Abstract
Over the past 5 years, many novel approaches to early diagnosis, prevention, and treatment of acute graft-versus-host disease (aGVHD) have been translated from the bench to the bedside. In this review, we highlight recent discoveries in the context of current aGVHD care. The most significant innovations that have already reached the clinic are prophylaxis strategies based upon a refinement of our understanding of key sensors, effectors, suppressors of the immune alloreactive response, and the resultant tissue damage from the aGVHD inflammatory cascade. In the near future, aGVHD prevention and treatment will likely involve multiple modalities, including small molecules regulating immunologic checkpoints, enhancement of suppressor cytokines and cellular subsets, modulation of the microbiota, graft manipulation, and other donor-based prophylaxis strategies. Despite long-term efforts, major challenges in treatment of established aGVHD still remain. Resolution of inflammation and facilitation of rapid immune reconstitution in those with only a limited response to corticosteroids is a research arena that remains rife with opportunity and urgent clinical need.
Introduction
Acute graft-versus-host disease (aGVHD) is a frequent and at times unpredictably severe inflammatory complication of allogeneic hematopoietic cell transplantation (HCT). Despite over 5 decades of extensive laboratory and clinical investigation into methods to prevent severe aGVHD, this complication remains a significant cause of morbidity and mortality in allogeneic HCT recipients. Each year, ∼6800 patients undergo HCT (Center for International Blood & Marrow Transplant Research [CIBMTR] data), and the majority will suffer some manifestations of aGVHD. Several advancements have led to novel aGVHD detection, prophylaxis, and treatment methods. In this review, we highlight aGVHD advancements in the context of current care. With many new modalities that target host and donor responses under investigation, an era of multimodal, personalized immunomodulation in HCT may emerge. However, significant challenges to elimination of aGVHD remain. These include developing treatment regimens that retain infectious immunity and graft-versus-tumor effects, as well as identification of effective therapy for steroid-refractory aGVHD.
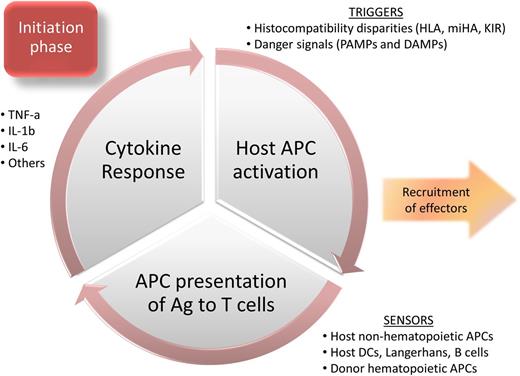
The classic description of aGVHD pathophysiology begins with activation of host antigen-presenting cells (APCs) by danger signals expressed on damaged tissues (damage-associated molecular patterns [DAMPs]) and/or pathogens (pathogen-associated molecular patterns [PAMPs], eg, lipopolysaccharide).1 Activated host APCs then present host antigens to donor T cells, leading to alloactivation and inflammatory cytokine release. These inflammatory cytokines then recruit and induce proliferation of additional immune effector cells, perpetuating the cycle of alloreactive tissue injury and inflammation. Although a simplified description of the complexity underlying graft-versus-host (GVH) interactions,2 it is the established foundation for disentangling the pathways involved. A more detailed overview of each phase of acute GVHD (the initiation, lymphocyte trafficking, expansion and effector, and treatment phases) will serve as background for emerging, novel diagnostic and therapeutic approaches.
Initiation phase
The initiation phase of aGVHD broadly involves triggers and sensors of GVH reactions (Figure 1). Many aGVHD triggers have been identified, with the most critical being disparities in major histocompatibility antigens (reviewed in detail by Petersdorf3 ). HLA-matched donors are not available for all eligible HCT recipients, making the identification of permissive mismatches of clinical interest. Several high-risk HLA allele mismatch combinations associated with severe aGVHD have been identified,4 although a provocative update suggests that HLA allele mismatches have had a lesser impact on outcomes in recent years (2002 and beyond) compared with HCT pre-2002.5 Recently, the identification of amino acid substitutions at peptide-binding pockets of HLA class I molecules HLA-B and HLA-C has been associated with increased GVHD risk.6 This report suggests that unrelated donor selection might be enhanced by avoiding donor/recipient amino acid substitutions at positions 99 and 116 of HLA-C, and at position 9 of HLA-B.6 In addition, avoiding mismatches at low expression loci (HLA-DP, DQ, and DRB3/4/5) could help further reduce adverse outcomes in 7 of 8 mismatched unrelated HCT.7 Minor histocompatibility antigens (miHAs) are also implicated as GVHD triggers, particularly in HLA-matched siblings. The best-described miHA differences involve immune responses against Y-chromosome–encoded antigens elicited in female to male HCT, leading to the preferential selection of male donors.8 Differences in autosomal miHA, especially HA-8 mismatches in related donor HCT, have also been associated with aGVHD.9 Finally, donor/recipient differences in killer immunoglobulin-like receptor (KIR) and KIR-ligand interactions can alter the risk of aGVHD,10 adding complexity to the immunogenetic determinants.
Several nongenetic triggers of aGVHD have been identified, predominantly danger signals: DAMPs and/or PAMPs. DAMPs include extracellular matrix components, adenosine triphosphate (ATP), and uric acid. Heparan sulfate, a component of extracellular matrix and endogenous Toll-like receptor 4 (TLR4) agonist, can promote alloreactive T-cell responses, is elevated in both murine and human GVHD, but is not increased by tissue damage related to conditioning.11 Heparan sulfate levels and GVHD severity could both be reduced by α1-antitrypsin treatment in a murine model.11 ATP released by dying cells can also induce inflammatory responses, and ATP neutralization or blockade of its receptor on immune cell subsets, P2X7R, reduced experimental GVHD.12 Uric acid can act as a DAMP, leading to NLRP3 inflammasome-mediated interleukin-1β (IL-1β) production, a key cytokine involved in aGVHD pathophysiology.13 In addition to endogenous danger signals, bacterial14 and viral15 PAMPs can both contribute to the inflammatory milieu. The role of danger signals and pattern recognition receptors in GVHD has been recently reviewed.16 Interestingly, not all PAMPs cause detrimental immune responses in aGVHD. Flagellin, a component of bacterial flagella and TLR5 agonist, has been shown to reduce GVHD while enhancing immune reconstitution in experimental HCT.17
APCs are the predominant sensors of GVHD-initiating signals. APCs capable of contributing to GVH reactions include residual host hematopoietic APCs that remain viable after the conditioning regimen, host nonhematopoietic APCs, and donor APCs transferred with the allograft. Because they are central to the initiation of GVHD,18,19 targeting host APCs is an attractive GVHD prevention strategy. However, recent animal models have challenged this dogma, as depletion of host dendritic cells (DCs),20 Langerhans cells,21 and B cells22 failed to prevent experimental GVHD. In contrast, depletion of host macrophages from lymphoid organs was shown to exacerbate GVHD, revealing an unexpected protective role.23 Therefore, it has been unclear which APC subsets are most critical for the initiation phase of aGVHD. Recent evidence suggests that nonhematopoietic APCs are the most potent GVHD inducers by 100- to 1000-fold, rendering host and donor professional hematopoietic APCs redundant or at least nonessential in GVH reactions.24 How to apply prophylaxis strategies related to this recent finding is not yet known.
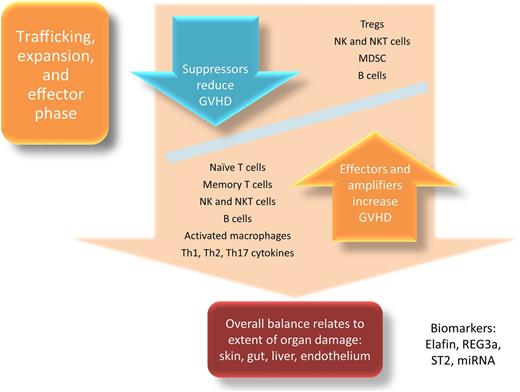
Lymphocyte trafficking, expansion, and effector phase
The net result of the initiation phase of aGVHD is the cytokine response made by sensors resulting in recruitment and activation of donor T cells which home to secondary lymphoid organs (nodes plus submucosal gastrointestinal and pulmonary sites) and proliferate. The effector phase of aGVHD consequently begins when cytolytic donor effector T cells, neutrophils, natural killer cells (NKs), NK T cells (NKTs), and macrophages begin to cause end-organ damage in a reaction that overwhelms any tolerance-promoting response from suppressor cells (Figure 2). Donor T cells are the best-studied effectors of aGVHD, and most prophylaxis and treatment strategies are aimed at attenuating their function. T-cell activation requires 2 stimulatory signals. First, the T-cell receptor (TCR) must be engaged and recognize antigens in the context of HLA. Recently, a small population of T cells expressing dual TCRs, potentially resulting from stochastic processes during thymic selection, was found to be disproportionately responsible for alloreactivity in human GVHD.25 Next, T cells must receive adequate costimulation from APCs to become activated and acquire effector functions. Many costimulatory ligand/receptor interactions and their involvement in aGVHD have been described, including the B7/CD28/CTLA4, CD30/CD30L, CD40/CD40L, OX40/OX40L, and ICOS/ICOS-L pathways (recently reviewed by Briones et al26 ). Once activated, these alloreactive T cells can proliferate and migrate in response to an inflamed environment27 and the activation of chemokine receptors, including CCR2,28 CCR5,29 CCR6,30 and CCR731 among others (reviewed by Kittan and Hildebrandt32 ). Homing of activated T cells to target tissues may yield change in these cell subsets in the peripheral blood. For example, fewer circulating CCR6+ T cells have been observed in patients with aGVHD due to homing toward CCL20+ target tissues.33 Similarly, fewer circulating CD8+CD45RA+β7 integrin+ T cells have also been observed in allogeneic HCT recipients experiencing gastrointestinal GVHD,34 although increased memory CD8+α4β7 integrin+ T cells have also been observed at the onset of aGVHD.35 Naïve donor T cells are central for clinical aGVHD manifestations.36,37 Memory T-cell subsets are less alloresponsive and do not appear to initiate GVHD to the same degree.38-40 However, even if less important as initiators of aGVHD, memory T cells may perpetuate GVH reactions.41
