In this issue of Blood, Wang et al report on the response of splenic-derived hematopoietic stem and progenitor cells from patients with myelofibrosis (MF) to the Janus kinase (JAK) inhibitor, AZD1480.1
In MF, MPN disease-propagating stem cells aberrantly mobilize from the bone marrow to the spleen. Several independent sources of data, including the current study, indicate that JAK2-mutant MPN stem cells are not effectively targeted by JAK inhibition. The current study by Wang et al suggests that following treatment with a JAK inhibitor, MPN progenitor cells in the spleens of patients with MF undergo apoptosis resulting in a reduction in splenomegaly. JAK inhibitor clinical trial data indicate that circulating proinflammatory cytokine levels decrease following JAK inhibition. Whether suppressing the inflammatory milieu through JAK inhibition could potentially delay progression or even reverse MF within the bone marrow, without selectively targeting the malignant hematopoietic clone, remains to be determined.
In MF, MPN disease-propagating stem cells aberrantly mobilize from the bone marrow to the spleen. Several independent sources of data, including the current study, indicate that JAK2-mutant MPN stem cells are not effectively targeted by JAK inhibition. The current study by Wang et al suggests that following treatment with a JAK inhibitor, MPN progenitor cells in the spleens of patients with MF undergo apoptosis resulting in a reduction in splenomegaly. JAK inhibitor clinical trial data indicate that circulating proinflammatory cytokine levels decrease following JAK inhibition. Whether suppressing the inflammatory milieu through JAK inhibition could potentially delay progression or even reverse MF within the bone marrow, without selectively targeting the malignant hematopoietic clone, remains to be determined.
A consistent finding in the JAK inhibitor clinical trials in MF is that a reduction in splenomegaly does not correlate with a reduction in JAK2V617F allele burden.2,3 Intrigued by this observation, Wang et al set about trying to provide a biological explanation for it. Analogous to the American bank robber Willie Sutton who, when asked why he robbed banks, reportedly responded, “Because that’s where the money is,” the authors turned to the spleens of patients with MF. The spleen is the major site of extramedullary hematopoiesis in MF and, in an earlier publication, the authors have elegantly demonstrated a splenic reservoir of myeloproliferative neoplasm (MPN)–propagating stem cells that can serially engraft NOD/SCID/IL2Rγnull (NSG) mice.4 Having established this MPN patient-derived xenograft model, Wang et al then focused on investigating the apparently discordant responses to JAK inhibition in the spleen and in the malignant hematopoietic clone observed in patients with MF.
The authors began by assessing the sensitivity of CD34+ cells isolated from either the peripheral blood (PB) or the spleens of patients with MF to in vitro treatment with the JAK inhibitor, AZD1480, and found that both were equally sensitive. Although CD34+ cells from patients with MF were more sensitive to in vitro treatment with AZD1480 than control CD34+ cells obtained from cord blood, the in vitro growth inhibition of MF cells following treatment with AZD1480 was not clearly enhanced in JAK2V617F-positive cells over JAK2-unmutated cells from the same patient. Importantly, the authors found no difference in the NSG-repopulating capacity of CD34+ cells from MF patients pretreated with AZD1480 as compared with cells pretreated with cytokines alone. In aggregate, this work provides further evidence in an MPN patient-derived xenograft model that JAK2V617F-positive MPN disease-propagating stem cells are not effectively targeted by JAK inhibition.
Supporting these findings, we have previously reported that a JAK2 inhibitor was effective at reducing splenomegaly but did not eradicate MPN disease-propagating stem cells in a Jak2V617F genetic murine model.5 Perhaps most compelling are the recently reported results of the COMFORT II study (ruxolitinib vs best available therapy) where, despite an ∼50% reduction in the risk of death in post hoc analysis at 3 years, the median change in the JAK2V617F allele burden from baseline was just −8.0% at 72 weeks,6 indicating that any survival benefit for ruxolitinib in MF is not due to preferentially targeting the malignant hematopoietic clone.
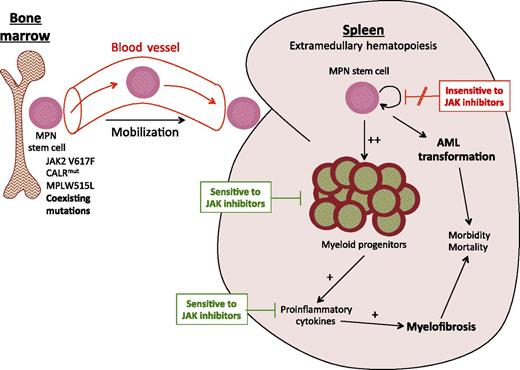
The work of Wang et al described in this issue of Blood suggests that splenic-derived MF hematopoietic progenitor cells are more sensitive to JAK inhibition, as compared with MF long-term hematopoietic stem cells (HSCs).1 Their model therefore proposes that the spleen shrinks because MF progenitor cells undergo apoptosis; however, the JAK2V617F allele burden does not fall because MF HSCs survive (see figure). In clinical trials in patients with MF, JAK2V617F allele burden is typically measured from PB DNA, which represents DNA derived mainly from granulocytes and lymphocytes, therefore reflecting downstream progeny of HSCs. Because myeloid progenitor cells are intermediates in the differentiation of HSCs to PB granulocytes, one might expect that if the expanded JAK2-mutant myeloid progenitor cell population in MF was preferentially sensitive to JAK2 inhibition (as compared with the JAK2-unmutated population), the JAK2V617F allele burden would fall to some degree, but this does not appear to occur in patients with MF treated with JAK inhibitors. Nevertheless, it is possible that PB JAK2V617F allele burden underestimates the effect of JAK inhibitors on erythroid progenitor cells, as terminally differentiated PB erythrocytes do not contain DNA. Because it is currently impossible to measure splenic JAK2V617F allele burden in patients with MF during treatment, we cannot determine whether JAK2-mutant hematopoietic cells within the spleen are preferentially sensitive to JAK inhibitor therapy in vivo. However, differences in the microenvironment of the bone marrow and the spleen could plausibly impact responses to JAK inhibition, with the presence of supporting stromal cells and/or differences in the levels of local inflammatory cytokines7 potentially rendering bone marrow JAK2-mutated cell populations less sensitive to JAK inhibition in vivo as compared with the same cellular populations within the spleen.
One caveat of the study by Wang et al is that mutational testing was limited to JAK2V617F and CALR mutations (cytogenetic analysis was also performed). Although Guglielmelli et al did not find any molecular predictors of response to ruxolitinib when they genotyped 14 MF-associated prognostically significant mutations in a subset of the COMFORT II cohort (166 of the 219 total patients were evaluated),8 earlier studies have indicated that JAK2V617F is not a strong driver of clonal expansion in the CD34+ compartment in MPN.9,10 This suggests that the presence of coexisting genetic alterations drives clonal evolution in JAK2-mutant MF and that genetic heterogeneity could influence the sensitivity of CD34+ cells from patients with MF to JAK inhibitor therapy. Comprehensive genomic characterization of patient-derived xenografts in MPN would provide valuable information when assessing responses to JAK inhibition and has the potential to identify novel therapeutic targets if combined with in vivo functional genomic and/or drug screening in xenografts.
Wang et al are to be commended for taking a fascinating clinical observation from the JAK inhibitor clinical trials and dissecting it in the laboratory in a true “beside-to-bench” style investigation. Although in the JAK inhibitor era the indication for splenectomy in MF and thus the supply of splenic-derived CD34+ cells for research is likely to be reduced, optimized MPN patient-derived xenografts provide an excellent model for additional studies to further dissect MF biology and therapeutic response. Through innovative thinking, and inspired by Sutton’s “wisdom,” the authors have opened the vault on a new avenue of MF research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal