Key Points
JAK2 inhibitors affect more mature MF progenitors, but spare disease-initiating stem cells.
Reduction in spleen size achieved with JAK2 inhibitor therapy in MF can be attributed to depletion of a subpopulation of MF progenitors.
Abstract
Dysregulation of Janus kinase (JAK)–signal transducer and activator of transcription signaling is central to the pathogenesis of myelofibrosis (MF). JAK2 inhibitor therapy in MF patients results in a rapid reduction of the degree of splenomegaly, yet the mechanism underlying this effect remains unknown. The in vitro treatment of splenic and peripheral blood MF CD34+ cells with the JAK1/2/3 inhibitor, AZD1480, reduced the absolute number of CD34+, CD34+CD90+, and CD34+CXCR4+ cells as well as assayable hematopoietic progenitor cells (HPCs) irrespective of the JAK2 and calreticulin mutational status. Furthermore, AZD1480 treatment resulted in only a modest reduction in the proportion of HPCs that were JAK2V617F+ or had a chromosomal abnormality. To study the effect of the drug on MF stem cells (MF-SCs), splenic CD34+ cells were treated with AZD1480 and transplanted into immunodeficient mice. JAK2 inhibitor therapy did not affect the degree of human cell chimerism or the proportion of malignant donor cells. These data indicate that JAK2 inhibitor treatment affects a subpopulation of MF-HPCs, while sparing another HPC subpopulation as well as MF-SCs. This pattern of activity might account for the reduction in spleen size observed with JAK2 inhibitor therapy as well as the rapid increase in spleen size observed frequently with its discontinuation.
Introduction
Primary myelofibrosis (PMF) as well as MF that develops during the course of essential thrombocythemia (ET) or polycythemia vera (PV; post-ET or PV MF) are characterized by the constitutive mobilization of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) and the establishment of extramedullary hematopoiesis.1-5 MF originates at the level of the HSC6 and is associated with a number of acquired mutations that activate Janus kinase–signal transducer and activator of transcription (JAK-STAT) signaling.7-22 Several JAK1/2 inhibitors including ruxolitinib have been shown to reduce spleen sizes in MF patients independent of their JAK2 mutational status.23-26
To date, the mechanism underlying the reduction of splenomegaly observed with JAK2 inhibitor therapy remains the subject of speculation. Recently, we documented that splenic (SP) MF-stem cells (MF-SCs) and those in the peripheral blood (PB) have distinct properties,27 suggesting that their responses to JAK2 inhibitors might differ. We, therefore, explored the effect of a JAK1/2/3 inhibitor, AZD1480, on paired SP and PB MF-HPCs and MF-SCs.
Materials and methods
Patient specimens and cell preparation
Surgically removed spleens were obtained from patients with advanced forms of MF requiring therapeutic splenectomy. All patients provided signed informed consent as approved by the institutional review board of the Icahn School of Medicine at Mount Sinai (ISMMS) and in accordance with the Declaration of Helsinki. Single-cell suspensions were prepared according to the method of Barosi and coworkers28 from the spleens of 12 patients with PMF or PV/ET-related MF who fulfilled the World Health Organization (WHO) diagnostic criteria29 (Table 1). PB was collected from these patients, except patients 11 and 12. Cord blood (CB) collections were provided by the New York Blood Center. Mononuclear cells (MNCs) were isolated by density gradient centrifugation using Ficoll-Paque (GE Healthcare Life Sciences). CD34+ cells were selected using a CD34+ cell selection kit (StemCell Technologies). CD34+ cells with a purity of ≥90% as analyzed using a FACSCanto Flow Cytometer (BD) were used in each experiment.
Clinical characteristics of MF patients studied
| Patient* . | Sex . | Age, y . | Diagnosis . | JAK2V617F allele burden, %† . | Chromosomal abnormalities (%)* . | CALR status . | Indication for splenectomy . |
|---|---|---|---|---|---|---|---|
| 1 | M | 65 | PMF | 0 | None | WT | Cytopenias |
| 2 | F | 70 | Post-PV MF | 85 | None | N/A | Prior to transplant |
| 3 | M | 74 | PMF | 0 | Unknown | Unknown | Cytopenias |
| 4 | F | 75 | PMF | 62 | t(3;3)(q21;q26) (73) del(20)(q11.2q13.2) (27) | N/A | Painful splenomegaly |
| 5 | M | 64 | PMF | 0 | None | WT | Cytopenias |
| 6 | M | 79 | PMF | 2.4 | del(20)(q11.1q13.3) (97) | WT | Cytopenias |
| 7 | M | 67 | PMF | 0 | t(8;12)(q13;q15) (100) | 46-bp deletion | Cytopenias |
| 8 | M | 66 | PMF | 0 | None | WT | Prior to transplant |
| 9 | F | 45 | Post-PV MF | 90 | del(20)(q11.1q13.3) (50) | N/A | Cytopenias |
| 10 | F | 64 | Post-PV MF | 78 | +der(9)t(1;9)(q12;q12) (35) del(20)(q11.2q13.1) (41) | N/A | Portal hypertension |
| 11 | F | 58 | Post-PV MF | 43 | del(13)(q12q22) (92) | N/A | Prior to transplant |
| 12 | M | 73 | PMF | 85.1 | Del(13)(q12q14) (20) | N/A | Palliation of advanced MF |
| Patient* . | Sex . | Age, y . | Diagnosis . | JAK2V617F allele burden, %† . | Chromosomal abnormalities (%)* . | CALR status . | Indication for splenectomy . |
|---|---|---|---|---|---|---|---|
| 1 | M | 65 | PMF | 0 | None | WT | Cytopenias |
| 2 | F | 70 | Post-PV MF | 85 | None | N/A | Prior to transplant |
| 3 | M | 74 | PMF | 0 | Unknown | Unknown | Cytopenias |
| 4 | F | 75 | PMF | 62 | t(3;3)(q21;q26) (73) del(20)(q11.2q13.2) (27) | N/A | Painful splenomegaly |
| 5 | M | 64 | PMF | 0 | None | WT | Cytopenias |
| 6 | M | 79 | PMF | 2.4 | del(20)(q11.1q13.3) (97) | WT | Cytopenias |
| 7 | M | 67 | PMF | 0 | t(8;12)(q13;q15) (100) | 46-bp deletion | Cytopenias |
| 8 | M | 66 | PMF | 0 | None | WT | Prior to transplant |
| 9 | F | 45 | Post-PV MF | 90 | del(20)(q11.1q13.3) (50) | N/A | Cytopenias |
| 10 | F | 64 | Post-PV MF | 78 | +der(9)t(1;9)(q12;q12) (35) del(20)(q11.2q13.1) (41) | N/A | Portal hypertension |
| 11 | F | 58 | Post-PV MF | 43 | del(13)(q12q22) (92) | N/A | Prior to transplant |
| 12 | M | 73 | PMF | 85.1 | Del(13)(q12q14) (20) | N/A | Palliation of advanced MF |
WT, wild-type.