Other immune effector cells have inflammatory roles in the pathophysiology of aGVHD, including neutrophils, NKTs, NKs, B cells, and mononuclear phagocytes, although their individual contributions to aGVHD reactions in humans can be difficult to isolate. Neutrophils engraft rapidly after allogeneic HCT and are secondary effectors of GVHD, recruited into target organs by cytokines including IL-8.42 Indirect evidence supports an inflammatory role of neutrophils in GVHD given the observation of increased rates of acute and chronic GVHD with granulocyte colony-stimulating factor administration post-HCT.43,44 Regarding NKs, bioluminescence studies have demonstrated rapid trafficking and homeostatic proliferation of NKs in a manner similar to allogeneic T cells after infusion, although NKs did not cause GVHD in this model.45 Classically, NKs have been implicated as a component of the effector phase in descriptions of GVHD pathophysiology,46 although in clinical HCT, an increased allograft NK dose is associated with less severe aGVHD,47 possibly due to host APC depletion by donor NKs. A contribution from B cells in the pathogenesis of human aGVHD is possible considering reports of a reduced incidence of aGVHD with B-cell depletion48,49 and reduced risks of chronic GVHD after rituximab-containing conditioning regimens.50 Finally, activated macrophages can be observed colocalized with T cells in areas of highest macrophage migration inhibitory factor expression in GVHD target organs,51 adding to the cytolytic effector-induced tissue damage during this phase of aGVHD.
Conversely, several cell populations have been demonstrated to function as suppressors of GVH reactions, dampening inflammation and promoting tolerance. Intense current investigation involves regulatory T cells (Tregs), with extensive preclinical and emerging clinical reports of their function in prevention and treatment of aGVHD.52-54 Tregs are CD4+ T cells that function to suppress runaway immune responses mediated by mature APCs and effector T cells.55 Mechanisms of Treg suppression of GVHD are pleiotropic and only partially known, but include downregulation of aGVHD-associated TLR5 expression.56,57 Another recently described CD4+ T-cell subset, T helper 9 (Th9) cells, secrete IL-9 and dampen interferon-γ–mediated GVHD while preserving graft-versus-tumor effects in a murine model.58 Whether this subset can suppress human aGVHD is not yet known. Myeloid-derived suppressor cells (MDSCs) can also mitigate GVHD via multiple mechanisms.59,60 Finally, even though these subsets have potential pathogenic roles in aGVHD, NKs,61 NKTs,62 B cells,63 and macrophages23 can also play tolerogenic roles in HCT, which adds further complexity to the landscape of effectors and suppressors of aGVHD.
The final components of the effector phase of aGVHD are cytokines. They can amplify or attenuate GVH reactions, and the balance between proinflammatory and anti-inflammatory cytokines (as opposed to differing concentrations of a single cytokine) shapes the overall milieu and GVHD response (reviewed by Reikvam et al64 ). The prototypic effector cytokines in aGVHD are tumor necrosis factor α (TNF-α), IL-1β, and IL-6, although IL-17,65 IL-23,66 and Th2 cytokines67 can also enhance the inflammatory milieu, depending upon organ involvement and other factors.
During the effector phase of aGVHD, inflammation escalates rapidly and target tissues are damaged. The inflammatory cascade and continued tissue damage can lead to release of biomarkers of aGVHD into the circulation, reflecting both effector activation and tissue injury. These biomarkers include soluble CD30 from activated T cells,68 elafin (skin-specific),69 regenerating islet-derived 3-α (REG3α; gut-specific),70 suppressor of tumorigenicity 2 (ST2; a member of the IL-1 receptor family–binding IL-33),71 microRNA,72 and others (reviewed by Paczesny73 ), even before the onset of clinical signs of aGVHD become apparent and potentially predicting therapy responses.
Treatment phase
At present, clinicians rely on history, physical examination, and routine clinical laboratory parameters to determine when treatment of aGVHD must commence. The classic target organs of aGVHD are the skin (severity ranging from maculopapular rash to erythroderma and bullae formation), the gastrointestinal tract (resulting in nausea, vomiting, abdominal cramps, and/or diarrhea), and the liver (resulting in hyperbilirubinemia, jaundice, and/or elevated transaminases). The hematopoietic system can also be targeted, resulting in complete donor lymphohematopoietic chimerism and the graft-versus-tumor response against hematologic malignancies. Endothelium,74,75 lungs, and other organs can also be targeted, although skin, gut, and liver involvement are the only organs scored in the current grading system.76 Clinical laboratory clues regarding the onset of aGVHD frequently include lymphopenia,77 eosinophilia,78 thrombocytopenia,79 and hypoalbuminemia.77 Histologic confirmation is routinely obtained to rule out opportunistic infections or other alternate explanation for the clinical symptoms, though the classic (mostly apoptotic) histologic findings of aGVHD are somewhat nonspecific and can be mimicked by conditioning regimen injury, infections, and other syndromes. Noninvasive imaging modalities that visualize processes at the cellular level may be a component of future GVHD diagnostic and monitoring techniques, but are not reliably accepted or available at the moment.80,81 The decision to treat is based upon severity of symptoms, with grade 1 skin involvement typically treated only with topical corticosteroids. For those requiring systemic therapy, the standard first-line treatment is oral or IV corticosteroids.
