2.35 (n = 3) | 6.87 ± 1.58 (n = 4) | 2.08 ± 0.40 | 1.62 ± 0.62 | 9.95 ± 0.65 | 1.48 ± 0.02 |
| D519V/E665V | 6.55 ± 1.34 (n = 2) | 12.58 ± 2.55 (n = 6) | 0.74 ± 0.10 | 0.43 ± 0.09 | 11.77 ± 0.82 | 1.87 ± 0.03 |
Kcat, constant for catalytic turnover; Km, Michaelis constant.
From kinetic assays. K1/2, the apparent dissociation constant, is calculated as described in Gale et al.5
FXase activity of thrombin-activated FVIII variants
Studies of FXa generation were performed to assess whether improved interactions with FIXa and FX can account for some of the enhanced chromogenic assay specific activity of D519V/E665V. Both D519V/E665V and BDD-FVIII followed Michaelis-Menton kinetics, with FXa generation increasing hyperbolically until saturation was achieved with increasing FVIIIa or FIXa (see supplemental Figure 1A-B on the Blood Web site). However, the rate of hyperbolic FXa generation with D519V/E665V was enhanced relative to that with BDD-FVIII regardless of whether FVIIIa or FIXa was varied. Furthermore, because the rate of FXa generation was similar with both D519V/E665V and BDD-FVIII (supplemental Figure 1C), the enhanced FXa generation was likely from improved FIXa-FVIIIa interactions. The calculated apparent affinity of D519V/E665V (vs BDD-FVIII) for FIXa reflects enhanced FXase activity of D519V/E665V over BDD-FVIII (Table 1; supplemental Figure 1). The relationship between A2 stabilization and enhancement in affinity of activated D519V/E665V for FIXa is unclear from the current data.
Increased thrombin generation response of D519V/E665V relative to BDD-FVIII
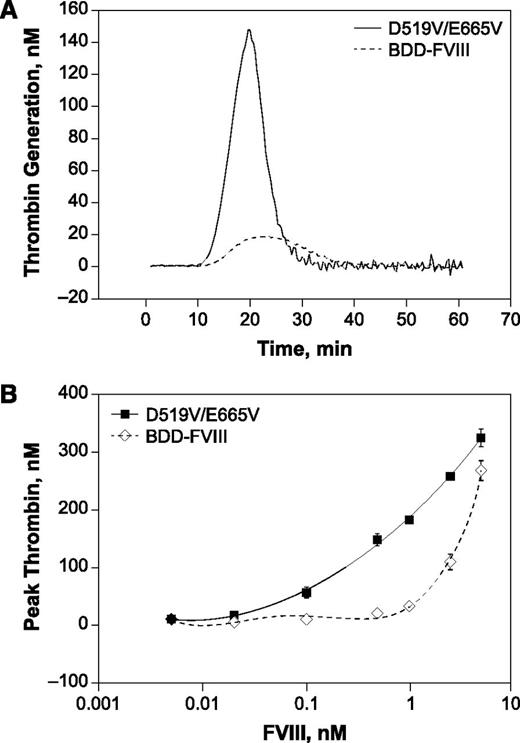
The effect of A2 subunit stabilization on potency and efficacy was further assessed by evaluating the ability of D519V/E665V to elicit thrombin generation in vitro. As shown in Figure 2A, both D519V/E665V and BDD-FVIII showed comparable lag times before the onset of the thrombin response, indicating similar initiation of thrombin generation. In contrast to the onset of the thrombin response, the peak and total thrombin potential with D519V/E665V was markedly enhanced compared with BDD-FVIII, consistent with the higher chromogenic-specific activity of D519V/E665V relative to BDD-FVIII (Figure 2B). The degree of enhancement in thrombin generation, however, was most marked at lower D519V/E665V levels, suggesting that positive effects of A2 stabilization may be more pronounced at low FVIII levels. This characteristic is also consistent with an FVIII variant with higher FIXa-FVIIIa affinity.