Indicates the percentage of primary MF spleen cells having the given chromosomal abnormality.
Indicates the granulocytic JAK2V617F allele burden as detected by real-time allele specific PCR assay.
JAK2V617F, CALR mutational analyses, and cytogenetic and FISH analyses
The JAK2V617F status of each MF patient was determined by analyzing PB granulocytes using a previously described real-time allele-specific polymerase chain reaction (AS-PCR) assay.30,31 Mutational analysis of calreticulin (CALR) was performed by DNA resequencing regions of known mutations in CALR as previously described.22 Cytogenetic and fluorescence in situ hybridization (FISH) analyses were performed as previously described.32,33 The JAK2V617F allele burden, CALR status, and the presence of a marker chromosomal abnormality in each patient is shown in Table 1.
Treatment of MF and CB CD34+ cells with AZD1480
SP or CB CD34+ cells (1 × 105/mL) were cultured in Iscove modified Dulbecco medium (IMDM; Lonza) containing 30% fetal bovine serum (FBS; HyClone Laboratories) supplemented with 100 ng/mL stem cell factor (SCF), 100 ng/mL feline McDonough sarcoma-like tyrosine kinase 3 ligand (FL), 100 ng/mL thrombopoietin (TPO), and 50 ng/mL interleukin-3 (IL-3; Amgen) in a humidified incubator maintained at 37°C with 5% CO2. After 16 hours, cells were exposed to AZ1480 (50 nM, 150 nM, 300 nM, and 500 nM, gift of AstraZeneca) for 3 days. In addition, cultures containing cytokines alone were performed in parallel. The determined optimal dose of AZD1480 identified (150 nM) was then used in subsequent investigations.
To determine whether AZD1480 affected normal HPCs, CB CD34+ cells (1 × 105/mL) were also cultured and treated with AZD1480 in an identical fashion.
Flow cytometric analysis of CD34+ cells
After 3 days, the cultured cells were labeled with anti–human CD34–allophycocyanin (APC), anti-human CD90–fluorescein isothiocyanate (FITC), and anti-human CXC chemokine receptor 4 (CXCR4)–phycoerythrin (PE; clone 12G5) monoclonal antibodies (mAbs; BD Biosciences). Each analysis was paired with a corresponding matched isotype control. Immediately before flow cytometric analysis, 1 μg/mL propidium iodide (PI; Sigma-Aldrich) was added to exclude nonviable cells. Cells were analyzed flow cytometrically, and at least 10 000 viable events were acquired from each sample (FACSDiva software version 6.1.2; BD).
The percentage of CD34+ cells undergoing apoptosis was determined using the Annexin V-FITC Apoptosis Detection kit (BD Biosciences). CD34+Annexin V+PI− cells were regarded as cells undergoing apoptosis.
Cells were also labeled with anti-CD34-APC, anti-Ki67-Alexa 700 (BD Biosciences), and PI and analyzed flow cytometrically. Ki67−PI− cells were considered to be in G0, Ki67+PI− cells in G1, and Ki67+PI+ cells in S/G2/M phase.
HPC assays
Cultured cells were assayed for HPCs in semisolid media.34 Briefly, 2500 cells were plated in duplicate culture dishes containing 1 mL of IMDM with 1.1% methylcellulose, 30% FBS, 5 × 10−5 mol/L 2-mercaptoethanol (StemCell Technologies), to which SCF, TPO, IL-3, IL-6, granulocyte macrophage colony-stimulating factor (GM-CSF), each at 100 ng/mL, and 4 U/mL erythropoietin (Amgen) were added. Colonies were enumerated after 12 to 14 days of incubation. Individual colonies were plucked and analyzed for the presence of JAK2V617F using a nested AS-PCR34 and the presence of a marker chromosomal abnormality using FISH as described above in “JAK2V617F, CALR mutational analyses, and cytogenetic and FISH analyses.”32 The percentage of JAK2V617F-positive colonies or colonies having chromosomal abnormalities was then determined.
NSG repopulating cell assay
Nonobese diabetic (NOD)/severe combined immune deficiency (SCID)/IL2Rγnull (NSG) mice were purchased from The Jackson Laboratory. All experiments were approved by the animal care committee of the ISMMS. The identical number of MF SP CD34+ cells (2.5 × 105 for patient 1 or 8-20 × 105 for patients 6-10) was cultured in the presence of cytokines alone or cytokines plus AZD1480 (150 nM) for 3 days. The starting number of SP CD34+ cells for each MF patient to determine the effect of AZD1480 treatment on SP MF-SCs was selected based on the primary MF CD34+ cell engraftment rate of each patient from previous experiments (data not shown). The total number of cells generated after culture were then transplanted via the tail vein into 8- to 9-week-old sublethally irradiated (240 cGy) NSG mice. Four or 6 months after the transplantation, the mice were killed and cells were recovered from the bone marrow (BM) of femurs, tibias, humeri, and spleens of the recipient mice. The presence of hCD45+ cells was determined by mAb staining and analyzed flow cytometrically. Cells obtained from mice not receiving human cells were analyzed in a similar fashion to exclude the possibility of false-positive immunostaining. The mAbs used did not cross-react with murine cells. We considered human engraftment to have occurred if human CD45+ cells were present at ≥0.1% of the nucleated cells in a murine BM or spleen.