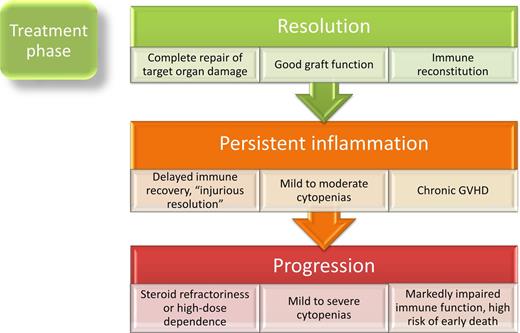
Once treatment begins, 3 primary immunologic outcomes are possible: the aGVHD inflammatory response (1) is completely resolved after therapy, (2) partially responds clinically, but incompletely resolves immunologically, leading to what may be a persistent state of “injurious resolution,”82 or (3) progresses despite therapy (Figure 3). It is not entirely known how steroids resolve aGVHD, but their lympholytic effects as well as alteration in cell adhesion molecules and chemokines in target organs are involved in reducing signs of GVHD.83 Symptoms can regress rapidly (within days), unless the patient is refractory, and plasma biomarkers of aGVHD respond accordingly.84 Reduction in lymphocyte and monocyte counts may be expected to a degree with corticosteroid therapy, although severe leukopenia (<200 cells/μL) and a major (median 10-fold) decrease of total circulating leukocytes during acute GVHD treatment are associated with a significantly lower likelihood of response to first-line therapy.85 In the wake of severe aGVHD, many patients are left with compromised hematopoietic and immune function, endothelial damage,86 and an increased risk of death even if without ongoing aGVHD signs and symptoms.87 In these patients, chronic GVHD can emerge through a variety of incompletely understood mechanisms that may include failure of NKs to eradicate CD4+ cells undergoing constant antigenic stimulation.88
It is difficult to predict at the onset of aGVHD who will respond fully or partially to first-line therapy, or who will be refractory. Approximately half of patients will not achieve a sustained complete response of aGVHD to first-line therapy with steroids,89 and timely determination of steroid nonresponse is important for institution of salvage therapies. Those at higher risk of treatment failure have been identified clinically as those with hyperacute GVHD,90 sex-mismatched HCT,90 or with a certain constellation of organ involvement.91 A recently described biomarker, ST2, the receptor for IL-33,71 correlates with resistance to initial GVHD therapy and death.92 During uncontrolled inflammation in refractory disease, additional rising biomarkers of epithelial cell death can be observed,93 macrophages heavily infiltrate into the skin in response to continued inflammation,94 and the chance of long-term survival drops dramatically. Survival is poor in steroid-resistant aGVHD, ∼15% at 2 years.90
Update in prevention and treatment
Prophylaxis
Based upon extensive preclinical studies in canines, post-HCT methotrexate (MTX) was the first widely used GVHD prophylaxis drug in clinical HCT,95 followed by cyclosporine (for historical perspective of early trials, see review by Vogelsang et al96 ). MTX and cyclosporine exert their antiproliferative effects on donor T cells by interfering with purine synthesis and calcium-dependent signal transduction pathways downstream of the TCR, respectively. Tacrolimus, with mechanism of action similar to cyclosporine, came to the fore in the 1990s showing similar or superior efficacy to cyclosporine in prospective, comparative trials97 and now is widely used in clinical HCT.
Today, the backbone of most T-cell replete conventional aGVHD prophylaxis regimens includes 2 drugs: (1) a calcineurin inhibitor plus (2) MTX or mycophenylate mofetil (MMF), the latter used more frequently in nonmyeloablative regimens and umbilical cord blood transplants.98 Steroids are typically not used as prophylaxis due to lack of proven benefit.99 Sirolimus, a mammalian target of rapamycin inhibitor, has also been demonstrated to have an impact in the treatment and prophylaxis of aGVHD. Comparison between sirolimus/tacrolimus to standard MTX/tacrolimus in a single-center study demonstrated an improved rate of aGVHD-free survival in the first 100 days posttransplant (43% vs 89%, P < .001).100 However, a multicenter, randomized phase 3 trial comparing these 2 regimens (Blood and Marrow Transplant Clinical Trials Network [BMT CTN] clinical trial 0402) did not show a significant difference in the primary end point of 114-day aGVHD-free survival. Furthermore, an increase in endothelial injury syndromes was observed in the sirolimus/tacrolimus arm, particularly following busulfan-containing conditioning.101,102
Additional methods of GVHD prophylaxis include T-cell depletion (ex vivo, ie, CD34+ positive selection or through T-cell subset depletion methods targeting CD3, α-β T cells,103 or in vivo via administration of antithymocyte globulin or alemtuzumab). Graft manipulation by CD34 selection decreases the numbers of T cells in the graft by several logs, although this alone does not completely abrogate aGVHD. This is particularly important in haploidentical HCT, where further immune modulation is required to avoid lethal GVHD given the significant HLA mismatch. High-dose cyclophosphamide (Cy) administered after stem cell infusion is currently used commonly in this setting, relying on the premise of direct lytic depletion of rapidly proliferating T cells with concomitant sparing of stem cells and regulatory T cells, possibly due to differential aldehyde dehydrogenase expression (higher in stem cells and Tregs).104 This simple in vivo graft manipulation is able to break the HLA barrier by allowing the infusion of haploidentical donor cells with similar or reduced rates of aGVHD compared with transplants using HLA-matched grafts.105,106
The results of these studies, as well as the results of 3 recently published novel though preliminary aGVHD prophylaxis trials, have prompted new multicenter comparative clinical trials. First, a phase 1/2 study of maraviroc, a CCR5 inhibitor that blocks lymphocyte chemotaxis while preserving effector functions, demonstrated very low rates of grade II-IV acute GVHD (∼15%) and no visceral GVHD at day 100 post-HCT in 35 evaluable patients.107 Infection and relapse rates were not significantly increased. Next, addition of bortezomib, an NF-κB–inhibiting immunomodulator, given on days +1, +4, and +7 in addition to standard tacrolimus and MTX in mismatched unrelated donor HCT, resulted in a day 180 cumulative incidence of grade II-IV aGVHD of 22%, notable given the mismatch donor source.108 Finally, a phase 1/2 study of vorinostat, used for its tolerogenic effects on APCs109 and apoptotic effects on alloreactive T cells,110 given from 10 days prior to transplant through day +100 in addition to the backbone of tacrolimus and MMF-based prophylaxis, resulted in a grade II-IV aGVHD rate of only 22% by day 100.111
Based upon the above studies, the BMT CTN is implementing 2 trials to identify optimal prophylaxis approaches. The first is in the setting of reduced-intensity conditioning, comparing 3 novel approaches: addition of maraviroc or bortezomib to a tacrolimus/MTX platform vs posttransplant Cy follow by tacrolimus/MMF. This trial (BMT CTN 1203) is a phase 2 trial that will compare the outcomes of a novel composite end point of GVHD-free, relapse-free survival to a contemporaneous control population receiving tacrolimus/MTX identified from the CIBMTR database. The second trial (BMT CTN 1301) is a 3-arm phase 3 that compares 2 approaches without calcineurin inhibitors (CD34+ cell selection and posttransplant Cy) to a standard tacrolimus/MTX approach in HLA-matched HCT using myeloablative conditioning. These 2 trials are part of the PROGRESS (Prevention and Reduction of GVHD and Relapse and Enhancing Survival after Stem Cell Transplant) initiative in the BMT CTN.
At present, several GVHD prophylaxis trials are ongoing, including sirolimus, cyclosporine plus MMF prior to nonmyeloablative HCT (NCT01251575), ustekinumab (IL-12/23 antagonist) in addition to a tacrolimus/sirolimus backbone (NCT01713400), bortezomib in addition to cyclosporine/MTX in pediatric HCT (NCT01926899), brentuximab vedotin (targeting CD30) in addition to tacrolimus plus MTX for mismatched HCT (NCT01700751), atorvastatin (NCT01665677), CD45RA+ (naïve) T-cell graft depletion (NCT01858740), extracorporeal photopheresis (NCT01174940), gene-modified donor T cells for haploidentical HCT (NCT01494103, NCT01744223), inducible Tregs (NCT01634217), and α-β T-cell depletion (NCT01810120). With many mechanisms to target in different HCT settings, it is unlikely that a 1-size-fits-all approach to aGVHD prophylaxis will be adopted in the near future. Importantly, promising results from phase 2 studies nearly always need broader testing in a multicenter comparative setting, such as in the US National Heart, Lung, and Blood Institute/National Cancer Institute/National Institute of Allergy and Infectious Diseases sponsored BMT CTN.
Initial therapy of aGVHD
The standard treatment of patients requiring systemic therapy is corticosteroids at a daily dose of 2 mg/kg,112 although there appears to be little detriment to a lower daily dose of 1 mg/kg for overall grade 1-2 aGVHD113,114 thereby sparing some of the side effects of high-dose steroids for milder disease. The determination of steroid-responsive or refractory disease should be made within a few days of initial therapy, recognizing nonresponse after 7 to 10 days and progression even sooner if the patient is clearly worsening 3 to 4 days after the start of high-dose steroids.115 The optimal duration of steroid therapy is unknown, but should be limited if possible to avoid side effects of long-term administration. The preferred rate to taper steroids for aGVHD has been rarely studied,116 but tapering limits have been included in some prospective trials.117 For trial purposes, important clinical end points for control of aGVHD include the day 28 response,118 day 56 aGVHD-free survival,118 and 6-month freedom from treatment failure,119 as well as rates of cGVHD, nonrelapse mortality, and survival. In an effort to safely improve responses to first-line steroid therapy, the BMT CTN recently conducted 2 clinical trials: BMT CTN 0302 and 0802. In BMT CTN 0302, patients were randomized to receive 1 of 4 immunomodulatory drugs in combination with steroids as first-line therapy: etanercept, MMF, denileukin diftitox, or pentostatin.120 In this study, the day 28 complete response rate for MMF was 60%, compared with 26% for etanercept, 53% for denileukin, and 38% for pentostatin. Therefore, MMF was selected as the agent of choice to be compared with placebo in a phase 3 trial, BMT CTN 0802. However, in 0802, addition of MMF did not improve outcomes compared with steroids alone for first-line therapy.117 At present, there are few registered trials of treatment of aGVHD, and they are often uncontrolled phase 1-2 pilots. For grade I/II aGVHD, a trial of cannabidiol is ongoing (NCT01596075), and for grades II-IV aGVHD, a trial of novel histone deacetylase inhibitor LBH589 (NCT01111526) is ongoing.