TGA responses of FVIII variants. Representative thrombin profiles at 0.5 nM (A) and the dose-dependent peak thrombin responses (B) for BDD-FVIII and D519V/E665V. The estimated EC50 for BDD and D519V/E665V are 3.3 and 0.7 nM, respectively, indicating a ∼fivefold difference in potency. The specific activities of BDD-FVIII and D519V/E665V tested were 6.02 and 11.25 U/µg, respectively.
TGA responses of FVIII variants. Representative thrombin profiles at 0.5 nM (A) and the dose-dependent peak thrombin responses (B) for BDD-FVIII and D519V/E665V. The estimated EC50 for BDD and D519V/E665V are 3.3 and 0.7 nM, respectively, indicating a ∼fivefold difference in potency. The specific activities of BDD-FVIII and D519V/E665V tested were 6.02 and 11.25 U/µg, respectively.
Thrombin profile comparison indicated that, in contrast to BDD-FVIII, D519V/E665V induced a rapid burst of thrombin generation. A consequence of this rapid burst in thrombin generation would be a lower D519V/E665V requirement to induce a similar level of peak thrombin as BDD-FVIII. Not surprisingly, the median effective concentration for peak thrombin was fivefold lower for D519V/E665V than for BDD-FVIII; that is, D519V/E665V was fivefold more potent than BDD-FVIII. The enhancement was even greater (∼10-fold) by total thrombin potential (data not shown). The degree of enhancement observed was greater than previously reported11,17 and is likely to reflect the different assay conditions used in the current study. Regardless of the degree of enhancement, the enhancement in quantitative thrombin responses with D519V/E665V is consistent with its enhanced chromogenic-specific activity (supplemental Data; supplemental Table 1) as a result of increased FVIIIa stability and improved interactions with FIXa.
However, despite the elevated thrombin burst with D519V/E665V, some characteristics of the thrombin response suggest that D519V/E665V may not induce excessive thrombin generation. The thrombin profile with D519V/E665V showed elevated peak thrombin that is inactivated rapidly (Figure 2A). Furthermore, the maximal responses achieved with both D519V/E665V and BDD-FVIII were comparable (Figure 2B). Taken together, these results suggest that A2 stabilization did not abrogate or suppress mechanisms regulating thrombin levels during coagulation.
Comparable PK profiles for D519V/E665V and BDD-FVIII
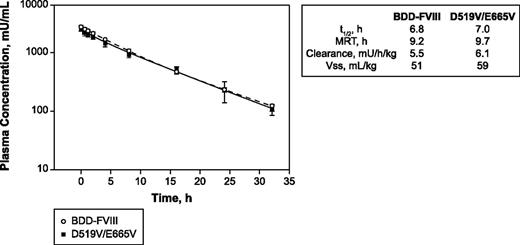
Because efficacy assessment would involve both acute and long-term protection in models of vascular injury in hemophilic mice, the PK characteristics of D519V/E665V relative to BDD-FVIII were determined in HemA mice (Figure 3). Administration of 200 IU/kg of either D519V/E665V or BDD-FVIII proteins to HemA mice resulted in comparable plasma half-life and clearance values (6.8 vs 7.0 hours and 5.5 vs 6.1 mU/h per kilogram for BDD-FVIII and D519V/E665V, respectively), indicating that the mutations introduced had minimal impact on PK parameters (half-life, mean residence time, clearance, and volume of distribution at steady state) for D519V/E665V.
Comparable profiles. PK profiles for D519V/E665V and BDD-FVIII in HemA mice dosed with 200 U/kg BDD-FVIII or D519V/E665V. MRT, mean residence time; t1/2, terminal half-life; Vss, volume of distribution at steady state.
