Key Points
Kinase-functional BTK is important in the development and expansion of CLL.
Both targeted genetic inactivation of BTK and inhibition of BTK by ibrutinib inhibit the development of CLL in the TCL1 mouse model.
Abstract
Chronic lymphocytic leukemia (CLL) is characterized by constitutive activation of the B-cell receptor (BCR) signaling pathway, but variable responsiveness of the BCR to antigen ligation. Bruton’s tyrosine kinase (BTK) shows constitutive activity in CLL and is the target of irreversible inhibition by ibrutinib, an orally bioavailable kinase inhibitor that has shown outstanding activity in CLL. Early clinical results in CLL with other reversible and irreversible BTK inhibitors have been less promising, however, raising the question of whether BTK kinase activity is an important target of ibrutinib and also in CLL. To determine the role of BTK in CLL, we used patient samples and the Eμ-TCL1 (TCL1) transgenic mouse model of CLL, which results in spontaneous leukemia development. Inhibition of BTK in primary human CLL cells by small interfering RNA promotes apoptosis. Inhibition of BTK kinase activity through either targeted genetic inactivation or ibrutinib in the TCL1 mouse significantly delays the development of CLL, demonstrating that BTK is a critical kinase for CLL development and expansion and thus an important target of ibrutinib. Collectively, our data confirm the importance of kinase-functional BTK in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is a common adult leukemia that is currently incurable outside of stem cell transplantation. Although response to IgM ligation is variable, the B-cell receptor (BCR) signaling pathway is aberrantly active in this disease, with antigen-dependent1,2 or -independent autonomous activation,3 leading to constitutive activation of kinases inducing cell survival and proliferation.4-7 One BCR pathway kinase that is uniformly overexpressed at the transcript level8 and constitutively phosphorylated in CLL is Bruton’s tyrosine kinase (BTK). Ibrutinib, an orally bioavailable irreversible inhibitor of BTK, has recently been shown to have outstanding clinical activity in CLL with extended durable remissions in both untreated and relapsed disease.9
BTK is a critical mediator of B-lymphocyte signaling and development. Mutations in various domains are responsible for X-linked agammaglobulinemia,10,11 a disorder characterized by developmental arrest of B cells and profound humoral immune deficiency in humans. A point mutation in the Pleckstrin homology domain is responsible for the milder X-linked immunodeficiency (XID) phenotype in the mouse,12,13 which is characterized by reduced numbers of circulating B cells and reduced serum immunoglobulins. BTK is also a critical mediator in B-cell signaling. It is recruited to the membrane-bound signalosome in the early stages of B-cell activation, and, following phosphorylation by Syk and Lyn, participates in the phosphorylation of phospholipase C, gamma 2 (PLCγ2), which leads to production of the second messengers diacylglycerol and inositol-1,4,5-triphosphate. This pathway is amplified in CLL and leads to prosurvival signals through its effects on phosphatidylinositol 3-kinase (PI3K), PLCγ2, and nuclear factor-κB (NF-κB).5,8,14,15 Inhibition of BTK by ibrutinib interrupts BTK autophosphorylation after IgM ligation and reduces the expression of downstream targets of BCR activation including extracellular signal-regulated kinase (ERK), NF-κB, and v-akt murine thymoma viral oncogene homolog (Akt).8
In addition to intracellular signaling, interaction of CLL cells with the microenvironment is controlled by BCR signaling and plays an important role in the survival and proliferation of malignant cells in this disease.16,17 Ibrutinib has been shown to inhibit microenvironment survival signals and block the protective effect of stromal coculture in vitro.8