Refractory and steroid-resistant aGVHD
Refractory aGVHD remains a vexing problem and leads to poor prognosis. It is a broad category that includes different clinical scenarios, usually requiring escalation of immunosuppressive therapy. Minimal, delayed, or absent response to first-line corticosteroids defines steroid-resistant aGVHD, as does inability to maintain aGVHD control upon tapering corticosteroid therapy. There is no standard treatment of refractory GVHD, however, anti-thymocyte globulin (ATG) or TNF inhibitors are most frequently used clinically, along with the other agents tested in BMT CTN 0302 (pentostatin, MMF, or denileukin diftitox). Although used clinically, agents such as ATG and pentostatin have a very low durable complete response rate (20% or less).121,122 Other immunosuppressants available include anti-IL-2 receptor–, anti-IL6 receptor–, anti-CD20–, and anti-CD52–targeted therapies. There is some data in support of extracorporeal photopheresis123 and infusion of mesenchymal stromal cells (MSCs)124 in refractory aGVHD. Current clinical trials available for the treatment of steroid-refractory aGVHD include a combination of basiliximab plus infliximab (combined targeting of the IL-2 receptor and TNFα, NCT01485055). tocilizumab (targeting the IL-6 receptor, NCT01475162, NCT01757197), α1 antitrypsin (NCT01523821, NCT01700036), and brentuximab vedotin (NCT01596218).
Emerging approaches
Many additional novel aGVHD prevention and treatment strategies are undergoing preclinical study or clinical development based upon a deeper understanding of the complex immunologic processes involved. These treatments include small molecules that target different checkpoints in the aGVHD cascade, cytokine/growth factor milieu-based therapies, cell-based therapies, alteration of host microbiota, infusion of Tregs or other suppressor populations, as well as new approaches to tolerize donor cells prior to collection. A summary of these new approaches is detailed in Table 1 (Brunstein et al,54 Highfill et al,59 Das et al,66 Valenzuela et al125 -Rotta et al158 ). Several of these have reached the early clinical trial stage or will be clinically tested in the coming years.
Emerging approaches for the prevention and treatment of aGVHD
| Treatment or pathway . | Potential mechanism(s) of action . | Level of evidence . | References . |
|---|---|---|---|
| Small molecules | |||
| PKC inhibitors, such as R524 (Rigel Pharmaceuticals), and sotrastaurin (Novartis) | Inhibition of PKCα/θ, proteins that maintain immunologic synapse between APC and effector T cell | Preclinical (mouse) Sotrastaurin being investigated in solid organ transplant clinical trials | 125,126 |
| Sphingosine 1-phosphate receptor agonist FTY720 (fingolimod; Gilenya Pharmaceuticals) | Modulates DC function and lymphocyte efflux from secondary lymphoid organs, enhancement of endothelial barrier function | Preclinical (mouse) | 127 |
| Hypomethylating agents azacitidine and decitabine | Induction of FOXP3 expression | Preclinical (mouse) phase 1/2 clinical | 128,129 |
| Retinoic acid signaling | Reduction of T-cell homing, reducing Th1 differentiation, inducing Tregs | Preclinical (mouse) | 130 |
| Tim-3/Gal-9 pathway | Increased activation-induced T-cell death in the absence of Tregs | Preclinical (mouse) | 131 |
| PDL-1 pathway | Coinhibitory molecule, conversion of Th1 cells to Tregs | Preclinical (mouse) | 132 |
| IDO | Rate-limiting enzyme in tryptophan (required for T-cell proliferation) metabolism | Preclinical (mouse, human) | 133,134 |
| Arginase-1 | L-arginine depletion, reducing T-cell signaling and inflammatory cytokines | Preclinical (mouse) | 59 |
| TLR/MyD88 signaling inhibitors | Interfere with danger signaling, especially via inhibitory oligonucleotides against TLR9, to reduce inflammation | Preclinical (mouse) | 135 |
| Notch/notch ligand inhibitors | Detal-like1/4 (notch ligand) inhibitor given peritransplant prevented GVHD, while Notch 1 inhibitor lead to intestinal toxicity | Preclinical (mouse) | 136 |
| Cytokine/growth factor modulation | |||
| JAK/STAT inhibition | Reduction in inflammatory cytokines | Preclinical, case report | 137,138 |
| IL-17 downregulation | Curcumin downregulates IFNγ and IL-17 production, ameliorating aGVHD | Preclinical (mouse) | 139 |
| IL-21 blockade | Enhances generation of inducible Tregs | Preclinical (mouse) | 140 |
| IL-22 augmentation | Protective factor for intestinal stem cells under immune attack | Preclinical (mouse) | 141 |
| IL-23 blockade | Reduces inflammatory cytokines, T-cell trafficking, gut protection | Preclinical (mouse) | 66,142 |
| Case report, phase 2 (ongoing) | |||
| Cell-based therapies | |||
