Key Points
A 20-gene gene expression-based assay accurately and robustly assigns COO subtypes of DLBCL using formalin-fixed paraffin-embedded tissue.
Abstract
The assignment of diffuse large B-cell lymphoma into cell-of-origin (COO) groups is becoming increasingly important with the emergence of novel therapies that have selective biological activity in germinal center B cell–like or activated B cell–like groups. The Lymphoma/Leukemia Molecular Profiling Project's Lymph2Cx assay is a parsimonious digital gene expression (NanoString)–based test for COO assignment in formalin-fixed paraffin-embedded tissue (FFPET). The 20-gene assay was trained using 51 FFPET biopsies; the locked assay was then validated using an independent cohort of 68 FFPET biopsies. Comparisons were made with COO assignment using the original COO model on matched frozen tissue. In the validation cohort, the assay was accurate, with only 1 case with definitive COO being incorrectly assigned, and robust, with >95% concordance of COO assignment between 2 independent laboratories. These qualities, along with the rapid turnaround time, make Lymph2Cx attractive for implementation in clinical trials and, ultimately, patient management.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of cancers classified together on the basis of morphology, immunophenotype, genetic alterations, and clinical behavior.1 The distinction of DLBCL into cell-of-origin (COO) categories based on patterns of gene expression reminiscent of germinal center B cell (the GCB group) and activated B cell (the ABC group), with a small number of unclassified cases, as defined and characterized by the Lymphoma/Leukemia Molecular Profiling Project (LLMPP),2,3 has profound biological,4 prognostic,5,6 and potential therapeutic implications.7-9 New therapeutic agents with selective activity in ABC and GCB DLBCL are under development. The original methods used to define these entities performed gene expression profiling (GEP) using microarrays on RNA derived from frozen tissue (FT). Subsequently, in an attempt to determine COO in standard practice using commonly available formalin-fixed paraffin-embedded tissue (FFPET), we and others used less precise10 but relatively inexpensive binary immunohistochemical (IHC) methods.11-13 Recently, the feasibility of quantitating gene expression in FFPET in lymphoma has been demonstrated.14-18 We sought to create a robust, highly accurate, validated molecular assay for COO distinction using GEP techniques applicable to FFPET.19
Study design
Studies were performed on FFPET biopsies of de novo DLBCL cases that had been classified using the original GEP methods and published algorithm (the gold standard method).6 Each case was centrally reviewed by a quorum of ≥4 LLMPP pathologists and had GEP performed on matched FT biopsies using Affymetrix U133 plus 2.0 microarrays. Data are available at www.ncbi.nlm.nih.gov/geo/query/acc.cgi (accession number GSE53786). The training cohort consisted of 51 cases (20 GCB, 19 ABC, and 12 unclassified). The independent validation cohort of 68 cases, drawn from the validation cohort in Lenz et al,6 had proportions of GCB (28 cases), ABC (30 cases), and unclassified (10 cases) typically observed in DLBCL populations. Patient characteristics of the cohorts are shown in supplemental Table 1 on the Blood Web site.
Tumors made up ≥60% of the surface area of the blocks. Ten-micrometer scrolls of FFPET were cut, to a surface area of 1 cm2, and tested in parallel at 2 independent laboratories: the Molecular Characterization Laboratory (MoCha), Frederick National Laboratory for Cancer Research (Frederick, MD), and the Centre for Lymphoid Cancer (CLC), BC Cancer Agency (Vancouver, BC, Canada). Nucleic acids were extracted using the Qiagen AllPrep FFPET kit, and digital GEP was performed on 200 ng RNA using NanoString technology (Seattle, WA). Details of study design are presented in the supplemental Methods.
To assign COO by IHC, tissue microarrays were made using 0.6-mm duplicate cores from 60 of 68 validation cohort cases and stained for CD10, BCL6, IRF4/MUM1, FOXP1, GCET1, and LMO2. Two expert hematopathologists independently assessed the proportion of tumor cells stained, with consensus on discordant cases reached with a third hematopathologist. For the validation studies, all individuals producing and analyzing the GEP and IHC data were blinded to the gold standard COO assignment.
