Abstract
Chronic graft-versus-host disease (GVHD) is a frequent and potentially life-threatening complication of allogeneic hematopoietic stem cell transplantation. Increased transplantation of older patients and the more frequent use of unrelated donors has led to increased numbers of patients with this painful complication. Recent advances have been made in understanding the pathophysiology of chronic GVHD and in establishing precise criteria for diagnosis and classification of disease manifestations. These advances will hopefully pave the way for improving both the prophylaxis and treatment of chronic GVHD.
Introduction
Chronic graft-versus-host disease (GVHD) is currently the leading cause of long-term morbidity and mortality following allogeneic hematopoietic stem cell transplantation (HSCT).1-3 Although early transplantation-related mortality after allogeneic HSCT has decreased through the introduction of reduced-intensity conditioning (RIC) regimens and more effective anti-infectious agents, little progress has been made in decreasing late transplantation-related mortality. This lack of success is mainly the result of our failure to reduce the incidence and severity of chronic GVHD. However, recent progress has been made in two directions. First, human studies and the development of new murine models have led to a much better understanding of the complex pathophysiology of chronic GVHD. Second, the National Institutes of Health (NIH) consensus conference on chronic GVHD that was held in 2005 has led to a better understanding of the wide spectrum of disease manifestations, a new clinical severity index to monitor disease progression and response to therapy, and the assessment of new therapies using innovative clinical trial designs.4-9
Incidence and definition
In the past, chronic GVHD included any clinical manifestations of GVHD that occurred beyond 100 days after hematopoietic cell transplantation. This definition clearly became imprecise and inadequate. The goals of the NIH consensus working group on diagnosis and staging were to (1) establish criteria for diagnosis of the disease, emphasizing the distinction between acute and chronic GVHD; (2) define criteria for scoring the severity of clinical manifestations in affected organs; and (3) propose categories describing the overall severity of the disease and the indications for treatment.4
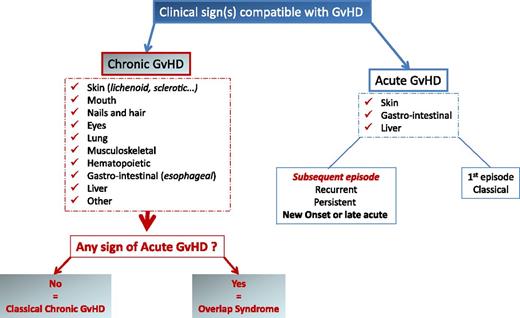
The NIH consensus conference recognized 2 main categories of GVHD, each with 2 subcategories. The broad category of acute GVHD includes classic acute GVHD (maculopapular erythematous rash, gastrointestinal symptoms, or cholestatic hepatitis) and also includes persistent, recurrent, or late-onset acute GVHD occurring more than 100 days after transplantation. The broad category of chronic GVHD includes classic chronic GVHD, presenting with manifestations that can be ascribed only to chronic GVHD. Chronic GVHD also includes an overlap syndrome, which has diagnostic or distinctive chronic GVHD manifestations together with features typical of acute GVHD (Figure 1).
Because the proposed NIH criteria were based on expert opinion, empirical studies were needed to assess their validity. Thus far, the largest retrospective validation study was performed by the Seattle group on patients who underwent allogeneic HSCT after myeloablative conditioning.15 Seven hundred forty patients required therapy for chronic GVHD that had been diagnosed according to the conventional day 100 landmark criteria. However, by using the NIH consensus criteria, the authors found that 352 (48%) of 740 patients did not meet the NIH criteria for chronic GVHD. At the Hospital Saint Louis, we retrospectively evaluated the incidence of chronic GVHD in a cohort of 177 patients who had received an RIC regimen prior to allogeneic HSCT.16 By using NIH consensus criteria, the 36-month cumulative incidence of chronic GVHD was 53.7%, and this was 20% lower when compared with previous conventional criteria. The incidence of late-onset acute GVHD was 2.8%, and this was lower than in previous retrospective studies (15% to 48%).15,17,18 However, previous studies included patients who underwent HSCT after myeloablative conditioning and RIC, and the Seattle study was restricted to patients who received systemic treatment for chronic GVHD.15 The incidence and prognostic impact of the overlap syndrome was assessed in a study of 427 patients with chronic GVHD from 9 centers. When 352 patients with overlap syndrome were compared with 75 patients with classic chronic GVHD, multivariable analysis showed that overlap patients had worse survival and higher nonrelapse mortality (NRM) than patients with classic chronic GVHD. All aspects of the overlap syndrome (erythematous rash, liver function abnormalities, upper or lower gastrointestinal involvement) seemed to be prognostic for survival and NRM.19
Thus, using diagnostic NIH criteria, the incidence of chronic GVHD might be much lower than previously reported. This is not just a semantic problem, since it affects the interpretation of older literature establishing the incidence of chronic GVHD in various settings, the analysis of risk factors, and results of all published clinical therapeutic trials. The results of all of these studies become difficult to interpret if 20% to 50% of chronic GVHD patients would be reclassified as acute GVHD patients. This uncertainty points out the urgent need for prospective validation of diagnostic and prognostic NIH criteria.20 Part of this validation has been performed in the United States by a consortium that analyzed several aspects of the NIH criteria; however, all Chronic GVHD Consortium studies are based on the analysis of a population that includes both prevalent and incident cases and thus cannot be fully considered as a validation cohort.17,19,21-30
Pathophysiology
For many years, studies of chronic GVHD that used experimental models were faced with the major drawback that these models did not fully recapitulate or mimic the human disease.11,31 Existing murine chronic GVHD models simulate 1 or more of the pathologic manifestations, such as increased anti-DNA antibodies, sclerotic chronic GVHD, fibrosis of skin and liver, and the less common immune complex–mediated glomerulonephritis. The type of multiorgan involvement and alloantibodies seen in patients often has not been well represented in these preclinical models. Moreover, some models do not involve conditioning regimens. Two recently developed murine models more closely recapitulate the clinical spectrum of the disease. The first one developed by Zeng’s group recapitulates a transition from acute GVHD to a scleroderma-like form of chronic GVHD with salivary gland involvement and serum antibodies.32 The second developed by Blazar’s group fully recapitulates an aggressive systemic disease with multiorgan involvement (including the lung).33 In the latter model, fibrosis was demonstrated in the lung and liver and was associated with CD4 T-cell and B-cell infiltration. Robust germinal-center reactions were present at the time of disease initiation, and blockade of germinal center formation suppressed the development of chronic GVHD.