Human CD45+ cells (purity ≥ 95%) in the BM and spleens of the recipient mice were isolated by mAb staining and cell sorting using an Aria BSL2 (BD). The JAK2V617F allele burden of the hCD45+ cells from the mice receiving SP CD34+ cells from 2 patients with a granulocyte JAK2V617F allele burden of 90% and 78%, respectively, was determined by real-time AS-PCR as previously described.30,31 Selected hCD45+ cells from the mice receiving SP CD34+ cells from 3 patients with marker chromosomal abnormalities were analyzed using FISH.32,33
Western blotting
SP MF and CB CD34+ cells (1 × 105/mL) were cultured in the presence of cytokines alone or cytokines + AZD1480 for 4 hours. Cells were then lysed with radioimmunoprecipitation assay (RIPA) buffer with a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Pierce Biotechnologies). Protein was resolved using 4% to 12% Nu-PAGE Bis-Tris gels (Invitrogen) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Membranes were blocked, incubated with indicated primary antibodies (Abs), and horseradish peroxidase (HRP)–conjugated secondary Abs (Cell Signaling Technologies), and detected using an enhanced chemiluminescence system (Pierce Biotechnology).
Statistical analysis
Results are reported as the mean ± standard deviation (SD) of data obtained from 3 to 5 individual experiments. Statistical significance was determined using 2-tailed Student t tests. All P values were 2-sided, and P values <.05 were considered significant.
Results
SP MF CD34+ cells respond to AZD1480 treatment in a dose-dependent fashion
AZD1480 was capable of suppressing the proliferation of SP MF CD34+ cells in a dose-dependent fashion (Figure 1A-B). By contrast, normal CD34+ cells were less responsive to the effects of AZD1480 (Figure 1A-B). AZD1480 at 150 nM was able to inhibit SP MF CD34+ cells by 55.0% ± 12.5%, whereas was only able to reduce normal CD34+ cells by 28.6% ± 13.50%. AZD1480 (150 nM) was then selected to further investigate the effect of AZD1480 on MF-HPCs and MF-SCs. Moreover, as shown in Figure 1C-E and G, the number of total CB cells, CD34+, CD34+CD90+ cells, and assayable HPCs in cultures exposed to AZD1480 were minimally affected as compared with cells exposed to cytokines alone. The absolute number of CB CD34+CXCR4+ cells was not affected by treatment with AZD1480 (Figure 1F). These findings suggest that AZD1480 treatment at the doses tested exhibited limited effects on normal CD34+ cells.
SP MF CD34+ cells responded to the treatment of AZD1480 in a dose-dependent fashion, whereas normal CB CD34+ cells were less responsive to the effects of AZD1480. (A-B) SP MF and CB CD34+ cells were cultured in the presence of AZD1480 at doses ranging from 50 nM to 500 nM for 3 days. After treatment, cells were analyzed by mAb staining and flow cytometry. The percentage of total cells (A) and CD34+ cells (B) generated in the cultures exposed to cytokines plus AZD1480 relative to those generated in the cultures exposed to cytokines alone is shown. *P < .05; **P < .01; ***P < .001 vs cytokines alone for SP MF or CB CD34+ cells, respectively (n = 4). (C-G) AZD1480 treatment did not affect normal CD34+ cells. The reduction in the absolute total number of CB cells (C), CD34+ cells (D), CD34+CD90+ cells (E), CD34+CXCR4+ cells (F), and assayable HPCs (G) in cultures exposed to cytokines plus AZD1480 compared with that detected in cultures exposed to cytokines alone was not statistically different. P all >.05 (n = 5).
SP MF CD34+ cells responded to the treatment of AZD1480 in a dose-dependent fashion, whereas normal CB CD34+ cells were less responsive to the effects of AZD1480. (A-B) SP MF and CB CD34+ cells were cultured in the presence of AZD1480 at doses ranging from 50 nM to 500 nM for 3 days. After treatment, cells were analyzed by mAb staining and flow cytometry. The percentage of total cells (A) and CD34+ cells (B) generated in the cultures exposed to cytokines plus AZD1480 relative to those generated in the cultures exposed to cytokines alone is shown. *P < .05; **P < .01; ***P < .001 vs cytokines alone for SP MF or CB CD34+ cells, respectively (n = 4). (C-G) AZD1480 treatment did not affect normal CD34+ cells. The reduction in the absolute total number of CB cells (C), CD34+ cells (D), CD34+CD90+ cells (E), CD34+CXCR4+ cells (F), and assayable HPCs (G) in cultures exposed to cytokines plus AZD1480 compared with that detected in cultures exposed to cytokines alone was not statistically different. P all >.05 (n = 5).
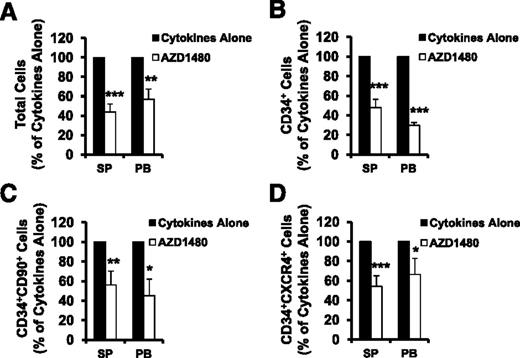
Effects of AZD1480 treatment on MF SP or PB CD34+ cells and HPCs
SP or PB MF CD34+ cells were treated with cytokines alone or cytokines plus AZD1480 (150 nM) for 3 days and were phenotypically characterized. The treatment of SP or PB MF CD34+ cells (1 × 105) with AZD1480 resulted in reductions in the absolute number of total cells, CD34+ cells, CD34+CD90+ cells as well as CD34+CXCR4+ cells as compared with the equal numbers of SP or PB MF CD34+ cells incubated with cytokines alone (supplemental Figure 1, see supplemental Data available on the Blood Web site). Next, we evaluated the response of SP or PB MF CD34+ cells to the action of this JAK2 inhibitor using the ratios of the absolute number of various cells generated in the cultures of SP or PB MF CD34+ cells treated with cytokines plus AZD1480 to those generated in the cultures treated with cytokines alone. As shown in Figure 2, the treatment of SP or PB MF CD34+ cells with 150 nM AZD1480 decreased the total number of cells, CD34+, CD34+CD90+, and CD34+CXCR4+ cells to a significant degree as compared with cells cultured with cytokines alone (P < .05 at least). Furthermore, the treatment of SP or PB MF CD34+ cells with AZD1480 resulted in equal reductions in the number of these cells (P all >.05). These findings suggest that both SP and PB MF CD34+ cells respond to a similar degree to AZD1480 treatment.