| MSCs | Suppress immune effector functions, secrete cytokines/growth factors for tissue repair and angiogenesis, can be obtained from related donors or third party | Phase 3, not yet reported in peer-reviewed literature (NCT00366145) | 124,143 |
| MAPCs | No expression of classical HLA class I markers (distinct from MSC), suppress T-cell activation via prostaglandin E2 synthesis, but only if colocalized with T cells at sites of activation | Preclinical (mouse) | 144,145 |
| Tregs | Expanded from umbilical cord blood, reduced aGVHD grade II-IV incidence from 61% to 43% in double UCB HCT (historical control); in haploidentical-related donors, Tregs reduced GVHD and enhanced immune reconstitution | Phase 1 | 54,146 |
| TRAIL+ T cells | Cytolytic mechanism against both tumor cells and alloreactive T cells | Preclinical (mouse) | 147 |
| NKs | GVHD protection only conferred if infusion was derived from Ly49-mismatched donor | Preclinical (mouse) | 148 |
| NKTs | Invariant NKTs attenuated murine GVHD in association with increased IL-2, IL-4, and IL-5 levels | Preclinical (mouse) | 149 |
| DCs | Tolerogenic DCs enhanced immunosuppressive cytokines in circulation, increased Tregs | Preclinical (mouse) | 150 |
| MDSCs | L-arginine depletion, contact-dependent immunosuppression | Preclinical (mouse) | 59 |
| Microbiota | |||
| α-defensins | Antimicrobial peptides secreted by intestinal Paneth cells, a target of GVHD | Preclinical (mouse) | 151 |
| Physiologic diversity | GVHD causes increase in Lactobacillales and decreases in Clostridiales, resulting in loss of physiologic diversity in gut bacteria | Preclinical (mouse and human) | 152 |
| Candida colonization | Patients colonized with Candida spp. had an increased incidence of grade II-IV GVHD (50% vs 32%) | Preclinical (human) | 153 |
| α-galactosylceramide (RGI-2001; RegImmune) | Produced by microbiome, can bind C1d and activate NKTs, induce Tregs | Preclinical (mouse) | 154,155 |
| Phase 1/2a (ongoing) | |||
| Donor-based immunomodulation | |||
| KGF (palifermin) | Epithelial, including thymic cytoprotection, inflammatory cytokine response, skewing toward Th2 cytokine response, although there was no reduction in GVHD when recipients were treated with palifermin in a phase 1/2 clinical trial | Preclinical (mouse) | 156,157 |
| Statins | Retrospective study demonstrated reduced grade III-IV GVHD in related HCT from statin-treated donors | Preclinical (mouse, human) | 158 |
| Phase 2 (ongoing) |
| Treatment or pathway . | Potential mechanism(s) of action . | Level of evidence . | References . |
|---|---|---|---|
| Small molecules | |||
| PKC inhibitors, such as R524 (Rigel Pharmaceuticals), and sotrastaurin (Novartis) | Inhibition of PKCα/θ, proteins that maintain immunologic synapse between APC and effector T cell | Preclinical (mouse) Sotrastaurin being investigated in solid organ transplant clinical trials | 125,126 |
| Sphingosine 1-phosphate receptor agonist FTY720 (fingolimod; Gilenya Pharmaceuticals) | Modulates DC function and lymphocyte efflux from secondary lymphoid organs, enhancement of endothelial barrier function | Preclinical (mouse) | 127 |
| Hypomethylating agents azacitidine and decitabine | Induction of FOXP3 expression | Preclinical (mouse) phase 1/2 clinical | 128,129 |
| Retinoic acid signaling | Reduction of T-cell homing, reducing Th1 differentiation, inducing Tregs | Preclinical (mouse) | 130 |
| Tim-3/Gal-9 pathway | Increased activation-induced T-cell death in the absence of Tregs | Preclinical (mouse) | 131 |
| PDL-1 pathway | Coinhibitory molecule, conversion of Th1 cells to Tregs | Preclinical (mouse) | 132 |
| IDO | Rate-limiting enzyme in tryptophan (required for T-cell proliferation) metabolism | Preclinical (mouse, human) | 133,134 |
| Arginase-1 | L-arginine depletion, reducing T-cell signaling and inflammatory cytokines | Preclinical (mouse) | 59 |
| TLR/MyD88 signaling inhibitors | Interfere with danger signaling, especially via inhibitory oligonucleotides against TLR9, to reduce inflammation | Preclinical (mouse) | 135 |
| Notch/notch ligand inhibitors | Detal-like1/4 (notch ligand) inhibitor given peritransplant prevented GVHD, while Notch 1 inhibitor lead to intestinal toxicity | Preclinical (mouse) | 136 |
| Cytokine/growth factor modulation | |||
| JAK/STAT inhibition | Reduction in inflammatory cytokines | Preclinical, case report | 137,138 |
| IL-17 downregulation | Curcumin downregulates IFNγ and IL-17 production, ameliorating aGVHD | Preclinical (mouse) | 139 |
| IL-21 blockade | Enhances generation of inducible Tregs | Preclinical (mouse) | 140 |
| IL-22 augmentation | Protective factor for intestinal stem cells under immune attack | Preclinical (mouse) | 141 |