In recent years, significant advances in our understanding of human chronic GVHD have been made. It is now evident that the clinical manifestations of chronic GVHD are the result of a highly complex immune pathology involving both donor B cells and T cells as well as other cells (Figure 2).
The pathophysiology of chronic GVHD. (A) General mechanisms. The acute graft-versus-host reaction is characterized by tissue damage mediated by inflammatory mediators, T cells, and cells from the innate immune system. Among target organs, two are particularly important for the development of subsequent chronic GVHD: (1) thymic epithelial cells (TECs) are damaged by alloreactive T cells leading to decreased generation of natural Tregs and release of self-reactive T cells. (2) Bone marrow microenvironment damage may explain disturbed B-cell homeostasis. The potent role of antigen-presenting cells (APCs) and the cross talk between B and T cells in chronic GVHD is poorly understood. (B) B cells and chronic GVHD. Patients with chronic GVHD have increased B-cell activation factor (BAFF):B-cell ratios, delayed reconstitution of naive B cells, and increased numbers of pregerminal center B cells. Patients with active chronic GVHD have elevated numbers of CD21– transitional B cells and a deficiency of memory CD27+ B cells. Patients who develop chronic GVHD have elevated levels of BAFF, a relative reduction in naive B cells, and relatively higher numbers of activated memory type B cells. Patients with hypogammaglobulinemia have elevated CD19+CD21low (immature) and CD19+CD21highCD38highIgMhigh (transitional) B cells. CD19+CD10–CD27–CD21high naive B cells are elevated in all patients with chronic GVHD. (C) Conventional T cells, Tregs, and chronic GVHD. An appropriate balance between Tregs and Tconv is critical for the maintenance of peripheral tolerance. In the setting of allogeneic HSCT, Tregs have been shown to play an important role in the establishment of tolerance between recipient tissues and donor-derived immunity. Monitoring of CD4+ T-cell subsets shows that Tregs rapidly expand after HSCT, but Treg levels subsequently decline in patients with prolonged CD4+ lymphopenia. This results in a relative deficiency of Tregs, which is associated with a high incidence of extensive chronic GVHD. In chronic lichenoid GVHD, a mixed Th1/Th17 signature with upregulated Th1/Th17 cytokine/chemokine transcripts and elevated numbers of interferon gamma (IFN-γ)– and IL-17–producing CD8+ T cells has been described. (D) Current issues in chronic GVHD pathophysiology. The hallmark of chronic GVHD is inflammatory fibrosis; putative mechanisms are described in the left part of the figure. Although a role of B- and T-cell subsets has been described, the cross talk between B and T cells is not well understood. Recent evidence from an experimental model suggests that a key player might be the T-follicular helper (TfH) cells. ICOS, inducible costimulatory [molecule]; NK, natural killer [cell]; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
The pathophysiology of chronic GVHD. (A) General mechanisms. The acute graft-versus-host reaction is characterized by tissue damage mediated by inflammatory mediators, T cells, and cells from the innate immune system. Among target organs, two are particularly important for the development of subsequent chronic GVHD: (1) thymic epithelial cells (TECs) are damaged by alloreactive T cells leading to decreased generation of natural Tregs and release of self-reactive T cells. (2) Bone marrow microenvironment damage may explain disturbed B-cell homeostasis. The potent role of antigen-presenting cells (APCs) and the cross talk between B and T cells in chronic GVHD is poorly understood. (B) B cells and chronic GVHD. Patients with chronic GVHD have increased B-cell activation factor (BAFF):B-cell ratios, delayed reconstitution of naive B cells, and increased numbers of pregerminal center B cells. Patients with active chronic GVHD have elevated numbers of CD21– transitional B cells and a deficiency of memory CD27+ B cells. Patients who develop chronic GVHD have elevated levels of BAFF, a relative reduction in naive B cells, and relatively higher numbers of activated memory type B cells. Patients with hypogammaglobulinemia have elevated CD19+CD21low (immature) and CD19+CD21highCD38highIgMhigh (transitional) B cells. CD19+CD10–CD27–CD21high naive B cells are elevated in all patients with chronic GVHD. (C) Conventional T cells, Tregs, and chronic GVHD. An appropriate balance between Tregs and Tconv is critical for the maintenance of peripheral tolerance. In the setting of allogeneic HSCT, Tregs have been shown to play an important role in the establishment of tolerance between recipient tissues and donor-derived immunity. Monitoring of CD4+ T-cell subsets shows that Tregs rapidly expand after HSCT, but Treg levels subsequently decline in patients with prolonged CD4+ lymphopenia. This results in a relative deficiency of Tregs, which is associated with a high incidence of extensive chronic GVHD. In chronic lichenoid GVHD, a mixed Th1/Th17 signature with upregulated Th1/Th17 cytokine/chemokine transcripts and elevated numbers of interferon gamma (IFN-γ)– and IL-17–producing CD8+ T cells has been described. (D) Current issues in chronic GVHD pathophysiology. The hallmark of chronic GVHD is inflammatory fibrosis; putative mechanisms are described in the left part of the figure. Although a role of B- and T-cell subsets has been described, the cross talk between B and T cells is not well understood. Recent evidence from an experimental model suggests that a key player might be the T-follicular helper (TfH) cells. ICOS, inducible costimulatory [molecule]; NK, natural killer [cell]; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