Comparable profiles. PK profiles for D519V/E665V and BDD-FVIII in HemA mice dosed with 200 U/kg BDD-FVIII or D519V/E665V. MRT, mean residence time; t1/2, terminal half-life; Vss, volume of distribution at steady state.
D519V/E665V mediates its procoagulant effects through enhanced platelet accumulation and fibrin formation after injury
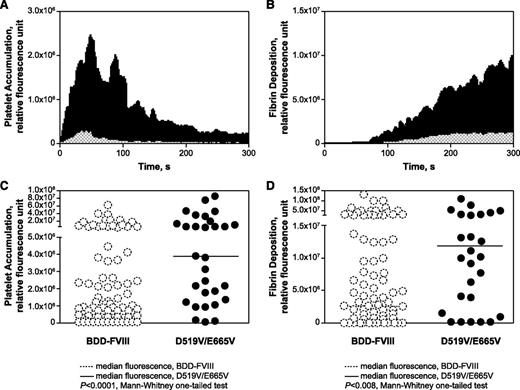
To further assess the efficacy of the variant, D519V/E665V or BDD-FVIII was infused into HemA mice; their hemostatic effects were assessed by intravital microscopy of vessels subjected to laser injury. Despite a comparable PK profile, HemA mice infused with 10 µg/kg D519V/E665V 24 hours before injury elicited enhanced hemostatic responses relative to BDD-FVIII controls when subjected to laser-induced injury (Figure 4A-B). The temporal pattern of platelet and fibrin deposition remained similar for D519V/E665V and BDD-FVIII at the injury site (Figure 4A-B). However, the higher thrombin-generating capacity of D519V/E665V led to markedly increased platelet accumulation and fibrin deposition that were ∼8- and fourfold greater, respectively, than BDD-FVIII (Figure 4C-D). Comparison of the median fluorescence values for platelet and fibrin accumulation with D519V/E665V vs BDD-FVIII showed statistically significant differences (P < .0001 and P < .008, respectively, by the 1-tailed Mann-Whitney U test). The degree of enhancement observed with the D519V/E665V over BDD-FVIII in the laser-injury model correlated well with the enhancement observed with the TGA but not the chromogenic assay.
Enhanced thrombin generation with D519V/E665V results in increased platelet and fibrin response to vascular injury. Intravital microscopy of laser-injured vessels was performed in HemA mice and the kinetics of platelet accumulation (A) and fibrin deposition (B) in response to D519V/E665V (filled black histograms) and BDD-FVIII (hatched histograms). The quantitation of the maximum clot size based on platelet-associated (C) and fibrin-associated (D) fluorescence is also shown. Comparison of the median maximum fluorescence (horizontal bars) shows a statistically significant difference between clots formed by animals dosed with BDD-FVIII (open circles) and D519V/E665V (filled circles); P < .0001 and P < .008 for platelet and fibrin maximum fluorescence, respectively. The BDD-FVIII data were derived from analysis of 85 clots from 9 mice; the D519V/E665V data were derived from analyzing 40 clots from 4 mice.
Enhanced thrombin generation with D519V/E665V results in increased platelet and fibrin response to vascular injury. Intravital microscopy of laser-injured vessels was performed in HemA mice and the kinetics of platelet accumulation (A) and fibrin deposition (B) in response to D519V/E665V (filled black histograms) and BDD-FVIII (hatched histograms). The quantitation of the maximum clot size based on platelet-associated (C) and fibrin-associated (D) fluorescence is also shown. Comparison of the median maximum fluorescence (horizontal bars) shows a statistically significant difference between clots formed by animals dosed with BDD-FVIII (open circles) and D519V/E665V (filled circles); P < .0001 and P < .008 for platelet and fibrin maximum fluorescence, respectively. The BDD-FVIII data were derived from analysis of 85 clots from 9 mice; the D519V/E665V data were derived from analyzing 40 clots from 4 mice.