| IL-23 blockade | Reduces inflammatory cytokines, T-cell trafficking, gut protection | Preclinical (mouse) | 66,142 |
| Case report, phase 2 (ongoing) | |||
| Cell-based therapies | |||
| MSCs | Suppress immune effector functions, secrete cytokines/growth factors for tissue repair and angiogenesis, can be obtained from related donors or third party | Phase 3, not yet reported in peer-reviewed literature (NCT00366145) | 124,143 |
| MAPCs | No expression of classical HLA class I markers (distinct from MSC), suppress T-cell activation via prostaglandin E2 synthesis, but only if colocalized with T cells at sites of activation | Preclinical (mouse) | 144,145 |
| Tregs | Expanded from umbilical cord blood, reduced aGVHD grade II-IV incidence from 61% to 43% in double UCB HCT (historical control); in haploidentical-related donors, Tregs reduced GVHD and enhanced immune reconstitution | Phase 1 | 54,146 |
| TRAIL+ T cells | Cytolytic mechanism against both tumor cells and alloreactive T cells | Preclinical (mouse) | 147 |
| NKs | GVHD protection only conferred if infusion was derived from Ly49-mismatched donor | Preclinical (mouse) | 148 |
| NKTs | Invariant NKTs attenuated murine GVHD in association with increased IL-2, IL-4, and IL-5 levels | Preclinical (mouse) | 149 |
| DCs | Tolerogenic DCs enhanced immunosuppressive cytokines in circulation, increased Tregs | Preclinical (mouse) | 150 |
| MDSCs | L-arginine depletion, contact-dependent immunosuppression | Preclinical (mouse) | 59 |
| Microbiota | |||
| α-defensins | Antimicrobial peptides secreted by intestinal Paneth cells, a target of GVHD | Preclinical (mouse) | 151 |
| Physiologic diversity | GVHD causes increase in Lactobacillales and decreases in Clostridiales, resulting in loss of physiologic diversity in gut bacteria | Preclinical (mouse and human) | 152 |
| Candida colonization | Patients colonized with Candida spp. had an increased incidence of grade II-IV GVHD (50% vs 32%) | Preclinical (human) | 153 |
| α-galactosylceramide (RGI-2001; RegImmune) | Produced by microbiome, can bind C1d and activate NKTs, induce Tregs | Preclinical (mouse) | 154,155 |
| Phase 1/2a (ongoing) | |||
| Donor-based immunomodulation | |||
| KGF (palifermin) | Epithelial, including thymic cytoprotection, inflammatory cytokine response, skewing toward Th2 cytokine response, although there was no reduction in GVHD when recipients were treated with palifermin in a phase 1/2 clinical trial | Preclinical (mouse) | 156,157 |
| Statins | Retrospective study demonstrated reduced grade III-IV GVHD in related HCT from statin-treated donors | Preclinical (mouse, human) | 158 |
| Phase 2 (ongoing) |
IDO, indoleamine 2,3 dioxygenase; IFN, interferon; JAK, Janus kinase; KGF, keratinocyte growth factor; MAPC, multipotent adult stromal cell; PDL, programmed death ligand; PKC, protein kinase C; STAT, signal transducer and activator of transcription; TRAIL, TNF-related apoptosis inducing ligand; UCB, umbilical cord blood.
Conclusion
Many new components of the aGVHD cascade have recently been identified, and translation of findings from bench to bedside has been faster than ever. Although prevention of aGVHD is clearly an important goal, finding new methods to resolve established aGVHD is critically needed today. Compared with prophylaxis studies, relatively fewer models dealing with the complicated problem of resistant aGVHD exist. Clinical features plus biomarkers may identify those most likely to fail and thereby classify those most suitable for study of intensified early treatment. Novel tolerance induction regimens that systematically and durably reprogram immune responses may become possible with ongoing research efforts. Identifying tolerizing factors and methods of manipulating microbiota may represent additional options for adjunctive aGVHD therapy that can be rapidly translated into clinical trials. Finally, more in-depth study of how to rescue patients from the cycle of incompletely resolved inflammation, where organ function has improved, but poor graft and immune function persist in the wake of severe aGVHD, remains an unmet need. Although we have seen significant innovation in methods to approach aGVHD in recent years, much work remains.
Acknowledgments
This work was supported in part by the Office of Research in Women’s Health and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development, Oregon Building Interdisciplinary Research Careers in Women’s Health, Award number 2K12HD043488-12 (S.G.H.).
Authorship
Contribution: S.G.H. wrote the paper; M.P. and D.J.W. edited the paper; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel J. Weisdorf, Blood and Marrow Transplant Program, University of Minnesota, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: weisd001@umn.edu.