Regarding B cells, it has been recognized since the early de-scription of the disease that patients with chronic GVHD frequently have circulating antibodies that are reactive with recipient cells.34,35 However, whether these antibodies are pathogenic or simply reflect a disturbed B-cell homeostasis is unknown. Two classes of recipient-reactive antibodies have been associated with chronic GVHD. The first class includes antibodies directed against antigens in the recipient that are not present in the donor. Antibodies directed against Y-chromosome–encoded (HY) proteins that develop in male patients who receive stem cell grafts from female donors exemplify this class of alloantibodies. HY antibodies have been detected in more than 80% of patients with chronic GVHD but only in male recipients who had female donors.36-38 HY antibodies very seldom develop in male recipients who have male donors. Anti-HY antibodies can be detected as soon as 3 months posttransplantation and seem to predict the subsequent development of chronic GVHD.39 These immunoglobulin G (IgG) alloantibodies recognize several HY proteins, but DBY and UTY proteins appear to be more highly immunogenic. One DBY epitope (DBY-2) appears to be a dominant epitope with more than 50% of male recipients who have female donors developing circulating donor B cells that express B-cell receptor (BCR) specific for DBY-2.40 Most studies of alloantibodies have focused on HY antigens because male recipients who have female donors are relatively common compared with mismatches for autosomal epitopes. Nevertheless, antibodies specific for alloantigens derived from autosomal disparities have also been identified.
Antibodies directed against nonpolymorphic autoantigens represent a second class of antibodies frequently present in patients with chronic GVHD. For example, antibodies specific for platelet-derived growth factor receptor (PDGFR) have been described in patients with systemic sclerosis and chronic GVHD.41,42 These antibodies recognize native PDGFR, induce tyrosine phosphorylation and accumulation of reactive oxygen species (ROS), and stimulate type 1 collagen gene expression through the Ha-Ras-ERK1/2-ROS signaling pathway. The biologic activity of these autoantibodies thus suggests a role in the development of fibrosis. This finding served as background for the use of imatinib in chronic GVHD but the role of these antibodies is still debatable. In addition to antibody production, accumulating evidence suggests that B cells contribute to the immune response by antibody-independent mechanisms such as antigen presentation, by production of cytokines and chemokines, and by acting as regulatory cells.35 Low B-cell counts and increased risk of infections have long been recognized in patients with chronic GVHD.43 As in autoimmune diseases, distortion of normal B-cell homeostasis exists in chronic GVHD45-48 (see legend to Figure 2). High levels of BAFF in the presence of low numbers of naive B cells have been proposed to foster the survival of activated alloreactive and autoreactive B cells, resulting in immune pathology.43,44 It was thus a logical step to introduce treatment with rituximab as a chronic GVHD therapeutic. Sarantopoulos et al49 showed that patients who failed to respond to rituximab had persistent elevation of BAFF and a predominance of circulating B cells that possessed an activated BAFF-Rlow/CD20low cell-surface phenotype. Patients with hypogammaglobulinemia had elevated immature and transitional B cells.48 Besides significantly higher BAFF:B-cell ratios, many more patients with hyper-IgG had autoantibodies compared with those with hypogammaglobulinemia.48 In the context of BAFF excess, activated B cells are resistant to apoptosis and exhibit increased BCR responsiveness with increased phosphorylation of BLNK and Syk.50
Donor T cells also clearly play an important role in the immune pathology of chronic GVHD. In humans, in vivo T-cell depletion is the only prophylactic measure that effectively decreases the incidence of chronic GVHD. Although early experimental studies have supported the paradigm of acute GVHD being a T-helper cell 1 (Th1) process and chronic GVHD being a Th2 process,11 this old concept has been revisited, and recent data in humans suggest that Th1 (TC1)-Th17 responses are present in skin GVHD. The immune response occurring in chronic lichenoid GVHD showed a mixed Th1/Th17 signature with upregulated Th1/Th17 cytokine/chemokine transcripts and elevated numbers of interferon gamma– and interleukin 17 (IL-17) –producing CD8+ T cells.51,52 Patients with active chronic GVHD also have a lower frequency of CD4+ regulatory T cells (Tregs) when compared with patients without chronic GVHD.51,53,54 Reconstitution of Tregs and CD4+ conventional T cells (Tconv) showed that thymic generation of naive Tregs was markedly impaired, and reconstituting Tregs had a predominantly activated/memory phenotype. In response to CD4+ lymphopenia after HSCT, Tregs underwent higher levels of proliferation than Tconv, but Tregs undergoing homeostatic proliferation also showed increased susceptibility to Fas-mediated apoptosis.54 Finally, recently increased mitochondrial apoptotic priming of human Tregs has also been reported (J.R., manuscript submitted December 2013).