Enhanced in vivo efficacy of D519V/E665V
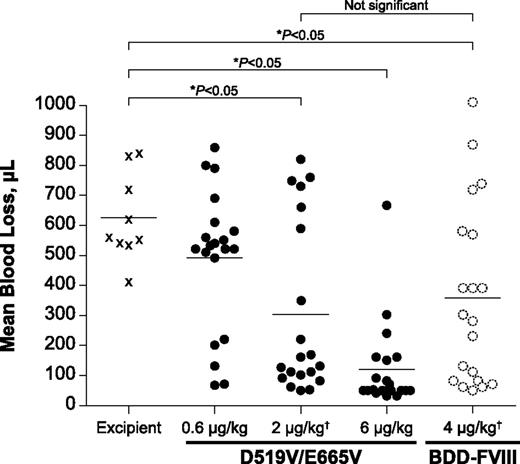
The tail clip model assesses acute severe bleeding. Both BDD-FVIII (data not shown) and the D519V/E665V variant provided dose-dependent reductions in blood loss after tail cut injury. At equal activity dosing at the median effective dose (ED50 = 0.6 U/kg BDD-FVIII), 2 µg/kg D519V/E665V and 4 µg/kg BDD-FVIII induced comparable blood loss in HemA mice dosed with the respective variants (Figure 5). Therefore, the greater specific activity of D519V/E665V vs BDD-FVIII offers comparable protection with reduced FVIII mass usage.
Tail clip injury model showing comparable efficacy as BDD-FVIII with half the mass dose for D519V/E665V. HemA mice were dosed with buffer (excipient); 0.6, 2, or 6 µg/kg D519V/E665V or 4 µg/kg BDD-FVIII. The BDD-FVIII dose represents the ED50 for this molecule in these studies. Statistical significance (*P < .05, 1-way ANOVA with Dunnett’s multiple comparison test) was achieved with 2 µg/kg D519V/E665V vs excipient, whereas 4 µg/kg BDD-FVIII was required to achieve the same level of statistical difference vs excipient. †0.6 U/kg = 2 µg/kg D519V/E665V = 4 µg/kg BDD-FVIII. ANOVA, analysis of variance.
Tail clip injury model showing comparable efficacy as BDD-FVIII with half the mass dose for D519V/E665V. HemA mice were dosed with buffer (excipient); 0.6, 2, or 6 µg/kg D519V/E665V or 4 µg/kg BDD-FVIII. The BDD-FVIII dose represents the ED50 for this molecule in these studies. Statistical significance (*P < .05, 1-way ANOVA with Dunnett’s multiple comparison test) was achieved with 2 µg/kg D519V/E665V vs excipient, whereas 4 µg/kg BDD-FVIII was required to achieve the same level of statistical difference vs excipient. †0.6 U/kg = 2 µg/kg D519V/E665V = 4 µg/kg BDD-FVIII. ANOVA, analysis of variance.
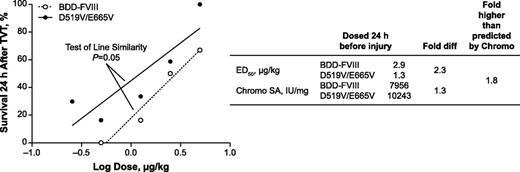
To determine whether D519V/E665V has hemostatic effects on delayed venous bleeds, TVT was performed on HemA mice treated with D519V/E665V or BDD-FVIII 24 hours before injury. Linear regression of the dose-response curves for the 2 variants indicated that the D519V/E665V dose response curve is different from BDD-FVIII (P = .05, analysis of covariance, GraphPad Prism 5). Estimation of the ED50 for survival in HemA mice dosed with the FVIII variants indicated that 2.9 µg/kg BDD-FVIII was required to protect 50% of the animals, whereas only 1.3 µg/kg D519V/E665V was required to confer the same level of protection (Figure 6). Therefore, D519V/E665V was 2.3-fold more efficacious when compared with BDD-FVIII by mass. To account for the difference in specific activity between the 2 FVIII forms, the ED50 values were normalized against their respective specific activities by chromogenic assay. With this normalization, the efficacy of D519V/E665V was found to be 1.8-fold higher than BDD-FVIII when administered on a per-unit basis (Figure 6). This twofold higher potency of D519V/E665V vs BDD-FVIII has been observed in 3 independent TVT studies. Interestingly, the enhancement observed with these vascular injury models correlated well with the enhancement in chromogenic activity.