Patients in the validation cohort received CHOP-type chemotherapy plus rituximab (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone [R-CHOP; n = 62]; R, C, mitoxantrone, O, P [n = 3]; R, C, H, etoposide, O, P [n = 1]; R, E, methylprednisolone, cytarabine, cisplatin [n = 1]; R-CHOP/hematopoietic stem cell transplantation [n = 1]). Progression-free survival and overall survival, defined as per the International Working Group response criteria,20 were estimated using the Kaplan-Meier method. Comparisons between groups were performed using the log-rank test.
This study was approved by the Institutional Review Boards of the participating centers in accordance with the Declaration of Helsinki.
Results and discussion
All FFPET biopsies yielded sufficient RNA at both laboratories for the NanoString technology analyses. A pilot study, using the training cohort, determined the FFPET gene expression by NanoString technology of 93 genes, identified in Lenz et al6 to differentiate ABC and GCB DLBCL subtypes. Fifteen genes, along with 5 housekeeping genes, were selected based on their ability to contribute to the accurate replication of the COO assignment model of Lenz et al.6 NanoString technology was then used to determine the expression of these 20 genes in FFPET-derived RNA from the training cohort, allowing a model to be built. As NanoString probes may vary in their hybridization efficiency from lot to lot, a synthetic oligonucleotide reference was run alongside the patient samples, with the gene expression of the samples adjusted for the results in the reference. This allows the model to be portable to new code set lots and also reduces other sources of assay variability. Details of the model’s performance in the training cohort are presented in the supplemental Methods. The locked model, including gene coefficients, thresholds, and quality criteria, was then applied to the independent validation cohort. This assay has been named the Lymph2Cx.
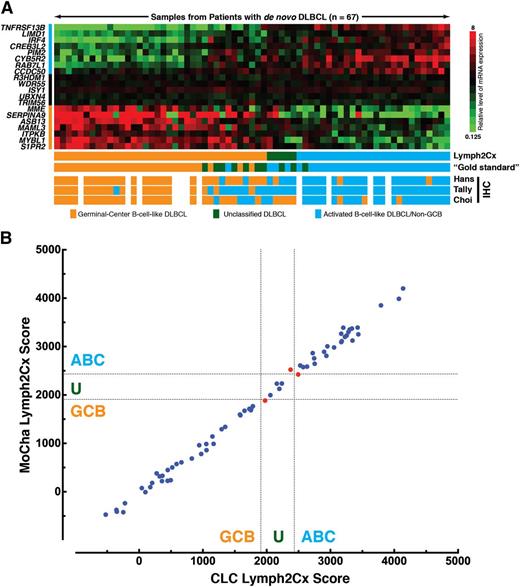
Ninety-nine percent of the independent validation cohort, with FFPET blocks ranging from 5 to 12 years of age, yielded gene expression of adequate quality (67 of 68 at both sites, with different single cases failing at each center). When considering the 58 cases designated as ABC or GCB by the gold standard method, the Lymph2Cx assay incorrectly assigned 1 case: an ABC by FT GEP assigned to GCB (Figure 1A). At 2%, this favorably compares with the 9%, 6%, and 17% rates of misassignment by the Hans, Tally, and Choi IHC-based algorithms, respectively (Figure 1A).11-13 Of note, the accuracy of the IHC-based algorithms observed here is consistent with the original descriptions11-13 but superior to that seen in a recent report that also used FT-based COO designation as the comparator.10 In replication of the original COO model and distinct from the binary IHC models, the Lymph2Cx assay recognizes a group of unclassified cases, where confident assignment cannot be made to either the ABC or GCB subtype. Among the 58 cases, 3 (5%) at the MoCha site and 4 (7%) at the CLC site were designated as unclassified. Full assignment data, including that of the unclassified cases by the gold standard method, are shown in supplemental Table 2.