Thus, data in humans support a role of both T and B cells in a highly complex network leading to chronic GVHD. How these T- and B-cell networks interact has not been resolved. At the 2013 American Society of Hematology Annual Meeting and Exposition, an experimental study by Blazar’s group suggested that the missing link might be the T follicular helper cells55 whose peripheral phenotype has been recently resolved in humans (Figure 2).
Diagnosis, risk factors, and prognostic factors
The signs and symptoms of chronic GVHD are summarized in Table1. From a clinical point of view (providing that NIH criteria are strictly applied), the diagnosis of chronic GVHD is often relatively easy for physicians with some expertise in HSCT18,56 (Figure 3). However, it should be emphasized that (1) the presentation of chronic GVHD can be very polymorphic, ranging from discrete lichenoid features in the mouth only, to a multisystemic appearance resembling an aggressive lupus or scleroderma-like disease; (2) although the NIH panel recommended that distinctive but not diagnostic features may require biopsy to confirm the diagnosis, this may not be easy (or without risk) for some disease locations such as fasciitis or myositis; (3) NIH criteria for lung involvement include only bronchiolitis obliterans (BO) and organizing pneumonia (formally called BOOP), and the NIH scoring system includes both clinical signs and results of pulmonary function tests. Although there is a strong statistical correlation between chronic GVHD and either BO or BOOP,33,47,57 the spectrum of syndromes, pathophysiology, and triggering agents leading to lung involvement in the setting of alloreactivity post-HSCT clearly warrants further clarification.
Signs and symptoms of chronic GVHD
| Organ or site . | Diagnostic (sufficient for diagnosis) . | Distinctive (insufficient alone for diagnosis) . | Common (seen in both acute and chronic GVHD) . |
|---|---|---|---|
| Skin | Poikiloderma, lichen planus-like, sclerosis or morphea | Depigmentation | Erythema, maculopapular rash |
| Nails | Dystrophy, onycholysis/nail loss | ||
| Scalp and body hair | Alopecia, scaling | ||
| Mouth | Lichen planus-like, hyperkeratotic plaques | Xerostomia, mucocele, ulcers, pseudomembrane* | Gingivitis, erythema |
| Eyes | Keratoconjunctivitis,* Sicca syndrome | ||
| Genitalia | Lichen planus-like, vaginal scarring or stenosis | Erosions,* fissures,* ulcers* | |
| Gastrointestinal tract | Esophageal web or stenosis* | Anorexia/nausea, diarrhea | |
| Liver | Mixed hepatitis | ||
| Lung | BO by lung biopsy | BO diagnosed by pulmonary function tests and radiology | BOOP |
| Muscle and fascia | Fasciitis, joint contractures | Myositis and polymyositis | |
| Hematopoietic | Thrombocytopenia, eosinophilia, hypo or hypergammaglobulinemia, autoantibodies | ||
| Other | Effusions† |
| Organ or site . | Diagnostic (sufficient for diagnosis) . | Distinctive (insufficient alone for diagnosis) . | Common (seen in both acute and chronic GVHD) . |
|---|---|---|---|
| Skin | Poikiloderma, lichen planus-like, sclerosis or morphea | Depigmentation | Erythema, maculopapular rash |
| Nails | Dystrophy, onycholysis/nail loss | ||
| Scalp and body hair | Alopecia, scaling | ||
| Mouth | Lichen planus-like, hyperkeratotic plaques | Xerostomia, mucocele, ulcers, pseudomembrane* | Gingivitis, erythema |
| Eyes | Keratoconjunctivitis,* Sicca syndrome | ||
| Genitalia | Lichen planus-like, vaginal scarring or stenosis | Erosions,* fissures,* ulcers* | |
| Gastrointestinal tract | Esophageal web or stenosis* | Anorexia/nausea, diarrhea | |
| Liver | Mixed hepatitis | ||
| Lung | BO by lung biopsy | BO diagnosed by pulmonary function tests and radiology | BOOP |
| Muscle and fascia | Fasciitis, joint contractures | Myositis and polymyositis | |
| Hematopoietic | Thrombocytopenia, eosinophilia, hypo or hypergammaglobulinemia, autoantibodies | ||
| Other | Effusions† |
Simplified from Filipovich et al.4
In all cases, infection, drug side effects, and malignancy must be excluded.
Pericardial, pleural, or ascites.
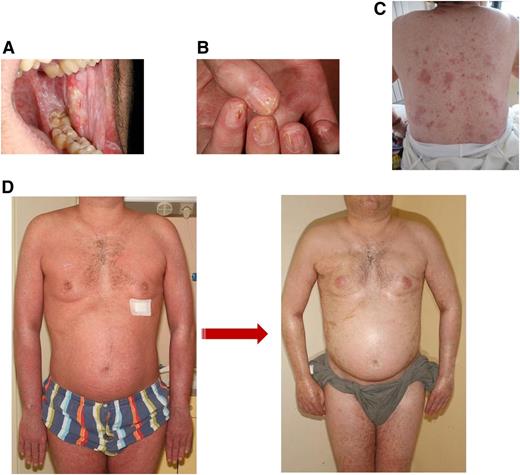
Clinical images of chronic GVHD. (A) Lichen planus-like lesions on buccal mucosa showing a lacework of white streaks and erosions. (B) Lichen planus lesions of the nails showing thinning of the nail plate, longitudinal lines, and pterygium formation. (C) Skin lichen planus lesions with shiny and violaceous papules of the back. (D) Edematous erythroderma with lichenoid features evolving into diffuse sclerodermatous changes of the skin.