It is apparent that BTK is critical for the development and function of normal B lymphocytes, and protein expression appears to be required for CLL development.18 However, the precise role of the kinase function of BTK in the initial development of CLL, as well as the disease expansion phase, is unclear. In addition, the concept of targeting a specific protein kinase in CLL, similar to targeting BCR-Abl in chronic myeloid leukemia, is one not generally believed to be feasible in CLL. Indeed, the lack of a ubiquitously amplified or mutated protein and overall heterogeneity of the disease suggests that multiple pathways would need to be targeted to achieve disease control. Ibrutinib covalently binds BTK at cysteine 481 within the hinge region and potentially cross-reacts with similar kinases that possess a homologous residue19 including some involved in B- and T-cell signaling such as B lymphocyte kinase, TEC, and interleukin-2 inducible T-cell kinase.19 Ibrutinib’s lack of selectivity raises the possibility that BTK is not the critical target in CLL and that alternative kinases or multiple kinases should be the focus of future drug development. Here we present a series of experiments using both primary CLL cells and the Eμ-TCL1 transgenic mouse model of CLL. In this model, the TCL1 oncogene is under the control of the VH promoter-IgH-Eμ enhancer,20 which is first expressed in B cells at the transition to pre-B cells.21 Similar to what is observed in primary human CLL cells,8 in vitro cytotoxicity of ibrutinib in murine TCL1 leukemic spleen lymphocytes is modest; however, signaling through the BCR is inhibited in vitro and in vivo.22 The work outlined below will demonstrate that BTK is an important target in CLL and likely the critical kinase targeted by ibrutinib and will thus validate BTK as a target for future drug development.
Materials and methods
CLL patient samples
Blood samples were collected from patients that satisfied standard morphologic and immunophenotypic criteria for B-cell CLL. Informed consent was obtained from all patients according to the Declaration of Helsinki, and approval for the study was obtained from the institutional human research committee at the Catholic University Hospital “A. Gemelli.” Mononuclear cells were isolated by Ficoll gradient centrifugation. CLL B cells were purified by negative selection with anti-CD3, anti-CD14, and anti-CD16 mouse monoclonal antibodies (kindly provided by Professor Fabio Malavasi, University of Turin, Turin, Italy) and Dynabeads coated with pan anti-mouse IgG antibody (Invitrogen Dynal AS, Oslo, Norway). The purity of the selected B-cell populations was determined by staining with anti-CD5 R-phycoerythrin (R-PE)–conjugated and anti-CD19 fluorescein (FITC)–conjugated antibodies (BD Biosciences, Franklin Lakes, NJ), followed by flow cytometry analysis on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences). The purity of the negatively selected CLL B-cell populations was >97%.
Small interfering RNA nucleofection experiments
For the BTK knockdown experiments, freshly isolated CLL cells were nucleofected with BTK Validated Stealth RNAi or Stealth RNAi Negative Control small interfering (si)RNA (Invitrogen, Carlsbad, CA). Nucleofections were performed on 6 to 8 × 106 CLL cells using Nucleofector Solution V, the Amaxa Nucleofector II device, and the U-013 program (Amaxa Biosystems, Cologne, Germany). Following nucleofection, CLL cells were resuspended at a cell density of 1 × 107/mL in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate (Invitrogen). Cells were cultured alone, in the presence of immobilized anti-human IgM (2 × 107/mL Dynabeads M-450 Epoxy [Invitrogen Dynal AS] coated with 20 µg goat anti-human IgM [Southern Biotechnology Associates]) or in CLL-bone marrow stromal cell cocultures (1 × 105/mL M2-10B4 bone marrow stromal cells [ATCC-LGC Standards, Middlesex, United Kingdom]), as described elsewhere.23 After 72 hours, the percentage of viable cells was determined by Annexin-A5-FITC conjugate/propidium iodide staining (Nexins Research, Kattendijke, The Netherlands) and flow cytometry. Down-regulation of BTK was evaluated by immunoblotting analysis of cellular extracts obtained from aliquots of the same samples. Cellular extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred on Immobilon-P polyvinilidene difluoride membranes (Millipore, Billerica, MA), and blotted with BTK, rabbit IgG-horseradish peroxidase, mouse IgG horseradish peroxidase–linked (Cell Signaling Technology, Danvers, MA) or β-actin (Sigma-Aldrich, St. Louis, MO) antibodies. Immunodetection and quantification were done on a Gel Logic 2200 Imaging System (Eastman Kodak, Rochester, NY), using ECL Plus enhanced-chemiluminescence detection reagents (Amersham Biosciences, Buckinghamshire, United Kingdom).