Enhanced efficacy of D519V/E665V in a TVT injury model. HemA mice were treated with BDD-FVIII or D519V/E665V at the indicated dose 24 hours before TVT. HemA mouse 24-hour survival plotted against the dose. ED50 values obtained for HemA mice dosed with BDD-FVIII or D519V/E665V are displayed, along with their respective chromogenic specific activities (right). Statistical significance (P = .05) was determined using analysis of covariance. Chromo SA, chromogenic specific activity.
Enhanced efficacy of D519V/E665V in a TVT injury model. HemA mice were treated with BDD-FVIII or D519V/E665V at the indicated dose 24 hours before TVT. HemA mouse 24-hour survival plotted against the dose. ED50 values obtained for HemA mice dosed with BDD-FVIII or D519V/E665V are displayed, along with their respective chromogenic specific activities (right). Statistical significance (P = .05) was determined using analysis of covariance. Chromo SA, chromogenic specific activity.
Discussion
In this study, we have demonstrated that the A2 domain of D519V/E665V is physically stabilized following thrombin activation compared with BDD-FVIII (Figure 1), which is consistent with the reduced distribution coefficient for A2 subunit interaction in the D519V/E665V FVIIIa variant.18 The consequence of reduced A2 dissociation is a markedly reduced FVIIIa decay rate as determined by FXase activity.11,19 Enhanced FXa generation, in turn, contributes to enhanced thrombin generation, platelet activation and accumulation, and fibrin deposition (Figures 2 and 4), all of which result in enhanced efficacy in 3 different vascular injury models using HemA mice (Figures 4-6).
Multiple animal models of vascular injury were used to determine whether the D519V/E665V variant offers an efficacy benefit and to quantify the degree of benefit. All the vascular injury models used clearly indicated that the D519V/E665V variant protected HemA mice from vascular injury. However, assessment of the degree of protection offered by D519V/E665V was complicated by the variable degree of benefit in the different animal models, in which vascular injuries varied in severity and in vascular beds. The differential responses observed in the models likely reflect differences in local hemodynamic, vascular, and hemostatic characteristics. It is well-established that hemostasis and thrombosis in different vascular beds depend on a variety of local conditions, including shear stress, endothelial cells, and the crosstalk between the endothelium and the surrounding cells of the vasculature.20 Furthermore, endothelial cells of large arteries are particularly enriched for endothelial protein C receptor, which is needed for protein C activation.21 Conceivably, activated protein C (APC) might play more dominant roles in large-vessel regulation of hemostasis. The lower relative degree of efficacy observed in the tail clip and TVT models is consistent with such a role for APC in these vessels because D519V/E665V does not protect against APC degradation. The availability of APC-resistant D519V/E665V17 would offer an opportunity to assess the relative contribution of A2 stabilization vs APC degradation to FVIII cofactor function in vivo.
Finally, the greater protection with the D519V/E665V variant in laser injury of cremaster vessels (Figure 4A-B) suggests a more prominent role for FVIII in microvascular bleeds. Because bleeding from small vessels is thought to be characteristic of joint bleeds,22 it is possible that D519V/E665V would be particularly effective in reducing joint arthropathy in hemophilia.
As with the animal models, the in vitro assays appeared to have differential sensitivities to various aspects of FVIII cofactor function. For example, the aPTT was relatively insensitive to the consequences of A2 stabilization (Table 1). On the other hand, the chromogenic assay and TGA were sensitive to changes in FVIIIa cofactor activity and efficacy. The significance of these in vitro assay differences requires further study, but it indicates that the assays are more or less sensitive to the changes made in novel FVIII variants. Therefore, both in vitro assays and animal models can be used to probe the role of specific FVIII cofactor functions in their response to specific vascular injuries.
From our studies in various animal models, it is clear that stabilization of the A2 domain likely accounts for the improved efficacy. However, the concomitant enhanced activated D519V/E665V affinity for FIXa also raises the possibility that the enhanced efficacy may also be related to the increased affinity, especially because the apparent dissociation constant (K1/2) between FIXa and activated D519V/E665V was also reduced threefold.
Further experimentation will be required to establish the relationship between the enhanced FIXa affinity and A2 domain stabilization. The unchanged or increased K1/2 values of an FVIII variant in which the A2 domain was covalently stabilized by disulfide bonding (C662/C1828)5 suggests that the enhanced FIXa-FVIIIa affinity may be independent of A2 stabilization. Instead, the enhanced affinity of D519V/E665V may be a consequence of a conformation more optimal for FIXa-FVIIIa interactions and increased FXa generation.
The enhanced efficacy of D519/E665V may provide numerous benefits in the treatment of hemophilia. Although successful adeno-associated viral–based gene delivery of FIX for treatment of hemophilia B has been achieved,23 FVIII gene therapy can potentially be improved by variants such as D519V/E665V. The high-activity FIX variant FIX-R338L has yielded promising results in a preclinical study and provides proof of principle for this strategy.24 Furthermore, attempts to increase circulating half-life of FVIII by PEGylation have led to limited success; further shielding of the molecule to simultaneously prevent von Willebrand factor binding and protease degradation may be required to transcend the 1.5- to twofold half-life increase ceiling.25 The enhanced activity of D519V/E665V may compensate for any loss of activity resulting from more extensive conjugation. Finally, APC-resistant D519V/E665V variants17 could potentially offer additional benefits above D519V/E665V, but that remains to be seen.
In conclusion, D519V/E665V represents the first FVIII molecule containing a noncovalently stabilized A2 domain with clear efficacy enhancement resulting from a specific mechanism of action. Also, because D519V/E665V FVIII efficacy is enhanced in all the injury models tested, we were able to observe differential efficacy enhancements among these HemA mouse models. From these efficacy results, the vessel beds, the types of injury studied, and the mechanism of action for D519V/E665V, we suggest some possible approaches that build on the D519V/E665V variant to improve FVIII performance in hemophilia.
Presented in oral presentation format at the XXIV Congress of the International Society on Thrombosis and Haemostasis; Amsterdam, The Netherlands; June 29-July 4, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Subramanian Yegneswaran, Vince Evans, Philip Ramsey, David Shiroma, Cornell Mallari, Yifan Xu, and other Bayer employees for their technical contributions to the study.
This work was supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (grant HL38199) (H.W. and P.J.F.).
Authorship
Contribution: J.E.M. conceived of and oversaw the study; P.J.F. and H.W. provided the D519V/E665V variant used in the study; T.T., P.J.K., and K.T. generated the proteins used in the study; L.L. and C.P. provided the in vitro data; P.L. and E.H. provided the in vivo data supporting the study; and J.E.M., P.J.F., L.L., C.P., D.S., P.J.K., K.T., and E.H. contributed to writing the manuscript.
Conflict-of-interest disclosure: L.L., D.S., C.P., K.T., P.L., E.H., T.T., P.J.K., and J.E.M. were employees of Bayer when this work was executed. H.W. and P.J.F. declare no conflicts of interest.
Correspondence: Lilley Leong, Bayer HealthCare Pharmaceuticals, 455 Mission Bay Blvd South, Suite 493, San Francisco, CA 94158; e-mail: Lilley.leong@bayer.com.