Clinical images of chronic GVHD. (A) Lichen planus-like lesions on buccal mucosa showing a lacework of white streaks and erosions. (B) Lichen planus lesions of the nails showing thinning of the nail plate, longitudinal lines, and pterygium formation. (C) Skin lichen planus lesions with shiny and violaceous papules of the back. (D) Edematous erythroderma with lichenoid features evolving into diffuse sclerodermatous changes of the skin.
Although the diagnosis of chronic GVHD is often clinical, pathologic examination is warranted in a significant number of cases and clearly helps either in confirming the diagnosis of chronic rather than acute GVHD by using validated histologic changes specific for chronic GVHD (eg, skin, bronchioles, salivary glands) or in making a differential diagnosis (eg, chronic GVHD vs carcinoma for mouth ulcerations).18 More recently, biomarkers have been explored for chronic GVHD that may facilitate diagnosis or predict treatment response. Several biomarkers of chronic GVHD have been described including soluble BAFF, anti-double-strand DNA antibodies, soluble IL-2 receptor α (IL-2Rα), soluble CD13, adiponectin, and more recently, soluble CXCL9.6,58-61 Although many of these biomarkers have been studied in relatively large numbers of patients, further confirmation of their utility in clinical diagnosis and defining prognosis will be needed in future prospective studies.
Numerous risk factors for developing chronic GVHD have been described and are summarized in Table 2.62 The most important, previous GVHD, warrants further commentary. Because NIH consensus criteria are now being widely applied, it will be of interest to reexamine de novo chronic GVHD (without preexisting acute GVHD) to determine the true incidence of this entity, establish the extent to which de novo chronic GVHD actually presents as an overlap syndrome, and identify risk factors for this disease. Both HLA mismatching and transplantation from unrelated donors account for the growing incidence of chronic GVHD.63-67 Among factors that have not been consistently found as risk factors for chronic GVHD is the intensity of the conditioning regimen.68 Although acute GVHD incidence might be lower (even if often delayed) after RIC, the incidence of chronic GVHD is not (although not often properly assessed by NIH criteria). In a single-center study that compared chronic GVHD incidence using either the classic day 100 definition or the NIH criteria,16 the cumulative incidence of chronic GVHD at 36 months was 74% using conventional criteria compared with 54% using NIH consensus criteria.
Risk and prognostic factors for chronic GVHD
| Factor . | Reference . | |
|---|---|---|
| Established risk factors | ||
| Previous acute GVHD, HLA disparities, recipient’s and donor’s age, peripheral blood stem cells, T-cell replete graft, controversial risk factors, male recipient/multiparous female donor, cord blood stem cells, viral infection, conditioning regimen | 62 | |
| Prognostic factors | 62 | |
| Thrombocytopenia, type of disease onset (progressive and overlap syndrome), extensive skin involvement, elevated bilirubin, lung involvement, gastrointestinal involvement (diarrhea), decreased performance status | ||
| Prognostic classifications | 18 | |
| Limited vs extensive | 79, 81 | |
| Johns Hopkins Hospital | 59 | |
| Center for International Blood and Marrow Transplant Research | 61, 63 | |
| NIH consensus classification | 4 |
| Factor . | Reference . | |
|---|---|---|
| Established risk factors | ||
| Previous acute GVHD, HLA disparities, recipient’s and donor’s age, peripheral blood stem cells, T-cell replete graft, controversial risk factors, male recipient/multiparous female donor, cord blood stem cells, viral infection, conditioning regimen | 62 | |
| Prognostic factors | 62 | |
| Thrombocytopenia, type of disease onset (progressive and overlap syndrome), extensive skin involvement, elevated bilirubin, lung involvement, gastrointestinal involvement (diarrhea), decreased performance status | ||
| Prognostic classifications | 18 | |
| Limited vs extensive | 79, 81 | |
| Johns Hopkins Hospital | 59 | |
| Center for International Blood and Marrow Transplant Research | 61, 63 | |
| NIH consensus classification | 4 |
The conventional classification of limited versus extensive chronic GVHD was proposed in 1980 on the basis of only 20 cases. Since then, numerous prognostic indexes or isolated risk factors have been described and are summarized in Table 2. Thrombocytopenia (<100 000 platelets per milliliter) is the first reported and most reproducible prognostic factor, even when using NIH criteria.69,70 Other prognostic factors such as diarrhea64 might be the result of the older definition of the disease or the worse prognosis of the overlap syndrome.19 The NIH Consensus Conference proposed a new global chronic severity score establishing mild, moderate, and severe forms of chronic GVHD based on a numerical scoring system for individual organs to calculate a summary scale.4 The Chronic GVHD Consortium produced an impressive amount of data,17,19,21-30 which aimed to validate the NIH consensus criteria. The global and organ-specific severity index analysis showed that, at study enrollment, 10%, 59%, and 31% of the patients had mild, moderate, or severe disease, respectively, that was associated with both NRM and survival. The 2-year overall survival was 62%, 86%, and 97% for the patients with severe, moderate, and mild disease, respectively.71 Other single-center retrospective analyses also found the NIH severity index to be useful for identifying patient groups with different expectations for survival.69,70,72 However, assessment of organ-specific severity, including gastrointestinal,22 oral,25,28 ophthalmologic,30 and quality of life,21,23 remains problematic because of discrepancies between the perceptions of patients and physicians of disease severity as well as which tools should be used to assess organ severity. In spring 2014, the NIH will reconvene experts to amend and update the 2005 NIH Consensus Conference.