Mice, mouse leukemia engraftment, and treatment with ibrutinib drinking water
All animal experiments were performed under a protocol approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee. TCL1 transgenic mice on a C3H/C57BL/6(B6) background have been previously described.20 This mouse strain has been extensively back-crossed with the B6 mouse to obtain a TCL1-expressing mouse on a pure B6 background. Strain purity was confirmed by microsatellite analysis (Charles River Laboratories, Wilmington, MA). To generate XID/TCL1 mice, homozygous B6/TCL1 mice were crossed with homozygous XID mice on a B6 background purchased from Jackson laboratories (Bar Harbor, ME). CB17/SCID mice for engraftments were purchased from Taconic Farms (Hudson, NY). Wild-type B6 mice for engraftments were purchased from Jackson Laboratories.
For all engraftment studies, leukemic spleen lymphocytes were obtained from B6/TCL1 mice with high white blood cell counts and splenomegaly. Spleens were manually pulverized, and spleen lymphocytes were purified by Ficoll density gradient separation. Flow cytometry was used to confirm that lymphocytes were leukemic, and then spleen lymphocytes were resuspended in sterile phosphate-buffered saline and injected through a lateral tail vein. For SCID engraftments, 1 × 106 cells were injected into each mouse. For XID and B6 engraftments, 5 × 106 to 5 × 107 cells were injected into each mouse, with equal amounts used for each individual experiment. Submandibular bleeding was used to obtain blood for smears and flow cytometry to evaluate disease progression. For experiments investigating survival, mice were killed for lethargy, difficulty walking due to spleen size, or loss of ≥20% body weight. Animals were evaluated daily, with formal measurements of weight weekly during engraftment studies and biweekly or monthly for long-term studies.
For ibrutinib treatment experiments, mice were provided drinking water via water bottles containing either 10% β-cyclodextrin (vehicle) or vehicle plus 0.16 mg/mL ibrutinib. Mice were allowed to drink liberally but were not provided another source of water. This drinking water formulation was kindly provided by Pharmacyclics. Mice receiving this as the only source of drinking water will receive ∼30 mg/kg/day of drug (supplemental Table 1; available on the Blood Web site).
Mouse flow cytometry
Flow cytometry to determine peripheral blood leukemia involvement was performed by staining fresh whole blood with anti-mouse CD45 APC, anti-mouse CD5 FITC, and anti-mouse CD19 PE (all from BD Biosciences). CountBright beads were obtained from Invitrogen and used according to manufacturer’s instructions. Cells were initially doublet-discriminated and then gated on the CD45-positive population. Flow cytometry was performed on a Beckman-Coulter FC-500 flow cytometer, and Kaluza software was used for data analysis.
Mouse immunoblot analysis
Whole cell lysates were prepared as previously described by our group.24 Equivalent amounts of protein were loaded into polyacrylamide gels and transferred onto nitrocellulose membranes. Following antibody incubations, proteins were detected with chemiluminescent substrate (SuperSignal; Pierce, Rockford, IL). pERK antibody was obtained from Millipore, ERK antibody was obtained from Cell Signaling Technology, and actin antibody was obained from Santa Cruz Biotechnology.
Statistical methods
Percent viability of CLL cells measured under different conditions is summarized with means and standard deviations and compared using paired Student t tests. For the mouse models, estimates of leukemia-free survival, overall survival (OS), and time to engraftment were obtained using the Kaplan-Meier method, and the log-rank test evaluated differences between curves. Leukemia-free survival was defined as the time from birth or engraftment to the date of flow cytometry showing CD5/CD19 in ≥10% of lymphocytes. OS was defined as the time from either birth or development of leukemia (noted in each individual experiment) until death. Time to engraftment was defined as the date of engraftment to the date of flow cytometry showing CD5/CD19 in ≥10% of lymphocytes. All tests were two-sided, and statistical significance was declared for P < .05.
Results
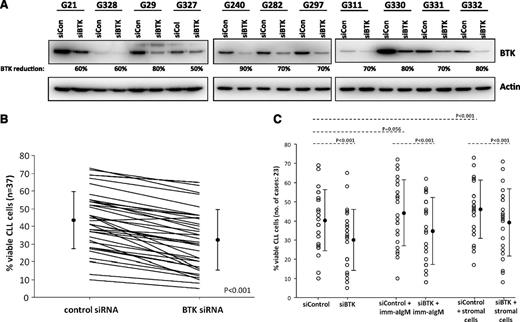
To first address the importance of BTK in CLL survival in vitro, we selectively down-regulated BTK protein by siRNA in primary tumor cells derived from 31 CLL patients (Figure 1A). Knockdown of BTK results in a significant decrease in tumor cell survival compared with control siRNA (P < .001; Figure 1B). Induction of apoptosis was also seen in the presence of BCR stimulation or stromal protection (Figure 1C; supplemental Figure 1), similar to what has been observed with CLL cells treated with ibrutinib in vitro.8 Collectively, these studies point toward the potential importance of this kinase in human CLL.
Specific knockdown of BTK impairs survival in CLL cells. (A) Knockdown of BTK by siRNA. Western blot analysis of BTK protein levels 72 hours after nucleofection with control or BTK-specific siRNA shows knockdown of BTK. This is representative of 37 patient samples. (B) CLL cell viability 72 hours after nucleofection with control or BTK siRNA. Following BTK knockdown, cells were cultured for 72 hours, and viability was determined at that time by percentage of cells negative for PI and Annexin V. Cells transfected with BTK siRNA had diminished viability compared with those treated with control siRNA. (C) Viability of CLL cells after transfection with BTK or control siRNA and coincubation with stromal cells or stimulation with IgM. BTK knockout reduced CLL cell viability even in the presence of stromal cells or after IgM stimulation.
Specific knockdown of BTK impairs survival in CLL cells. (A) Knockdown of BTK by siRNA. Western blot analysis of BTK protein levels 72 hours after nucleofection with control or BTK-specific siRNA shows knockdown of BTK. This is representative of 37 patient samples. (B) CLL cell viability 72 hours after nucleofection with control or BTK siRNA. Following BTK knockdown, cells were cultured for 72 hours, and viability was determined at that time by percentage of cells negative for PI and Annexin V. Cells transfected with BTK siRNA had diminished viability compared with those treated with control siRNA. (C) Viability of CLL cells after transfection with BTK or control siRNA and coincubation with stromal cells or stimulation with IgM. BTK knockout reduced CLL cell viability even in the presence of stromal cells or after IgM stimulation.