AZD1480 treatment inhibited both SP and PB MF CD34+ cells. SP or PB MF CD34+ cells were treated with cytokines alone or cytokines plus AZD1480 (150 nM) for 3 days and were phenotypically characterized. The absolute number of total cells (A), CD34+ cells (B), CD34+CD90+ (C), and CD34+CXCR4+ cells (D) generated following the culture of SP or PB CD34+ cells exposed to cytokines plus AZD1480 relative to that generated in the culture exposed to cytokines alone is shown.*P < .05; **P < .01; ***P < .001 (n = 10).
AZD1480 treatment inhibited both SP and PB MF CD34+ cells. SP or PB MF CD34+ cells were treated with cytokines alone or cytokines plus AZD1480 (150 nM) for 3 days and were phenotypically characterized. The absolute number of total cells (A), CD34+ cells (B), CD34+CD90+ (C), and CD34+CXCR4+ cells (D) generated following the culture of SP or PB CD34+ cells exposed to cytokines plus AZD1480 relative to that generated in the culture exposed to cytokines alone is shown.*P < .05; **P < .01; ***P < .001 (n = 10).
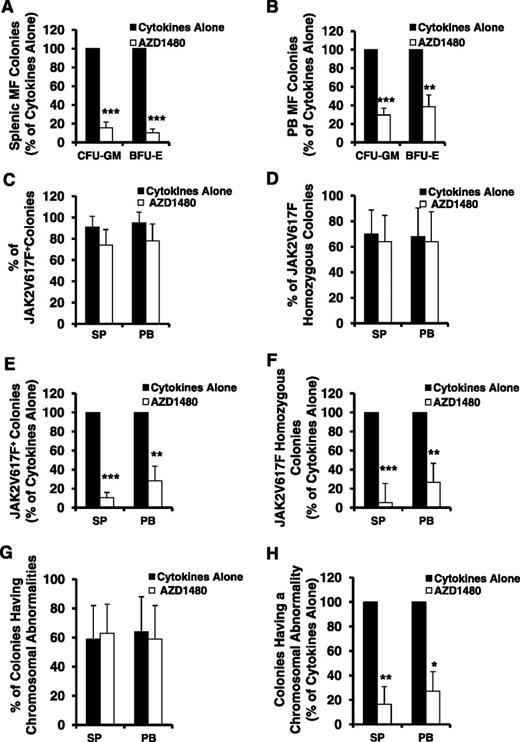
We next assessed the inhibitory effect of AZD1480 on the numbers of both SP and PB MF-HPCs. As shown in Figure 3A-B, the number of assayable HPCs, colony-forming unit–granulocyte-macrophage (CFU-GM), and burst-forming unit-erythrocyte (BFU-E), generated in the cultures of SP or PB MF CD34+ cells exposed to cytokines plus AZD1480 was significantly decreased as compared with that generated in the cultures of SP or PB MF CD34+ cells with cytokines alone (SP: CFU-GM and BFU-E, P both <.001; PB: CFU-GM, P < .001; BFU-E, P < .01). Moreover, a similar level of reduction in the number of CFU-GM and BFU-E was observed following the treatment of equal number of either SP or PB MF CD34+ cells with AZD1480 (P both >.05). These findings suggest that AZD1480 is equally capable of inhibiting both SP and PB MF-HPCs.
AZD1480 treatment resulted in a reduction in both SP and PB MF HPCs. The inhibitory effect of AZD1480 on both SP and PB MF HPCs was assessed by determining a proportion of colonies generated in cultures of SP or PB MF CD34+ cells treated with cytokines or cytokines plus AZD 1480 in semisolid media. (A-B) The percentage of CFU-GM and BFU-E generated in the cultures of SP (A) or paired PB (B) MF CD34+ cells treated with cytokines plus AZD1480 relative to that generated in the cultures with cytokines alone. **P < .01; ***P < .001 (n = 10). (C-D) The percentage of JAK2V617F-positive (C) and JAK2V617F homozygous (D) colonies generated in cultures of SP or PB MF CD34+ cells containing cytokines plus AZD1480 or cytokines alone. Twenty-one to 35 individual colonies were randomly plucked and genotyped for the presence of JAK2V617F in each paired SP or PB MF CD34+ cell specimen cultured under each experimental condition from each of 4 patients. (E-F) The percentage of the absolute number of JAK2V617F-positive (E) and JAK2V617F homozygous colonies (F) generated in cultures of SP or PB MF CD34+ cells treated with cytokines plus AZD1480 relative to that generated in cultures treated with cytokines alone. **P < .01; ***P < .001 (n = 4). (G) The percentage of colonies having a chromosomal abnormality generated in cultures of SP or PB MF CD34+ cells treated with cytokines plus AZD1480 or cytokines alone. Twenty-one to 35 individual colonies were randomly plucked and genotyped for the presence of a chromosomal abnormality in each paired SP or PB MF CD34+ cell specimen cultured under each experimental condition from each of 4 patients. (H) The percentage of colonies having a chromosomal abnormality generated in cultures of SP or PB MF CD34+ cells treated with cytokines plus AZD1480 relative to that generated in cultures treated with cytokines alone. **P < .01; ***P < .001 (n = 4).
AZD1480 treatment resulted in a reduction in both SP and PB MF HPCs. The inhibitory effect of AZD1480 on both SP and PB MF HPCs was assessed by determining a proportion of colonies generated in cultures of SP or PB MF CD34+ cells treated with cytokines or cytokines plus AZD 1480 in semisolid media. (A-B) The percentage of CFU-GM and BFU-E generated in the cultures of SP (A) or paired PB (B) MF CD34+ cells treated with cytokines plus AZD1480 relative to that generated in the cultures with cytokines alone. **P < .01; ***P < .001 (n = 10). (C-D) The percentage of JAK2V617F-positive (C) and JAK2V617F homozygous (D) colonies generated in cultures of SP or PB MF CD34+ cells containing cytokines plus AZD1480 or cytokines alone. Twenty-one to 35 individual colonies were randomly plucked and genotyped for the presence of JAK2V617F in each paired SP or PB MF CD34+ cell specimen cultured under each experimental condition from each of 4 patients. (E-F) The percentage of the absolute number of JAK2V617F-positive (E) and JAK2V617F homozygous colonies (F) generated in cultures of SP or PB MF CD34+ cells treated with cytokines plus AZD1480 relative to that generated in cultures treated with cytokines alone. **P < .01; ***P < .001 (n = 4). (G) The percentage of colonies having a chromosomal abnormality generated in cultures of SP or PB MF CD34+ cells treated with cytokines plus AZD1480 or cytokines alone. Twenty-one to 35 individual colonies were randomly plucked and genotyped for the presence of a chromosomal abnormality in each paired SP or PB MF CD34+ cell specimen cultured under each experimental condition from each of 4 patients. (H) The percentage of colonies having a chromosomal abnormality generated in cultures of SP or PB MF CD34+ cells treated with cytokines plus AZD1480 relative to that generated in cultures treated with cytokines alone. **P < .01; ***P < .001 (n = 4).
Individual colonies from 4 JAK2V617F-positive MF patients (JAK2V617F allele burden: 62%-90%) treated with cytokines alone or cytokines plus AZD1480 were plucked and analyzed for the JAK2V617F.34 As shown in Figure 3C-D, the treatment of JAK2V617F-positive SP or PB MF CD34+ cells with AZD1480 resulted in similarly modest reductions in the percentage of JAK2V617F-positive colonies and JAK2V617F homozygous colonies. However, because the treatment of both SP and PB MF CD34+ cells with AZD1480 resulted in a significant reduction in the number of CFU-GM and BFU-E (Figure 3A-B), as shown in Figure 3E-F, a significant reduction in absolute numbers of JAK2V617F-positive and homozygous colonies (SP: P both <.001; PB: P both <.01) were observed.
Similarly, although the percentage of the colonies having marker chromosomal abnormalities in cultures of either SP or PB MF CD34+ cells exposed to cytokines plus AZD1480 was nearly equal to that in cultures exposed to cytokines alone (Figure 3G), a significant reduction in the absolute number of colonies possessing the marker chromosomal abnormality was observed following exposure to AZD1480 (Figure 3H). These data suggest that AZD1480 treatment is able to impair the in vitro generation of MF-HPCs and thereby leads to a reduction but not elimination of the number of malignant HPC.
Effects of AZD1480 on MF CD34+ cells are independent of JAK2V617F status
The clinical responses of patients with MF to ruxolitinib occur independent of their JAK2 mutational status.23,24 We therefore investigated whether the action of AZD1480 on both SP and PB CD34+ cells differed when JAK2V617F-negative and JAK2V617F-positive CD34+ cells were studied. As shown in Figure 4A-E, the numbers of total cells, CD34+, CD34+CD90+, CD34+CXCR4+ cells, and assayable HPCs were reduced to a similar degree when JAK2V617F-positive or JAK2V617F-negative SP MF CD34+ cells were exposed to AZD1480 (P all >.05). Similar patterns of response were observed when either JAK2V617F-positive or JAK2V617F-negative PB MF CD34+ cells were treated with AZD1480 (Figure 4A-D,F; P all >.05). Moreover, as shown in supplemental Figure 2, AZD1480 treatment significantly inhibited both SP and PB CD34+, CD34+CD90+, CD34+CXCR4+ cell, and HPC generation from patient 7 having a CALR mutation (46-bp deletion). These findings suggest that AZD1480 has similar effects on JAK2V617F-positive and JAK2V617F-negative as well as CALR mutant-positive SP and PB MF-HPCs.
Inhibitory effects of AZD1480 on SP and PB MF CD34+ cells are independent of JAK2V617F status. (A-D) The percentage of the absolute number of total cells (A), CD34+ cells (B), CD34+CD90+ (C), and CD34+CXCR4+ cells (D) generated in cultures of either JAK2V617F-positive or -negative SP or PB MF CD34+ cells exposed to cytokines plus AZD1480 relative to the number generated in cultures exposed to cytokines alone. The numbers of total cell, CD34+, CD34+CD90+, CD34+CXCR4+ cells were equally reduced in the cultures of either JAK2V617F-positive or -negative SP or PB MF CD34+ cells exposed to AZD1480. (E-F) A similar reduction in the number of assayable HPCs (CFU-GM, BFU-E) was achieved by the treatment of either JAK2V617F-positive or -negative SP (E) or PB (F) MF CD34+ cells with AZD1480. P all >.05. JAK2V617F-positive and JAK2V617F-negative (each n = 5).
Inhibitory effects of AZD1480 on SP and PB MF CD34+ cells are independent of JAK2V617F status. (A-D) The percentage of the absolute number of total cells (A), CD34+ cells (B), CD34+CD90+ (C), and CD34+CXCR4+ cells (D) generated in cultures of either JAK2V617F-positive or -negative SP or PB MF CD34+ cells exposed to cytokines plus AZD1480 relative to the number generated in cultures exposed to cytokines alone. The numbers of total cell, CD34+, CD34+CD90+, CD34+CXCR4+ cells were equally reduced in the cultures of either JAK2V617F-positive or -negative SP or PB MF CD34+ cells exposed to AZD1480. (E-F) A similar reduction in the number of assayable HPCs (CFU-GM, BFU-E) was achieved by the treatment of either JAK2V617F-positive or -negative SP (E) or PB (F) MF CD34+ cells with AZD1480. P all >.05. JAK2V617F-positive and JAK2V617F-negative (each n = 5).
Effects of treatment with AZD1480 on MF-NRCs
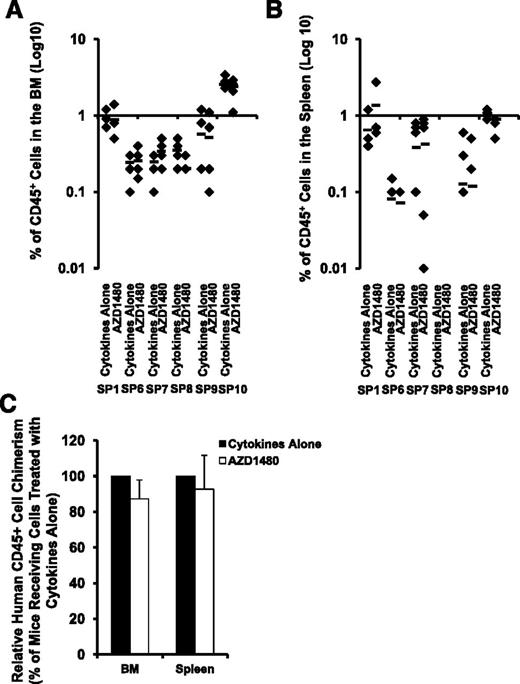
We examined the effect of AZD1480 on SP MF-SCs by transplanting SP MF CD34+ cells from 6 patients treated with cytokines alone or cytokines plus AZD1480 into NSG mice. Because the engraftment capacity of SP CD34+ cells from each individual patient differs greatly, we first performed statistical analyses of the degree of human cell chimerism (percentage of hCD45+ cells) achieved in mice receiving total cells generated in cultures of equal number of SP MF CD34+ cells exposed to cytokines alone and cytokines plus AZD1480 from each individual patient. As shown in Figure 5A-B, 4 or 6 months after the transplantation, similar numbers of hCD45+ cells were detected in both the BM and spleens of recipient mice receiving SP MF CD34+ cells treated with cytokines alone or cytokines plus AZD1480 (each individual patient: P all >.05). Next, we determined the effect of AZD1480 on SP MF NSG repopulating cells (NRCs) by analyzing the relative hCD45+ cell chimerism achieved in mice receiving cells treated with cytokine plus AZD1480 to that achieved in mice receiving cells treated with cytokines alone. Again, as shown in Figure 5C, AZD1480 treatment did not result in a reduction in hCD45+ cell chimerism achieved in either the BM or spleens of the recipient mice. Furthermore, hCD45+ cells were harvested and isolated from the BM and spleen of these mice and analyzed for JAK2V617F or a marker chromosomal abnormality. Modest reductions (9% and 7%) in JAK2V617F allele burden of hCD45+ cells were observed in the BM and the spleen of mice transplanted with SP10 CD34+ cells treated with AZD1480, respectively, as compared with that observed in mice transplanted with SP10 CD34+ cells treated with cytokines alone (Table 2). As also shown in Table 2, similar number of hCD45+ cells isolated from both the marrow and spleen of mice transplanted with SP7 CD34+ cells contained the marker chromosomal abnormality. Moreover, all of the human BM CD45+ cells isolated from the mice transplanted with SP8 SP CD34+ cells treated with either cytokines alone or cytokines plus AZD1480 possessed the marker chromosomal abnormality (Table 2). These findings suggest that JAK2 inhibitor treatment does not affect malignant SP MF-SCs. Surprisingly, none of the hCD45+ cells in the BM and the spleen of mice transplanted with SP9 were observed to harbor either JAK2V617F, or the chromosomal abnormalities (Table 2), that were present in primary CD34+ cells, indicating that either normal NRCs or malignant NRCs which lacked JAK2V617F or the marker chromosomal abnormalities were responsible for the donor cell chimerism in this case.
Effects of treatment with AZD1480 on MF NRCs. (A-B) The degree of SP MF hematopoietic cell engraftment is indicated by the percentage of hCD45+ cells detected within the marrow (A) and spleen (B) of the NSG mice. P all >.05 for each patient studied (n = 6). Horizontal bars indicate the mean of percentage of hCD45+ cells in the BM and spleen of NSG mice. (C) Relative hCD45+ cell chimerism achieved in the BM and spleens of mice receiving SP MF CD34+ cells treated with cytokine plus AZD1480 to that achieved in mice receiving cells treated with cytokines alone. BM and spleen: P > .05 (n = 6).
Effects of treatment with AZD1480 on MF NRCs. (A-B) The degree of SP MF hematopoietic cell engraftment is indicated by the percentage of hCD45+ cells detected within the marrow (A) and spleen (B) of the NSG mice. P all >.05 for each patient studied (n = 6). Horizontal bars indicate the mean of percentage of hCD45+ cells in the BM and spleen of NSG mice. (C) Relative hCD45+ cell chimerism achieved in the BM and spleens of mice receiving SP MF CD34+ cells treated with cytokine plus AZD1480 to that achieved in mice receiving cells treated with cytokines alone. BM and spleen: P > .05 (n = 6).
The presence of a marker chromosomal abnormality or JAK2V617F status of SP MF-NRCs following the treatment with AZD1480
| Patient no. . | NRCs assayed . | |||
|---|---|---|---|---|
| CD34+ cells treated with cytokines alone, % . | CD34+ cells treated with cytokines + AZD1480, % . | |||
| JAK2V617F in BM hCD45+ cells | JAK2V617F in spleen hCD45+ cells | JAK2V617F in BM hCD45+ cells | JAK2V617F in spleen hCD45+ cells | |
| SP9 | 0 | 0 | 0 | 0 |
| SP10 | 88 | 72 | 79 | 65 |
| BM hCD45+ cells with chromosomal abnormality | Spleen hCD45+ cells with chromosomal abnormality | BM hCD45+ cells with chromosomal abnormality | Spleen hCD45+ cells with chromosomal abnormality | |
| SP7 | 81 | 96 | 98 | 100 |
| SP8 | 100 | 100 | 100 | 100 |
| SP9 | 0 | 0 | 0 | 0 |
| Patient no. . | NRCs assayed . | |||
|---|---|---|---|---|
| CD34+ cells treated with cytokines alone, % . | CD34+ cells treated with cytokines + AZD1480, % . | |||
| JAK2V617F in BM hCD45+ cells | JAK2V617F in spleen hCD45+ cells | JAK2V617F in BM hCD45+ cells | JAK2V617F in spleen hCD45+ cells | |
| SP9 | 0 | 0 | 0 | 0 |
| SP10 | 88 | 72 | 79 | 65 |
| BM hCD45+ cells with chromosomal abnormality | Spleen hCD45+ cells with chromosomal abnormality | BM hCD45+ cells with chromosomal abnormality | Spleen hCD45+ cells with chromosomal abnormality | |
| SP7 | 81 | 96 | 98 | 100 |
| SP8 | 100 | 100 | 100 | 100 |
| SP9 | 0 | 0 | 0 | 0 |
Mechanisms underlying the inhibitory effects of AZD1480 treatment on MF CD34+ cells
Inhibition of JAK-STAT activity in CD34+ cells from MF patients
We determined phosphorylated (p) JAK2, pSTAT3, and pSTAT5 levels in primary SP MF CD34+ cells from 1 JAK2V617F-negative and CALR mutant-positive patient (SP 7), 1 JAK2V617F-negative and CALR-negative patient (SP 8), and 2 JAK2V617F-positive and CALR-negative patient specimens (SP 11, 12) using western blotting. Sufficient numbers of cells were only available from spleens of patients 11 and 12, which were used to assess the inhibitory effects of AZD1480 treatment on CD34+ cells and HPCs (supplemental Figure 3). Although primary JAK2V617F-positive SP MF CD34+ cells were characterized by increased levels of pJAK2 as compared with primary JAK2V617F-negative/CALR mutant-positive MF CD34+ cells and normal CB CD34+ cells, both primary JAK2V617F-positive and JAK2V617F-negative/CALR mutant-positive MF CD34+ cells showed similar increased levels of pSTAT3 and pSTAT5 compared with normal CB CD34+ cells, suggesting that MF CD34+ cells are characterized by enhanced JAK-STAT activity, irrespective of their JAKV617F and CALR status (Figure 6A). Moreover, AZD1480 treatment resulted in inhibition of pJAK2 and/or pSTAT3/5 levels to varying degrees in both JAK2V617F-positive and JAK2V617F-negative/CALR mutant-positive MF CD34+ cells. By contrast, limited inhibition of JAK-STAT activity was observed following the treatment of normal CB CD34+ cells (Figure 6B). In addition, the inhibition of JAK-STAT activity in MF CD34+ cells was sustained for the entire 48-hour test period (data not shown).
Mechanisms underlying the inhibitory effects of AZD1480 treatment on MF CD34+ cells. (A) pJAK2, pSTAT3, and pSTAT5 levels in 1 JAK2V617F-negative and CALR mutant-positive (SP 7), 1 JAK2V617F-negative and CALR negative (SP 8) and 2 JAK2V617F-positive (SP 11, 12) primary SP MF CD34+ cells were determined using western blotting. Both primary JAK2V617F-positive and -negative/CALR mutant-positive MF CD34+ cells showed similar degrees of enhanced JAK-STAT activity as compared with normal CB CD34+ cells. (B) pJAK2, pSTAT3, and pSTAT5 levels in SP MF CD34+ cells following the treatment with AZD1480 for 4 hours were determined using western blotting. AZD1480 treatment resulted in the inhibition of pJAK2 and/or pSTAT3/5 levels to varying degrees in both JAK2V617F-positive and -negative/CALR mutant-positive MF CD34+ cells, whereas, only limited inhibition of JAK-STAT activity was observed with normal CB CD34+ cells. (C) AZD1480 treatment induced apoptosis of SP MF CD34+ cells. Two days after the culture, the percentage of CD34+Annexin V+PI− cells was greater in SP MF CD34+ cells treated with cytokines plus AZD1480 as compared with that observed in SP MF CD34+ cells treated with cytokines alone (n = 10) or CB CD34+ cells treated with cytokines plus AZD1480 (n = 5) . ***P < .001; **P < .01. (D) AZD1480 treatment induced G0/G1 cell-cycle arrest in MF CD34+ cells. A significant accumulation of CD34+ cells in the G0 phase and a significant decrease of CD34+ cells in the G1 phase was observed in SP MF CD34+ cell (n = 10) treated with 150 nM AZD1480 for 3 days. By contrast, AZD1480 treatment did not alter the cell-cycle status of CB CD34+ cells (n = 5). *P < .05; #P = .07.
Mechanisms underlying the inhibitory effects of AZD1480 treatment on MF CD34+ cells. (A) pJAK2, pSTAT3, and pSTAT5 levels in 1 JAK2V617F-negative and CALR mutant-positive (SP 7), 1 JAK2V617F-negative and CALR negative (SP 8) and 2 JAK2V617F-positive (SP 11, 12) primary SP MF CD34+ cells were determined using western blotting. Both primary JAK2V617F-positive and -negative/CALR mutant-positive MF CD34+ cells showed similar degrees of enhanced JAK-STAT activity as compared with normal CB CD34+ cells. (B) pJAK2, pSTAT3, and pSTAT5 levels in SP MF CD34+ cells following the treatment with AZD1480 for 4 hours were determined using western blotting. AZD1480 treatment resulted in the inhibition of pJAK2 and/or pSTAT3/5 levels to varying degrees in both JAK2V617F-positive and -negative/CALR mutant-positive MF CD34+ cells, whereas, only limited inhibition of JAK-STAT activity was observed with normal CB CD34+ cells. (C) AZD1480 treatment induced apoptosis of SP MF CD34+ cells. Two days after the culture, the percentage of CD34+Annexin V+PI− cells was greater in SP MF CD34+ cells treated with cytokines plus AZD1480 as compared with that observed in SP MF CD34+ cells treated with cytokines alone (n = 10) or CB CD34+ cells treated with cytokines plus AZD1480 (n = 5) . ***P < .001; **P < .01. (D) AZD1480 treatment induced G0/G1 cell-cycle arrest in MF CD34+ cells. A significant accumulation of CD34+ cells in the G0 phase and a significant decrease of CD34+ cells in the G1 phase was observed in SP MF CD34+ cell (n = 10) treated with 150 nM AZD1480 for 3 days. By contrast, AZD1480 treatment did not alter the cell-cycle status of CB CD34+ cells (n = 5). *P < .05; #P = .07.
AZD1480 treatment induces the apoptosis of MF CD34+ cells
As shown in Figure 6C, 2 days after the culture, the percentage of CD34+Annexin V+PI− cells was significantly greater following the treatment of MF SP CD34+ cells with cytokines plus AZD1480 than that observed with corresponding MF SP CD34+ cells treated with cytokines alone (P < .001). Remarkably, treatment of CB CB34+ cells with the same doses of AZD1480 in the presence of cytokines was not associated with the same degree of apoptosis (P = .23).
AZD1480 treatment induces G0/G1 cell-cycle arrest in MF CD34+ cells
We next examined the effect of AZD1480 treatment on MF CD34+ cell-cycle status. As shown in Figure 6D, treatment of SP MF CD34+ cells with 150 nM AZD1480 for 3 days resulted in a significant accumulation of CD34+ cells in the G0 phase (P < .05) and a significant reduction of CD34+ cells in the G1 phase (P < .05) as compared with cells treated with cytokines alone. By contrast, AZD1480 treatment did not alter the cell-cycle status of CB CD34+ cells. These findings suggest that the inhibitory effect of AZD1480 on MF CD34+ cells was associated with the induction of apoptosis and G0/G1 cell-cycle arrest through the inhibition of JAK-STAT signaling which may account for the differential sensitivity to AZD1480 treatment observed between MF and CB CD34+ cells.
Discussion
Dysregulation of the JAK-STAT signaling pathway represents a central mechanism in the pathogenesis of myeloproliferative neoplasms (MPNs), and small-molecule inhibition of this pathway (primarily through JAK2 blockade) results in rapid relief of splenomegaly and debilitating MF-related constitutional symptoms, both in patients with JAK2V617F and in patients with wild-type JAK2.23,24 However, at present, current JAK inhibitors have been shown to have little impact in modifying the JAK2 mutant allele burden, marrow histology, or reducing the risk of transformation to acute myelogenous leukemia (AML). These finding are dramatically different than those that are observed with tyrosine kinase inhibitor therapy for another MPN, chronic myeloid leukemia (CML), where imatinib therapy is associated with a high proportion of patients achieving durable complete hematological cytogenetic and molecular remissions.35 Even in patients who are in complete cytogenetic remissions, however, Bhatia and coworkers detected residual BCR/ABL+ HPCs indicating that imatinib therapy, although capable of altering the natural history of CML, is not able to eliminate every primitive CML HPCs or SCID repopulating cells (SRCs).36 Several small-molecule inhibitors of JAK1/2 have been reported to be capable of inhibiting but not eliminating JAK2V617F+ progenitor cells.34,37 Furthermore, Mullaly and coworkers using a JAK2V617F+ animal model have provided data which indicated that although the JAK2 inhibitor TG101348 had therapeutic efficacy against serially transplanted JAK2V617F-evoked MPN, resulting in reduction in spleen size and the erythroid precursor cell number, treatment with the drug did not eliminate the MPN-initiating population in vivo.9 In this report, we have examined the effects of a potent JAK1/2/3 inhibitor on primary MF progenitor cells and NRCs. For these studies, we have used CD34+ cells that were isolated from the spleens of MF patients which we have previously shown to contain a higher frequency of malignant MF-SCs than that present in PB.27 Treatment with AZD1480 resulted in the depletion of both SP and PB MF-HPCs (CFU-GM and BFU-E) but did not affect the malignant NRCs. These data provide direct evidence that JAK inhibitors may affect only a subpopulation of progenitors, but spare another reservoir of malignant HPCs and stem cells. Our studies would account for the rapid but incomplete shrinkage in spleen size associated with JAK2 inhibitor therapy and the rapid enlargement in spleen size that not infrequently occurs if the drug is discontinued. These observations can be attributed to the persistence of a reservoir of malignant HPCs and NRCs which survive exposure to a JAK1/2/3 inhibitor, but which rapidly proliferate once the tyrosine kinase inhibitor therapy is halted. These data also highlight the possible limitations of JAK2 inhibitor therapy in eliminating MF-SCs. Because both interferon α38 and sequential treatment with a DNA methyltransferase inhibitor and a histone deacetylase inhibitor30 have been previously shown to be capable of depleting MPN stem cells, one might anticipate that these classes of drugs might be more likely to be more effective than JAK1/2 inhibitors in targeting MPN stem cells with the goal of affecting the evolution of MF to leukemia.
In PMF and post-PV or post-ET MF patients, hematopoiesis occurs not only in the marrow but also in a variety of extramedullary sites including the spleen and liver. In this report, the possibility that the dramatic effects on the spleen could be attributed to an increased sensitivity of SP MF-HPCs as compared with PB HPCs was examined by assessing the effects of AZD1480 on CD34+ cells isolated from the PB and spleens of the same MF patients. Treatment with AZD1480 reduced to a similar extent the numbers of CD34+, CD34+CD90+, CD34+CXCR4+ cells and assayable HPCs (CFU-GM, BFU-E) from both the spleen and PB of MF patients. AZD1480 treatment of either SP or PB CD34+ cells resulted in only a modest reduction of the fraction of colonies containing JAK2V617F-positive cells. The similar efficacy of the JAK2 inhibitor on both SP and PB MF-HSCs/HPCs suggests that the reduction in spleen size achieved with JAK1/2 inhibitor therapy cannot be attributed to the greater sensitivity of SP HPCs than PB HPCs to this class of compounds.
To date, none of the currently available JAK2 inhibitors has been shown to be specific to the mutant as opposed to wild-type JAK2.23-25,39 We have shown that AZD1480 treatment decreased the number of CD34+, CD34+CD90+, CD34+CXCR4+ cells, and assayable HPCs (CFU-GM, BFU-E) isolated from JAK2V617F+ and JAK2V617F−/CALR mutant+ MF patients to a similar degree. These findings are consistent with the clinical responses observed in patients with both JAK2V617F+ and JAK2V617F−/CALR mutant+ MF. We have further shown that MF CD34+ cells are characterized by enhanced JAK-STAT activity, irrespective of their JAKV617F and CALR status, whereas AZD1480 treatment resulted in the inhibition of JAK-STAT activity in both JAK2V617F-positive and negative/CALR mutant-positive MF CD34+ cells, leading to apoptosis and G0/G1 cell-cycle arrest. Anand et al have reported that JAK-STAT signaling was enhanced to a similar degree in patients with or without mutated JAK2 and that inhibition of intracellular signaling in MF CD34+ cells by the JAK1/2 inhibitor TG101209 was independent of the JAK2 mutational status.40
Although JAK2 inhibitor therapy clearly results in relief for patients from MF-related symptoms and the consequences of splenomegaly, the inability of AZD1480 to substantially eliminate the number of MF HPCs or to deplete MF-SCs as observed in this report leads one to question whether this class of agents has the potential to substantially alter the natural history of MF. More effective therapeutic strategies that would be able to not only block the JAK-STAT signaling pathway but also selectively target JAK2V617F and target at least MF-HPCs and possibly MF-SCs are clearly needed if drug treatments that substantially reduce the malignant MF cell burden are to become a reality.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from National Cancer Institute at the National Institutes of Health (1P01CA108671; R.H.).
Authorship
Contribution: X.W. designed and performed the experiments, analyzed the data, and wrote the paper; F.Y., C.S.H., J.T., Y.L., J.N., and J.Q. performed some of the experiments; V.N. reviewed FISH data; R.R. performed CALR mutational analysis and reviewed the paper; and R.H. designed the experiments, interpreted the data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Hoffman, Division of Hematology/Oncology, Tisch Cancer Institute, Department of Medicine, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Place, Box 1079, New York, NY 10029; e-mail: ronald.hoffman@mssm.edu.