The immune deficiency associated with chronic GVHD
Posttransplantation immune deficiency is due to a variety of factors (including conditioning-induced thymic damage, age-associated thymic involution, thymic GVHD, or GVHD prophylaxis or treatment) and is a major cause of morbidity and mortality from infections and relapse. In recent years, new strategies have been explored to enhance posttransplantation T-cell recovery, and several of those are now in clinical evaluation.73 Immune reconstitution occurs gradually over time (generally 12 to 18 months) and is slower for allogeneic recipients, particularly in survivors with GVHD or those who have received prolonged immunosuppression.74 Chronic GVHD is the major factor affecting immune reconstitution of B cells and CD4– and CD8– T cells. Donor source and the degree of HLA compatibility between donor and recipient also affect the pace of immune reconstitution. Low B-cell count, inverted CD4:CD8 ratio, and a decreased IgA level are all risk factors associated with late infections. Susceptibility to encapsulated bacteria has been well documented, especially in patients with current or previous chronic GVHD. Late fungal or cytomegalovirus infections are rare and occur primarily in patients with ongoing immune suppression for GVHD. Finally, late Pneumocystis carinii infections are more common in patients receiving active treatment of chronic GVHD.
Late effects after transplantation and chronic GVHD
As reviewed elsewhere,74 chronic GVHD and its associated immunodeficiency contribute directly or indirectly to most malignant and nonmalignant late complications of allogeneic HSCT. Nonmalignant complications involving ocular, bone, joint, and cardiovascular systems and impaired quality of life are very often directly or indirectly (through treatment) linked to chronic GVHD. Among secondary malignancies, squamous cell carcinomas (particularly of the head and neck) have been associated with chronic GVHD.75 In immune-suppressed patients, oncogenic viruses, such as human papillomavirus, may contribute to squamous cell cancers of the skin and buccal mucosa. The observed excess risk of squamous cell cancers of the buccal cavity and skin is unexplained but may be indicative of an interaction between chronic lichen planus-like erosions, ionizing radiation, immunodeficiency and, conceivably, factors such as smoking or alcohol consumption.
Current issues in chronic GVHD prophylaxis and treatment
Prophylaxis.
Randomized studies aimed at reducing the incidence of GVHD are summarized in Table 3.76,77 Thus far, only anti-thymocyte globulin (ATG) included within conditioning regimens has successfully lowered the incidence and severity of chronic GVHD.78-81 However, it should be noted that (1) the first trial by the Italian group included only patients who received transplants from an unrelated donor using bone marrow as the stem cell source and at a time when HLA typing by molecular techniques was not available.80,81 (2) The second German/French trial included only patients who received transplants from an unrelated donor using peripheral blood and HLA typing by molecular techniques.78,79 This trial also demonstrated that ATG improved the likelihood of survival without any immunosuppressive therapy and without chronic GVHD. Both trials were conducted after myeloablative conditioning. Thus, lessons from randomized trials to prevent the occurrence of chronic GVHD after transplantation from an HLA-identical sibling or after RIC is currently unknown (even if a retrospective analysis suggested worse outcome without reduced chronic GVHD after RIC that include ATG82 ). Recently published phase 2 trials of prophylactic rituximab83,84 seem to be promising but clearly await confirmatory results from randomized trials.
Prophylaxis regimens and treatments for chronic GVHD
| Randomized trial . | Main results on chronic GVHD . | Reference . |
|---|---|---|
| Acute GVHD prophylaxis | ||
| Calcineurin inhibitors +/– methotrexate | No effect | 10, 76 |
| Prolonged cyclosporine | No effect | 10, 76 |
| Ex vivo T-cell depletion | No effect | 10, 76, 77 |
| Antithymocyte globulin | Decreased incidence | 72-75 |
| First-line treatment | ||
| Prednisone +/– azathioprine | Prednisone better | 79 |
| Prednisone + cyclosporine vs prednisone | Reduced prednisone exposure with combination | 80 |
| Prednisone + cyclosporine vs prednisone + cyclosporine + thalidomide | No benefit of thalidomide | 81, 82 |
| Prednisone + cyclosporine vs prednisone + cyclosporine + mycophenolate mofetil | No benefit of mycophenolate mofetil | 83 |
| Prednisone +cyclosporine vs prednisone +cyclosporine + hydroxychloroquine | No benefit of hydroxychloroquine | 84 |
| Randomized trial . | Main results on chronic GVHD . | Reference . |
|---|---|---|
| Acute GVHD prophylaxis | ||
| Calcineurin inhibitors +/– methotrexate | No effect | 10, 76 |
| Prolonged cyclosporine | No effect | 10, 76 |
| Ex vivo T-cell depletion | No effect | 10, 76, 77 |
| Antithymocyte globulin | Decreased incidence | 72-75 |
| First-line treatment | ||
| Prednisone +/– azathioprine | Prednisone better | 79 |
| Prednisone + cyclosporine vs prednisone | Reduced prednisone exposure with combination | 80 |
| Prednisone + cyclosporine vs prednisone + cyclosporine + thalidomide | No benefit of thalidomide | 81, 82 |
| Prednisone + cyclosporine vs prednisone + cyclosporine + mycophenolate mofetil | No benefit of mycophenolate mofetil | 83 |
| Prednisone +cyclosporine vs prednisone +cyclosporine + hydroxychloroquine | No benefit of hydroxychloroquine | 84 |
Ancillary therapy and supportive care.
If any progress has been made in the treatment of chronic GVHD in the past 30 years, we believe that it is because of our progress in supportive care of these patients. The most extensive review of ancillary therapy and supportive care was published by the NIH Consensus Conference in 2006.8 That review established extensive guidelines, including treatments for symptoms and recommendations for patient education, preventive measures, and appropriate follow-up. It provided guidelines for prevention and management of infections and other common complications of treatment of chronic GVHD. And it highlighted that optimal care of patients with chronic GVHD often requires a multidisciplinary approach.
First and secondary therapy.
To date, only 6 randomized phase 3 studies have been reported for initial treatment of chronic GVHD.85-90 The study by Koc et al86 was the only one that indicated benefit. Results of this study suggested that treatment with cyclosporine reduced the amount of glucocorticoid treatment needed to control the disease, as indicated by a decreased frequency of avascular necrosis. The generally recommended approach for treatment of chronic GVHD involves continued administration of the calcineurin inhibitor used for GVHD prophylaxis together with prednisone initially at 1 mg/kg per day.10,12,91,92 Strategies for tapering the dose of prednisone vary considerably, but as a general principle, efforts should be made to use the minimum dose that is sufficient to control GVHD manifestations. The median duration of treatment of chronic GVHD is approximately 2 to 3 years. The current therapeutic approach functions primarily to prevent immune-mediated damage, while awaiting the development of tolerance. Evidence to suggest that current treatments accelerate the development of immunologic tolerance is mostly lacking. The mechanisms that facilitate development of tolerance have not been well defined.91
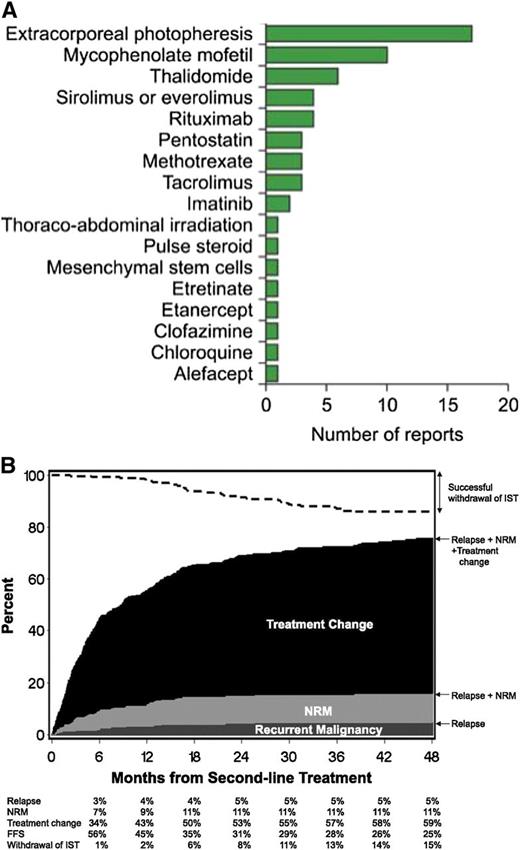
Indications for secondary treatment include worsening manifestations in a previously affected organ, development of manifestations in a previously unaffected organ, absence of improvement after 1 month of treatment, or inability to decrease the dose of prednisone below 1.0 mg/kg per day within 2 months.10,12,91,93 Numerous clinical trials have been carried out to evaluate approaches for secondary treatment of chronic GVHD. To date, no consensus has been reached regarding the optimal choice of agents for secondary treatment, and clinical management is generally approached through empirical trial and error.12,91 Treatment choices are based on physician experience, ease of use, need for monitoring risk of toxicity, and potential exacerbation of preexisting comorbidity. Treatments previously evaluated in 60 reports and included in a literature review are displayed in Figure 4A. It is worth noting that a recent analysis from the Seattle group94 found that failure-free survival after second-line treatment was only 20% (Figure 4B). Second-line treatments include extracorporeal photochemotherapy (ECP), mycophenolate mofetil, rituximab, sirolimus (rapamycin), or imatinib based on results from previous phase 2 trials.10,12,91,95-102 Both ECP and rituximab provide encouraging results, especially in patients with extensive skin or oral chronic GVHD. The Dana-Farber group recently presented a new approach that used low-dose IL-2 in a limited number of patients (n = 29) with advanced disease.103 Results were promising and were supported by strong immunologic data showing a series of changes in Treg homeostasis, including increased proliferation, increased thymic export, and enhanced resistance to apoptosis with only minimal effect on conventional T cells.104 A Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) trial comparing strategies involving ECP, rapamycin plus steroids, or calcineurin inhibitor plus steroids has apparently been prematurely closed as a result of lack of accrual in one arm and for futility to show any difference between the other 2 arms.