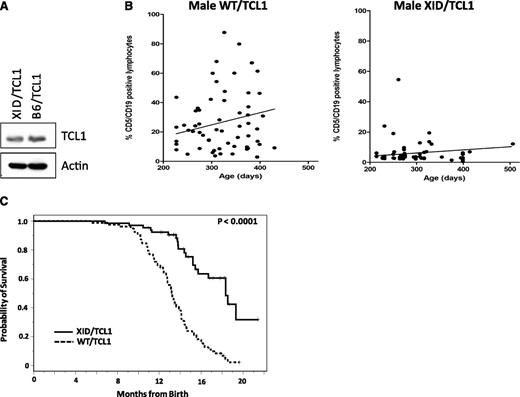
As we have seen that BTK knockdown leads to decreased CLL cell viability, we sought to determine whether the BTK kinase is a viable target in CLL in vivo. To evaluate this we used the well-characterized TCL1 mouse model, which has been shown to possess genetic, epigenetic, and pharmacologic properties of human CLL.20,25 B6/TCL1 mice were crossed with the well-characterized XID mouse, which has a point mutation in BTK preventing PH domain binding and therefore kinase activity.12,13,26 Cells derived from XID/TCL mice continue to express TCL1 protein (Figure 2A) and do not respond to IgM stimulation as robustly as WT/TCL1 mice (supplemental Figures 2 and 3). They have modestly decreased B cells and similar numbers of T cells compared with WT/TCL1 littermates (average 1843 B cells/μL and 1379 T cells/μL in XID/TCL1 vs 2157 B cells/μL and 1236 T cells/μL in WT/TCL1 mice). We next performed peripheral blood flow cytometry for CD5 and CD19 co-expressing leukemia cells on 59 WT/TCL1 and 61 XID/TCL1 mice of varying ages. At similar time points, the WT/TCL1 mice showed significantly higher percentage and number of leukemic cells than XID/TCL1 mice (Figure 2B). Furthermore, survival analysis of all male mice born within a 12-month period, which included 78 WT/TCL1 and 65 XID/TCL1, demonstrated superior survival in the XID/TCL1 cohort. Median OS for WT/TCL1 mice was 13.2 months (95% confidence interval: 12.6-14.0 months) vs 18.3 months for XID/TCL1 mice (95% confidence interval: 15.4 months to not reached; Figure 2C; P < .0001). All XID/TCL1 mice that have been killed had flow cytometric evaluation of the spleen to detect leukemic cells. In a cohort of 49 mice that had spleen cells available for flow cytometry, 61% had developed a CD5/CD19 coexpressing leukemia and 18% had developed a T-cell leukemia, which is also seen in the TCL1 mouse model. The delay in leukemia development and improvement in OS thus demonstrate that kinase functional BTK is important for leukemia development in the TCL1 model of CLL and suggest that BTK is therefore an important target of ibrutinib.
Kinase-functional BTK is necessary for the development and expansion of TCL1 leukemia. (A) TCL1 protein expression is retained after the cross with the XID mouse. Splenic lymphocytes from a pool of 3 1-month-old female XID/TCL1 mice and 1-month-old female B6/TCL1 mice were purified and B cell selected using the EasySep Mouse B Cell Enrichment Kit (Stem Cell Technologies) and then lysed; 50 μg of protein from each mouse was used for western blot analysis of TCL1 protein expression. TCL1 is present in both the B6/TCL1 and the XID/TCL1 mice. (B) WT/TCL1 mice have a higher percentage of leukemic lymphocytes in the peripheral blood compared with XID/TCL1 mice. Peripheral blood flow cytometry for CD5 and CD19 was performed on 59 WT/TCL1 and 61 XID/TCL1 mice. XID/TCL1 mice had a lower percentage of leukemic CD5/CD19 coexpressing cells than did WT/TCL1 mice. (C) OS is improved for XID/TCL1 mice compared with WT/TCL1 mice. All male XID/TCL1 and WT/TCL1 mice born within a 1-year time period, which included 65 XID/TCL1 and 78 WT/TCL1 mice, were followed for survival. OS is significantly prolonged in the XID/TCL1 cohort (P < .0001).
Kinase-functional BTK is necessary for the development and expansion of TCL1 leukemia. (A) TCL1 protein expression is retained after the cross with the XID mouse. Splenic lymphocytes from a pool of 3 1-month-old female XID/TCL1 mice and 1-month-old female B6/TCL1 mice were purified and B cell selected using the EasySep Mouse B Cell Enrichment Kit (Stem Cell Technologies) and then lysed; 50 μg of protein from each mouse was used for western blot analysis of TCL1 protein expression. TCL1 is present in both the B6/TCL1 and the XID/TCL1 mice. (B) WT/TCL1 mice have a higher percentage of leukemic lymphocytes in the peripheral blood compared with XID/TCL1 mice. Peripheral blood flow cytometry for CD5 and CD19 was performed on 59 WT/TCL1 and 61 XID/TCL1 mice. XID/TCL1 mice had a lower percentage of leukemic CD5/CD19 coexpressing cells than did WT/TCL1 mice. (C) OS is improved for XID/TCL1 mice compared with WT/TCL1 mice. All male XID/TCL1 and WT/TCL1 mice born within a 1-year time period, which included 65 XID/TCL1 and 78 WT/TCL1 mice, were followed for survival. OS is significantly prolonged in the XID/TCL1 cohort (P < .0001).
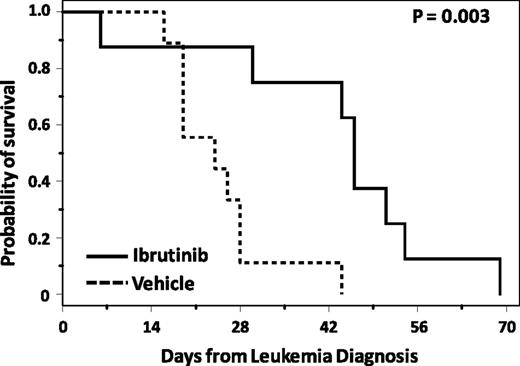
Next, we sought to determine whether ibrutinib is effective in the TCL1 mouse model, and whether ibrutinib could inhibit CLL development in a manner similar to genetic manipulation of BTK kinase activity. Prior studies with ibrutinib showing effects on disease progression and BCR signaling have used a serially transplanted TCL1 leukemia cell line model that was initially antigen selected to create consistent BCR expression.22 Although this is an excellent tool for BCR signaling evaluation, it is not representative of the primary TCL1 leukemia cells that show more heterogeneous signaling (supplemental Figure 4). To determine whether ibrutinib was also effective in spontaneous TCL1 leukemia, TCL1 leukemic spleen lymphocytes were transplanted into CB17/SCID mice. This model has the advantage of producing a CLL-like phenotype including peripheral blood leukemia, splenomegaly, and occasionally lymphadenopathy in a short period of time compared with the long latency period of the original TCL1 mouse model. At the time of leukemia diagnosis by flow cytometry, defined as ≥10% of lymphocytes coexpressing CD5 and CD19, mice were randomized to treatment with a drinking water containing 10% cyclodextrin (vehicle) or ibrutinib at a concentration providing ∼30 mg/kg/day and that has been shown to occupy ∼80% of available BTK in vivo (supplemental Table 1; supplemental Figure 5). BCR signaling, using phosphorylation of ERK as a surrogate, is inhibited in these mice (supplemental Figure 2). From the time of leukemia diagnosis, mice treated with ibrutinib survived significantly longer (46 vs 24 days, P = .003) with ibrutinib compared with vehicle (Figure 3). Unlike human CLL, in this model, no lymphocytosis was observed after treatment with ibrutinib. These data confirm that ibrutinib is effective in transplanted primary TCL1 leukemia cells not selected for enhanced BCR signaling and also that this drug inhibits the expansion phase of CLL in this model.
Ibrutinib improves OS in mice transplanted with TCL1 leukemia. After transplantation with leukemic TCL1 spleen lymphocytes, SCID mice were followed for leukemia development, and once leukemic, randomized to treatment with ibrutinib or vehicle. Mice treated with ibrutinib (n = 8) survived significantly longer than those treated with vehicle (n = 9; P = .003).
Ibrutinib improves OS in mice transplanted with TCL1 leukemia. After transplantation with leukemic TCL1 spleen lymphocytes, SCID mice were followed for leukemia development, and once leukemic, randomized to treatment with ibrutinib or vehicle. Mice treated with ibrutinib (n = 8) survived significantly longer than those treated with vehicle (n = 9; P = .003).
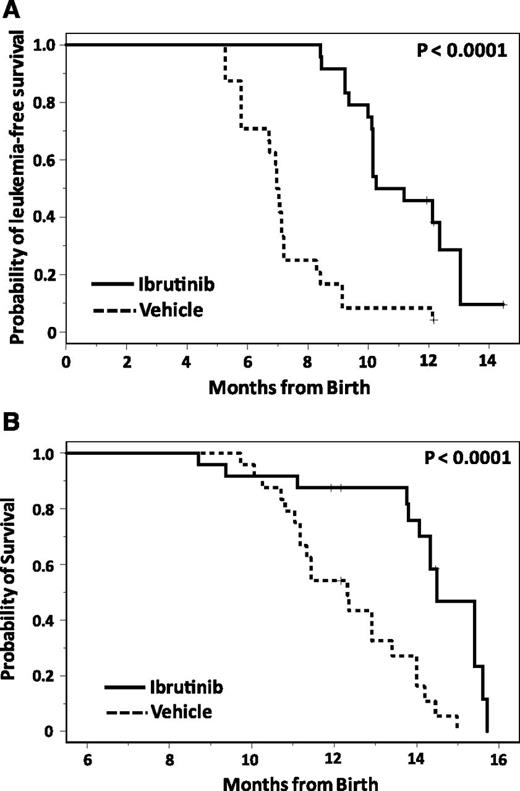
We next sought to determine whether inhibition of BTK kinase function by ibrutinib can prevent CLL development similar to that observed in XID/TCL1 mice outlined above. To evaluate this, TCL1 pups on both the B6 and C3H/B6 background were treated continuously starting at 1 month of age with ibrutinib or vehicle drinking water. Monthly assessment of CD5/CD19 leukemia cells by flow cytometry was performed for both groups. Mice treated with ibrutinib had a significantly prolonged time to leukemia development compared with vehicle (median 10.7 months [95% confidence interval: 5.8-7.2] vs 7.0 months [95% confidence interval: 10.1-13.0]; P < .0001; Figure 4A). OS was also extended in mice treated with ibrutinib compared with vehicle (median 12.3 months [95% confidence interval: 11.2-13.4] vs 14.5 months [95% confidence interval: 14.1-15.6], P < .0001; Figure 4B). This confirms that ibrutinib treatment inhibits the development of TCL1 leukemia similar to genetic inactivation of BTK.
Ibrutinib improves outcomes in continuously-treated TCL1 mice. (A) Continuous administration of ibrutinib inhibits the development of CLL in TCL1 mice. TCL1 mice were randomized at 1 month of age to treatment with vehicle drinking water (n = 24) or ibrutinib drinking water (n = 24), and monitored for leukemia development by monthly peripheral blood flow cytometry. Ibrutinib significantly impairs leukemia development (P < .0001). (B) Continuous administration of ibrutinib improves survival in TCL1 mice. TCL1 mice were randomized at 1 month of age to treatment with vehicle drinking water (n = 24) or ibrutinib drinking water (n = 24) and followed for survival. Survival was significantly improved by ibrutinib administration compared with vehicle (P < .0001).
Ibrutinib improves outcomes in continuously-treated TCL1 mice. (A) Continuous administration of ibrutinib inhibits the development of CLL in TCL1 mice. TCL1 mice were randomized at 1 month of age to treatment with vehicle drinking water (n = 24) or ibrutinib drinking water (n = 24), and monitored for leukemia development by monthly peripheral blood flow cytometry. Ibrutinib significantly impairs leukemia development (P < .0001). (B) Continuous administration of ibrutinib improves survival in TCL1 mice. TCL1 mice were randomized at 1 month of age to treatment with vehicle drinking water (n = 24) or ibrutinib drinking water (n = 24) and followed for survival. Survival was significantly improved by ibrutinib administration compared with vehicle (P < .0001).
Discussion
Collectively, our data show that kinase-functional BTK is critical to the development and expansion of CLL. Although the inhibition in disease expansion demonstrated by the survival advantage in established disease is expected given the dramatic responses to this drug seen in the clinic, more remarkable is the significant delay in leukemia onset after targeted inactivation of BTK through either the XID/TCL1 model or the administration of ibrutinib. The XID/TCL1 cross definitively demonstrates that BTK is an important kinase in CLL, and the similar results seen with the BTK inhibitor ibrutinib suggest that this is the most relevant target of ibrutinib in CLL. Although our data do not rule out the contribution of other related kinases to the efficacy of ibrutinib in patients, it does provide further support that BTK is indeed an important target of the drug.
The efficacy that has been seen with ibrutinib has led to preliminary development of more selective next-generation BTK inhibitors, but the discouraging preliminary results with more selective inhibitors (AVL-292/CC-292)27 questions whether ibrutinib is effective because it targets BTK or because it has multiple alternative targets including interleukin-2 inducible T-cell kinase, TEC, and B lymphocyte kinase.19 The dramatic leukemia inhibition seen with the XID/TCL1 cross shows that targeted inhibition of BTK kinase activity is sufficient to inhibit leukemia development in the TCL1 mouse model and suggests that clinical investigation of selective BTK inhibitors is reasonable.
This conclusion is important to our understanding of CLL, as ibrutinib therapy is the first instance where selective kinase inhibition is broadly effective in a disease without a uniform mutation or genetic modification. The model of selective kinase inhibition is effective with imatinib in chronic myeloid leukemia and vemurafenib in the subset of melanoma patients with the V600E v-raf murine sarcoma viral oncogene homolog B (BRAF) mutation.28 Critically, however, in the studies that have examined recurrent DNA mutations in CLL, BTK has not been shown to be mutated in a significant subset of patients.29-31 These data therefore indicate that pathway activation, even in the absence of observed genetic modification, can serve as an effective therapeutic target in an otherwise genetically complex disease. BTK is an ideal candidate for selective inhibition as the severe phenotype of BTK dysfunction reveals its importance in B cells; however, the model may be applicable to other kinases in CLL and even other diseases.
It has recently been demonstrated that BTK expression is necessary for the development of leukemia in the IgH.ETμ mouse model,18 and it is notable that in this model where BTK was knocked out entirely, no leukemia was observed. In our model of the XID/TCL1 cross, leukemia developed very late, but did still develop in the majority of animals. We believe that the data presented here are more applicable to human CLL, as drug development now and in the future will focus on inhibition of kinase function of BTK rather than gene knockout. In addition, the TCL1 mouse model used in the experiments described here has been extensively characterized so that the similarities to human CLL are well established,20,25 and the use of ibrutinib in this model is directly relevant to human CLL. The fact that genetic knockout of BTK produced no disease, where disease is seen in the XID/TCL1 cross and the ibrutinib-treated TCL1 mice, suggests that resistance to ibrutinib is mediated through BTK itself. In such case, the absence of BTK cannot be overcome, but drug inhibition and even genetic mutation can. This finding is important to future work investigating mechanisms of resistance associated with ibrutinib.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported in part by the Leukemia and Lymphoma Society, D. Warren Brown Foundation, Mr and Mrs Michael Thomas, the Harry Mangurian Foundation, National Institutes of Health, National Cancer Institute grants P50 CA140158, K12 CA133250-05, R01 CA177292, and P01 CA095426, and the Italian Association for Cancer Research (AIRC IG_12939).
Authorship
Contribution: J.A.W., J.C.B., and A.J.J. designed and performed research, analyzed data, and wrote the paper; E.B., L.L., and D.G.E. designed and performed research and analyzed data; A.S.R. analyzed data; M.R.S., V.M.G., K.A.S., L.L.S., J.A.D., W.H.T., J.M., B.K.H., S.G., and M.E.D. performed research; and B.Y.C. and J.J.B. contributed vital reagents.
Conflict-of-interest disclosure: B.Y.C. and J.J.B. are employees of Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Amy J. Johnson, 445C Wiseman Hall, 410 W 12th Ave, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.