Performance of the Lymph2Cx assay in the independent validation cohort. (A) The Lymph2Cx model is shown in the form of a gene expression heatmap (upper) with 67 patient samples from the independent validation cohort arrayed left to right in ascending order of the assay score. The 20 genes that contribute to the model are shown at the left, with the top 8 genes being overexpressed in ABC, the middle 5 genes being housekeeping genes, and the lower 7 genes being overexpressed in GCB. (Lower) The cell-of-origin assignments are shown for the assay, the gold standard method using previously published algorithms6 on gene expression from FT and 3 immunohistochemistry-based algorithms. The Lymph2Cx results shown are from the Molecular Characterization Laboratory (FNLCR, Frederick, MD), with 1 of the 68 cases in the independent validation cohort having failed. Results from the Centre for Lymphoid Cancer, BC Cancer Agency, Vancouver, BC Canada, are shown in supplemental Figure 2. (B) Comparison of the Lymph2Cx scores in the validation cohort from the 2 independent laboratories: the Molecular Characterization Laboratory (MoCha) (Frederick National Laboratory for Cancer Research) and the CLC, BC Cancer Agency. The dotted lines represent the thresholds between GCB, unclassified, and ABC. The 66 of 68 cases where both laboratories generated results are shown. The 3 cases that gave discordant COO assignments are shown in red. The concordance is 98%, when considering the ABC and GCB cases by the gold standard method, and 95% if the unclassified cases are included. The R2 is 0.996, and the slope of the line of best fit is 1.015. Comparisons in the training and total cohorts are shown in supplemental Figure 3.
Performance of the Lymph2Cx assay in the independent validation cohort. (A) The Lymph2Cx model is shown in the form of a gene expression heatmap (upper) with 67 patient samples from the independent validation cohort arrayed left to right in ascending order of the assay score. The 20 genes that contribute to the model are shown at the left, with the top 8 genes being overexpressed in ABC, the middle 5 genes being housekeeping genes, and the lower 7 genes being overexpressed in GCB. (Lower) The cell-of-origin assignments are shown for the assay, the gold standard method using previously published algorithms6 on gene expression from FT and 3 immunohistochemistry-based algorithms. The Lymph2Cx results shown are from the Molecular Characterization Laboratory (FNLCR, Frederick, MD), with 1 of the 68 cases in the independent validation cohort having failed. Results from the Centre for Lymphoid Cancer, BC Cancer Agency, Vancouver, BC Canada, are shown in supplemental Figure 2. (B) Comparison of the Lymph2Cx scores in the validation cohort from the 2 independent laboratories: the Molecular Characterization Laboratory (MoCha) (Frederick National Laboratory for Cancer Research) and the CLC, BC Cancer Agency. The dotted lines represent the thresholds between GCB, unclassified, and ABC. The 66 of 68 cases where both laboratories generated results are shown. The 3 cases that gave discordant COO assignments are shown in red. The concordance is 98%, when considering the ABC and GCB cases by the gold standard method, and 95% if the unclassified cases are included. The R2 is 0.996, and the slope of the line of best fit is 1.015. Comparisons in the training and total cohorts are shown in supplemental Figure 3.
It has become increasingly apparent that preanalytical, analytical, and interobserver variability contribute to poor reproducibility of COO assignment by IHC methods.21 The independent testing of the FFPET biopsies at 2 laboratories, beginning after the scrolling of the blocks, allowed determination of interlaboratory concordance and the robustness and portability of the assay. The Lymph2Cx scores produced at the 2 sites show a very high degree of concordance (Figure 1B).
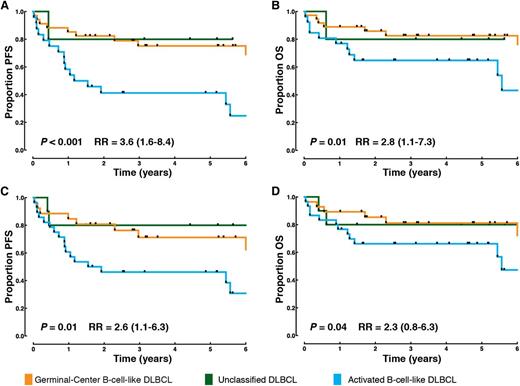
Outcomes in the validation cohort were used to determine whether the COO assignments made by the Lymph2Cx assay maintained the prognostic significance previously demonstrated for the original FT sample-based method.6 The gold standard method– and Lymph2Cx-defined ABC groups both had significantly worse outcome than the GCB groups (Figure 2). Larger cohorts will need to be examined to provide the statistical power to determine whether this prognostic power is independent of other biomarkers, particularly the International Prognostic Index.22 Outcomes in the COO groups assigned by IHC were not significantly different in this cohort (supplemental Figure 1).