Second-line treatment of chronic GVHD. (A) Number of reports on second-line treatment of chronic GVHD (reprinted with permission from Martin et al91 ). (B) Failure-free survival after second-line treatment of chronic GVHD (reprinted with permission from Inamoto et al94 ). FFS, failure-free survival; IST, immunosuppressive therapy.
Second-line treatment of chronic GVHD. (A) Number of reports on second-line treatment of chronic GVHD (reprinted with permission from Martin et al91 ). (B) Failure-free survival after second-line treatment of chronic GVHD (reprinted with permission from Inamoto et al94 ). FFS, failure-free survival; IST, immunosuppressive therapy.
There is thus an urgent need for well-designed phase 2 and phase 3 trials with prespecified short-term end points and objective response criteria in steroid-resistant chronic GVHD.9 The NIH Consensus Conference previously proposed such response criteria and possible clinical trial designs7,105-107 that have been retrospectively (at least partly) validated. Increased understanding of the complex immune pathology of chronic GVHD will hopefully help identify new potential therapeutic interventions targeting B cells, effector T cells, or Tregs individually or in combination. If these novel approaches are successful in patients with steroid-resistant chronic GVHD, it will be possible to undertake studies to evaluate these new approaches for prevention as well as primary therapy for this difficult and persistent long-term complication of allogeneic HSCT.
Acknowledgments
We apologize to hundreds of colleagues worldwide whose excellent reports we were unable to cite in this review because of space constraints. The authors thank Dr Jean David Bouaziz for critical reading and for illustrations of skin GVHD.
This work was supported by National Institutes of Health, National Cancer Institute grants CA142106, CA183559, and CA183560 (J.R.).
Authorship
Contribution: G.S. and J.R. wrote the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerard Socié, Hematology/Transplantation, Hospital Saint Louis, 1 Avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail; gerard.socie@sls.aphp.fr.

![Figure 2. The pathophysiology of chronic GVHD. (A) General mechanisms. The acute graft-versus-host reaction is characterized by tissue damage mediated by inflammatory mediators, T cells, and cells from the innate immune system. Among target organs, two are particularly important for the development of subsequent chronic GVHD: (1) thymic epithelial cells (TECs) are damaged by alloreactive T cells leading to decreased generation of natural Tregs and release of self-reactive T cells. (2) Bone marrow microenvironment damage may explain disturbed B-cell homeostasis. The potent role of antigen-presenting cells (APCs) and the cross talk between B and T cells in chronic GVHD is poorly understood. (B) B cells and chronic GVHD. Patients with chronic GVHD have increased B-cell activation factor (BAFF):B-cell ratios, delayed reconstitution of naive B cells, and increased numbers of pregerminal center B cells. Patients with active chronic GVHD have elevated numbers of CD21– transitional B cells and a deficiency of memory CD27+ B cells. Patients who develop chronic GVHD have elevated levels of BAFF, a relative reduction in naive B cells, and relatively higher numbers of activated memory type B cells. Patients with hypogammaglobulinemia have elevated CD19+CD21low (immature) and CD19+CD21highCD38highIgMhigh (transitional) B cells. CD19+CD10–CD27–CD21high naive B cells are elevated in all patients with chronic GVHD. (C) Conventional T cells, Tregs, and chronic GVHD. An appropriate balance between Tregs and Tconv is critical for the maintenance of peripheral tolerance. In the setting of allogeneic HSCT, Tregs have been shown to play an important role in the establishment of tolerance between recipient tissues and donor-derived immunity. Monitoring of CD4+ T-cell subsets shows that Tregs rapidly expand after HSCT, but Treg levels subsequently decline in patients with prolonged CD4+ lymphopenia. This results in a relative deficiency of Tregs, which is associated with a high incidence of extensive chronic GVHD. In chronic lichenoid GVHD, a mixed Th1/Th17 signature with upregulated Th1/Th17 cytokine/chemokine transcripts and elevated numbers of interferon gamma (IFN-γ)– and IL-17–producing CD8+ T cells has been described. (D) Current issues in chronic GVHD pathophysiology. The hallmark of chronic GVHD is inflammatory fibrosis; putative mechanisms are described in the left part of the figure. Although a role of B- and T-cell subsets has been described, the cross talk between B and T cells is not well understood. Recent evidence from an experimental model suggests that a key player might be the T-follicular helper (TfH) cells. ICOS, inducible costimulatory [molecule]; NK, natural killer [cell]; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/3/10.1182_blood-2014-01-514752/4/m_374f2.jpeg?Expires=1769080160&Signature=DLRVXQjPExU8F7pneCSwDFseE4TiE2oxSd2KghnUGCYCGjXe80DjMM-pJdhemkBsBcnaHfX~ToW4bgykbmfIYQgG6ZGl83ccIjKV1iHhE01QJ8VaNqGw4ghnhkngZ-zhZnmZ3URI2U2CdiGfJCA2o-176z~f5Ozftp4JYpctsh8nDp5yZTXdywb3zokSF36bnzdExD5gSgso7vGiZMFHVly-byCmsS50Z08xoVvzlBmzHZ8XFkFmsUgBuLdOKZ9afKZhx8qzX1KGCZAKfu2YbiMtHdM6PJV2AdGQh~8dAvLtH6pBbjfBcAYTMtcB-82nLnm0bxPAx9DgpPzdlK-8GQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)