Patient outcomes according to COO in the independent validation cohort. (A) Progression-free survival in the COO groups as determined by the Lymph2Cx assay. (B) Overall survival in the COO groups as determined by the Lymph2Cx assay. (C) Progression-free survival in the COO groups determined by the gold standard method applying the previously described model6 to gene expression on FT. (D) Overall survival in the COO groups determined by the gold standard method. The P values are from log-rank tests comparing the ABC and GCB groups. The log-rank tests are 1 sided in the direction of greater hazard for ABC. RR, relative risk (with the 95% confidence interval in brackets) associated with the ABC group compared with the GCB group. The groupings in A and B are from the results at the Molecular Characterization Laboratory (Frederick National Laboratory for Cancer Research). Results from the Centre for Lymphoid Cancer, BC Cancer Agency, Vancouver, BC Canada, are shown in supplemental Figure 4.
Patient outcomes according to COO in the independent validation cohort. (A) Progression-free survival in the COO groups as determined by the Lymph2Cx assay. (B) Overall survival in the COO groups as determined by the Lymph2Cx assay. (C) Progression-free survival in the COO groups determined by the gold standard method applying the previously described model6 to gene expression on FT. (D) Overall survival in the COO groups determined by the gold standard method. The P values are from log-rank tests comparing the ABC and GCB groups. The log-rank tests are 1 sided in the direction of greater hazard for ABC. RR, relative risk (with the 95% confidence interval in brackets) associated with the ABC group compared with the GCB group. The groupings in A and B are from the results at the Molecular Characterization Laboratory (Frederick National Laboratory for Cancer Research). Results from the Centre for Lymphoid Cancer, BC Cancer Agency, Vancouver, BC Canada, are shown in supplemental Figure 4.
In summary, herein we described a robust method for COO assignment, applicable to FFPET biopsies that are generated as part of routine diagnostic workflow. Tested against the gold standard FT Affymetrix-based method, the rates of correct assignment are very high, and the prognostic significance is maintained. Thus, the Lymph2Cx assay brings to fruition the potential to use gene expression-based COO assignment, first described more than a decade ago,2 in standard practice. With this demonstrated accuracy and portability, combined with a rapid turnaround time of less than 36 hours, the application of this assay will enable prospective selection of patients for therapeutic clinical trials and, ultimately, will guide appropriate patient management in clinical practice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge the contributing efforts of the past members of the LLMPP, our collaborators on previous DLBCL projects, and administrative support.
The project was funded by National Cancer Institute grant 1U01CA157581-01. D.W.S. is supported by a Canadian Institutes of Health Research Post-Doctoral Fellowship. A.M. is supported by a fellowship award by the Mildred Scheel Cancer Foundation.
Authorship
Contribution: D.W.S. and G.W.W. designed and performed research and wrote the manuscript; P.M.W. designed research and read and approved the final manuscript; C.-J.L., W.W., A.M., and B.J.G.-G. performed research and read and approved the final manuscript; E.S.J., A.R., E.C., W.C.C., R.M.B., G.O., J.D., R.R.T., J.R.C., D.D.W., T.C.G., and K.F. contributed vital reagents, performed research, and read and approved the final manuscript; J.M.C. performed research, curated clinical and survival data, and read and approved the final manuscript; E.B.S. contributed vital reagents and read and approved the final manuscript; L.M.S. designed and performed research and read and approved the final manuscript; and R.D.G. and L.M.R. designed and performed research and edited and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lisa M. Rimsza, 1501 N Campbell Ave, PO Box 245043, Tucson, AZ 85724-5043; e-mail: lrimsza@email.arizona.edu.
References
Author notes
D.W.S. and G.W.W. contributed equally to the work.
L.M.S., R.D.G., and L.M.R. contributed equally to the work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal